Controlled Drug Delivery Systems: Current Status and Future Directions
Abstract
:1. Introduction
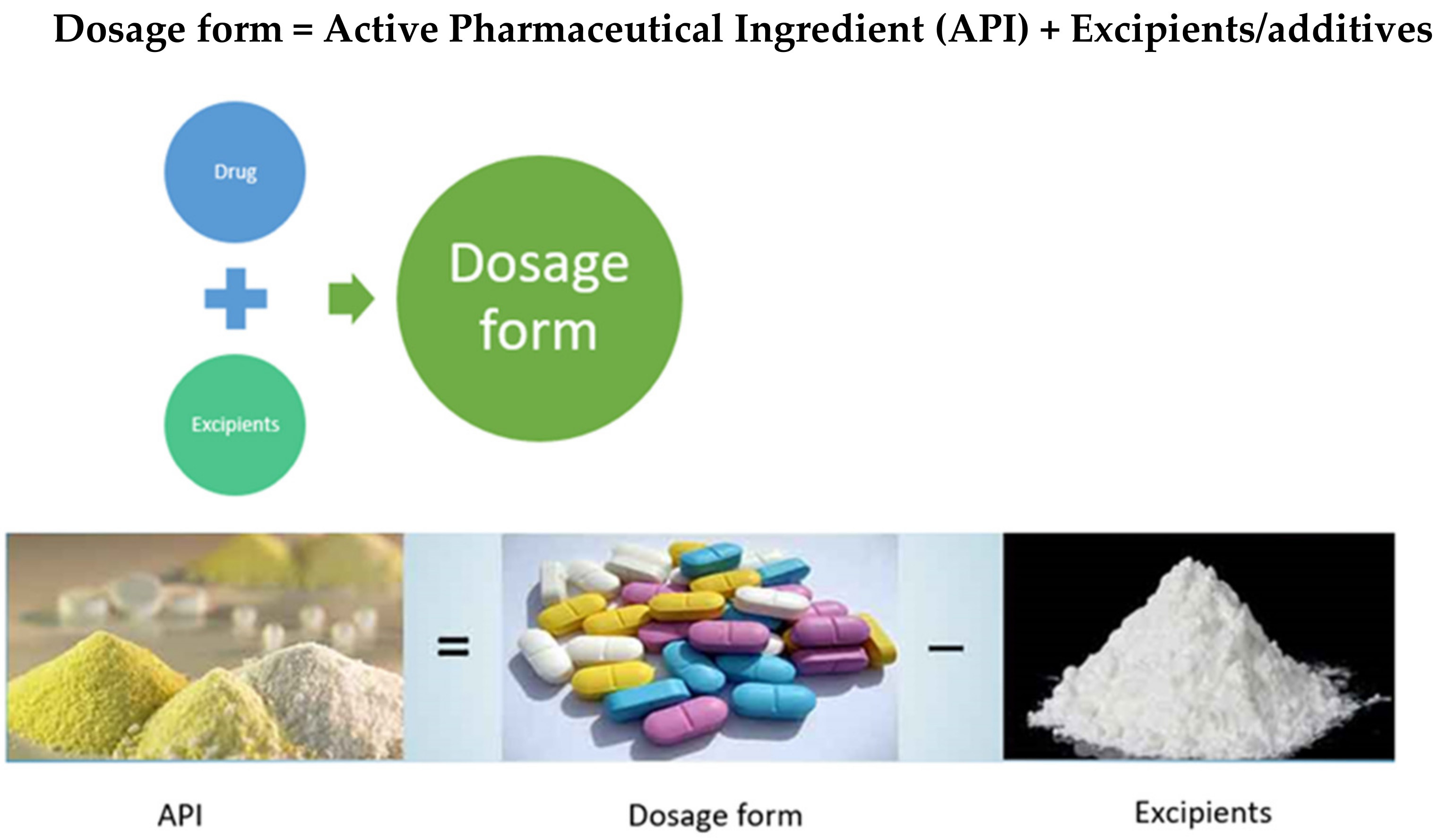
1.1. Need for a Dosage Form
1.2. Excipients
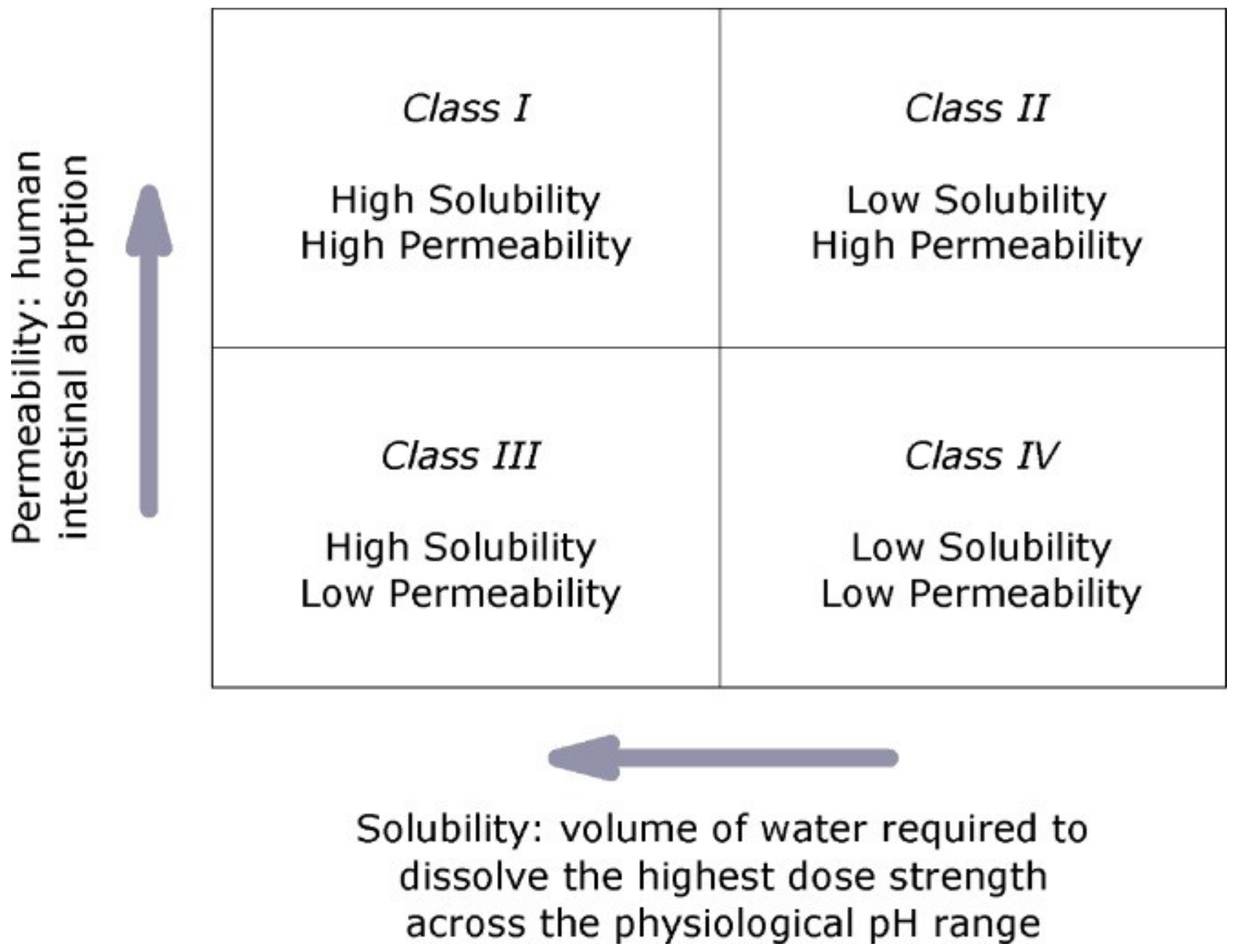
1.3. Biopharmaceutics Classification System (BCS) Classification of Drugs
1.4. Different Routes of Drug Administration
2. Classification of Dosage Forms
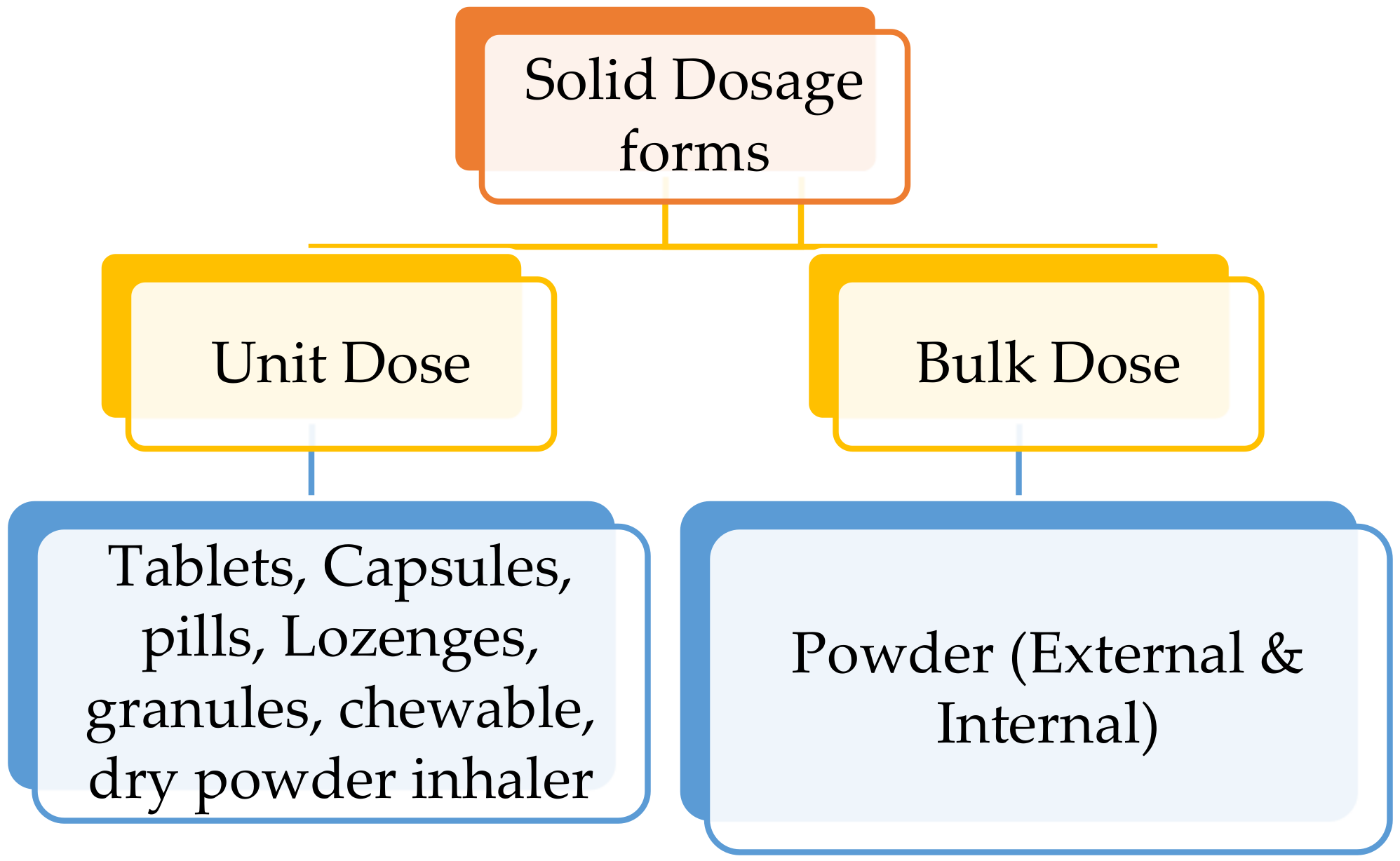
2.1. Classification of Solid Dosage Forms
2.1.1. Tablets
2.1.2. Capsules, Lozenges, Pills and Granules
2.1.3. Bulk Solid Dosage Forms
2.2. Semisolid Dosage Forms
2.2.1. Ointments
2.2.2. Creams
2.2.3. Gels (Jellies) and Lotions
2.2.4. Pastes
2.2.5. Transdermal Patches
2.2.6. Suppositories
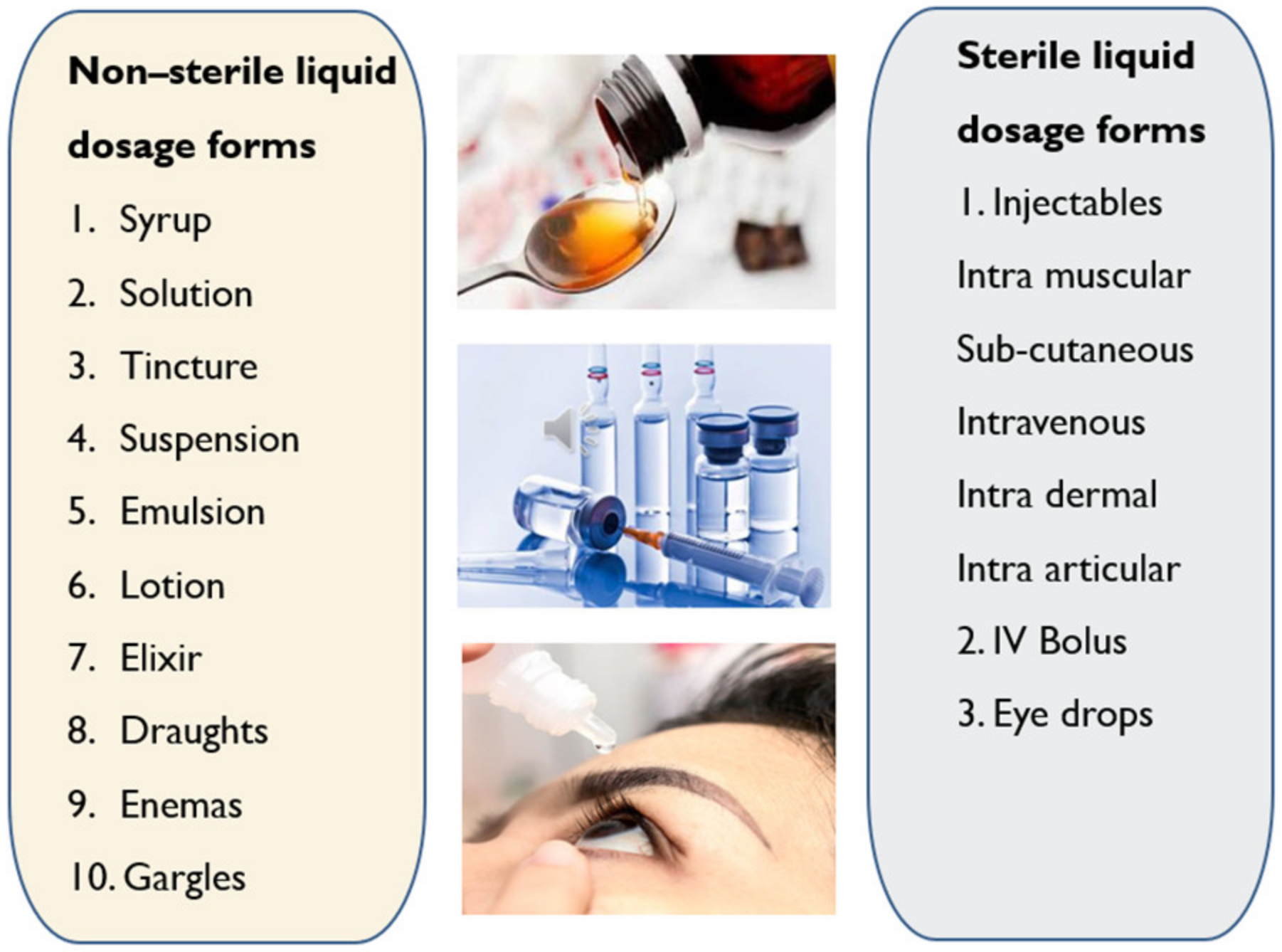
2.3. Liquid Dosage Forms
- Oral solutions are monophasic clear liquids for oral use comprising of one or more active ingredients dissolved in a suitable solvent system [24].
- Oral emulsions are biphasic liquids for oral use where the drug is present in oil-in-water emulsion either in single or dual phases [25].
- Oral suspensions are biphasic liquid dosage forms for oral use comprising of one or more APIs suspended in a suitable solvent. They tend to sediment with time; nevertheless, they can be readily re-dispersed by shaking into a uniform suspension that remains appropriately stable to allow the accurate dose to be delivered [24].
- Syrup is a concentrated aqueous sugar solution, usually sucrose, in which APIs are dissolved. Flavoured syrups are suitable to mask the unpleasant taste of drugs [25].
- Elixir is monophasic clear liquids for oral use for administering potent or nauseous drugs by adding pleasant flavours. The vehicle comprises a high amount of ethanol or sucrose along with antimicrobial preservatives to enhance the stability of the formulation [25].
- Linctuses are viscous oral liquids made of a high amount of syrup and glycerol which have a demulcent effect on the membranes of the throat and are used for cough relief. These are taken in smaller doses (<5 ml) and undiluted to prolong the demulcent action [26].
- Oral drops are either solutions, suspensions or emulsions that are administered in very small volumes (<1 ml) into the eyes, nose or ears [27].
- Gargles are concentrated aqueous solutions that need to be diluted with warm water before use to wash the mouth and throat by holding the liquid in the throat and agitate it by the air from the lungs [28].
3. Pharmacokinetics of Drug Delivery Systems
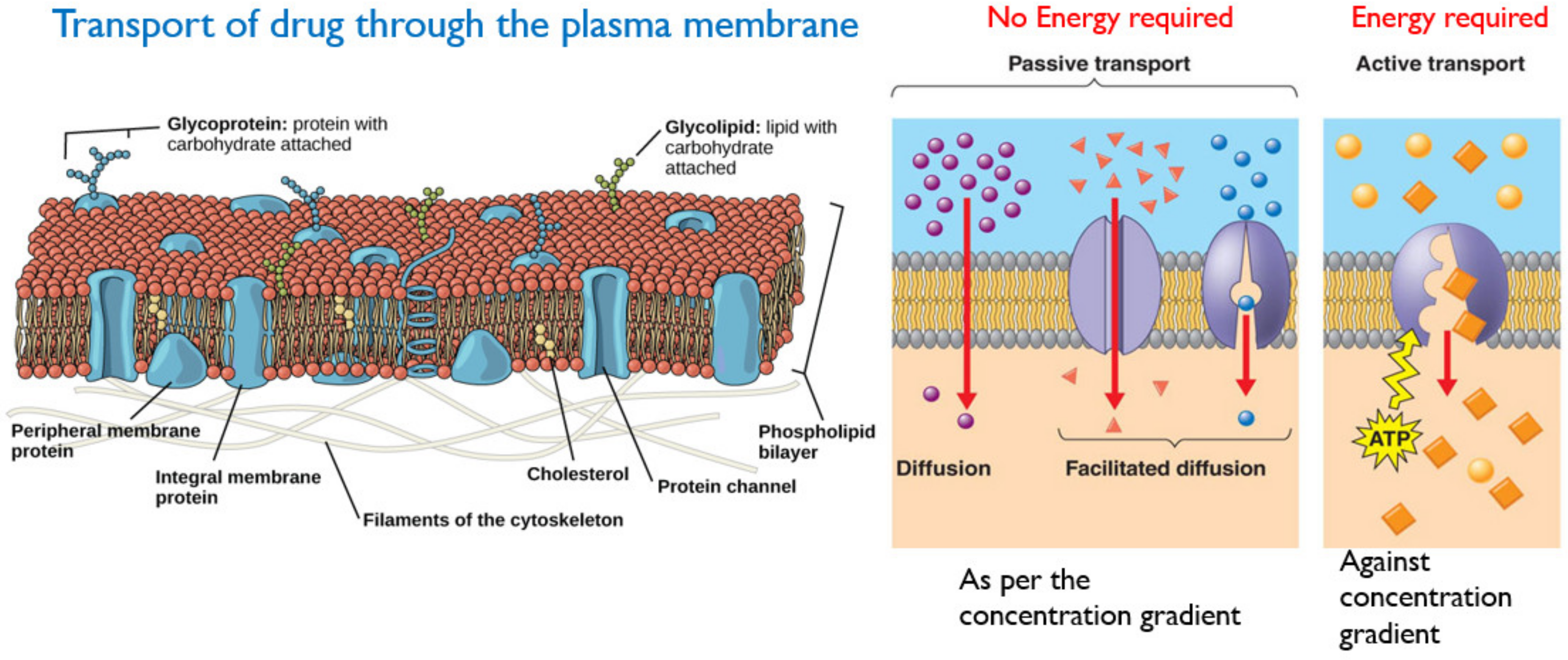
3.1. Absorption
- (a)
- Passive Transport involves the movement of the drug across the cell membrane from the high drug concentration region (such as gastrointestinal tract), to the low drug concentration region (such as blood). This is a passive process and no energy is required, and the rate of drug diffusion is directly proportional to the concentration gradient [32]. Other factors influencing passive transport include the physicochemical properties of the drug, such as its lipid solubility, molecular size, degree of ionization and the absorptive surface area available to the drug [30].
- (b)
- Active transport requires energy to facilitate the transport of drug molecules against a concentration gradient, which usually occurs at specific sites in the small intestine. The majority of drugs that are absorbed via active transport share a similar structure with endogenous substances such as ions, vitamins, sugars and amino acids [30,33]. A schematic of active and passive transport is given in Figure 11.
3.2. Distribution
3.3. Metabolism
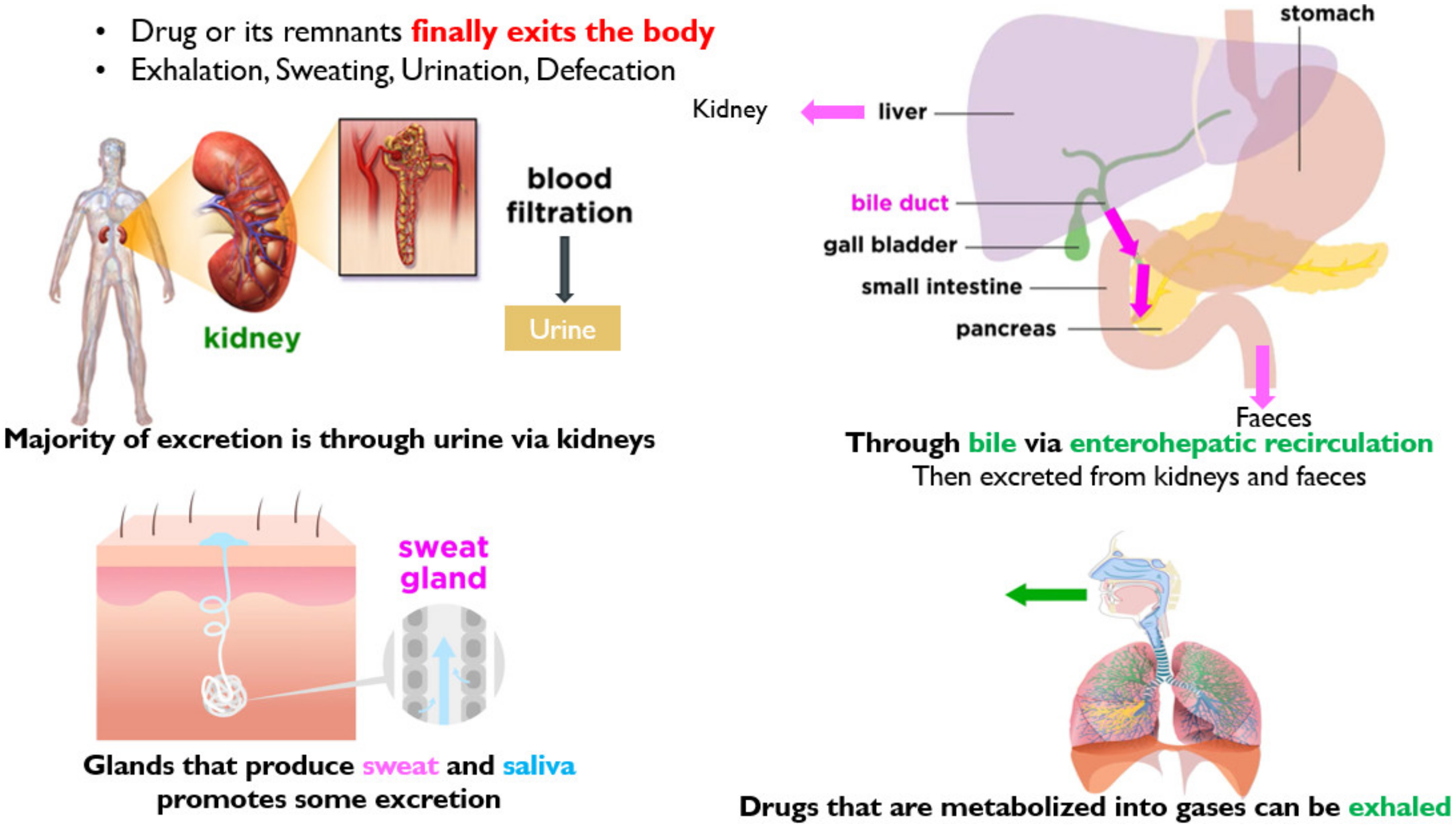
3.4. Excretion
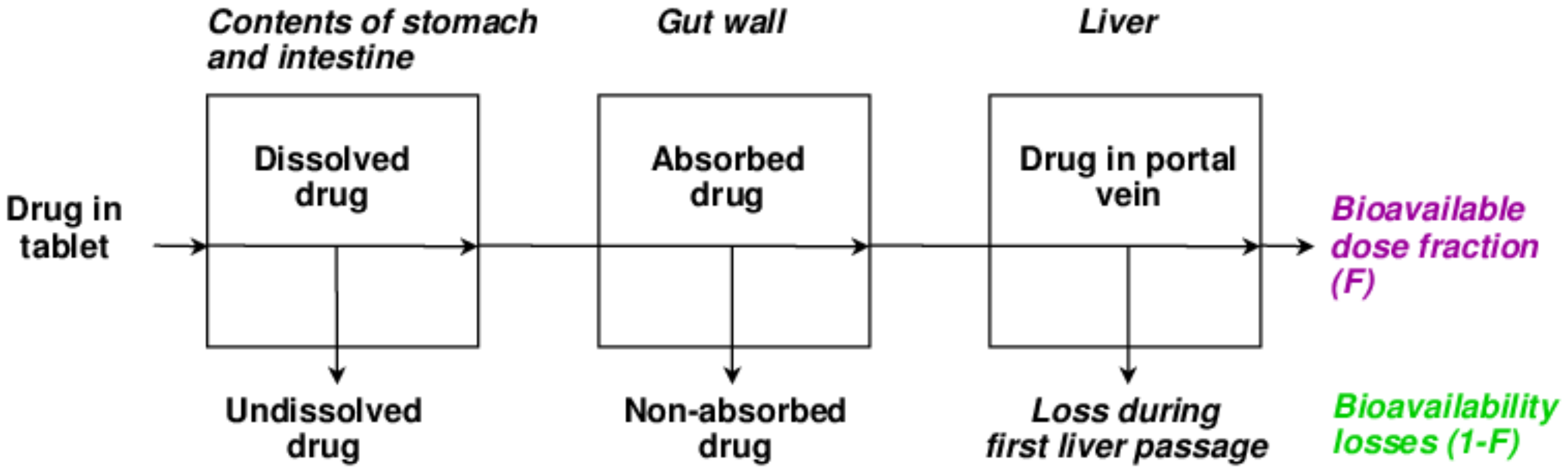
3.5. Bioavailability
3.6. Biological Half-Life (t1/2)
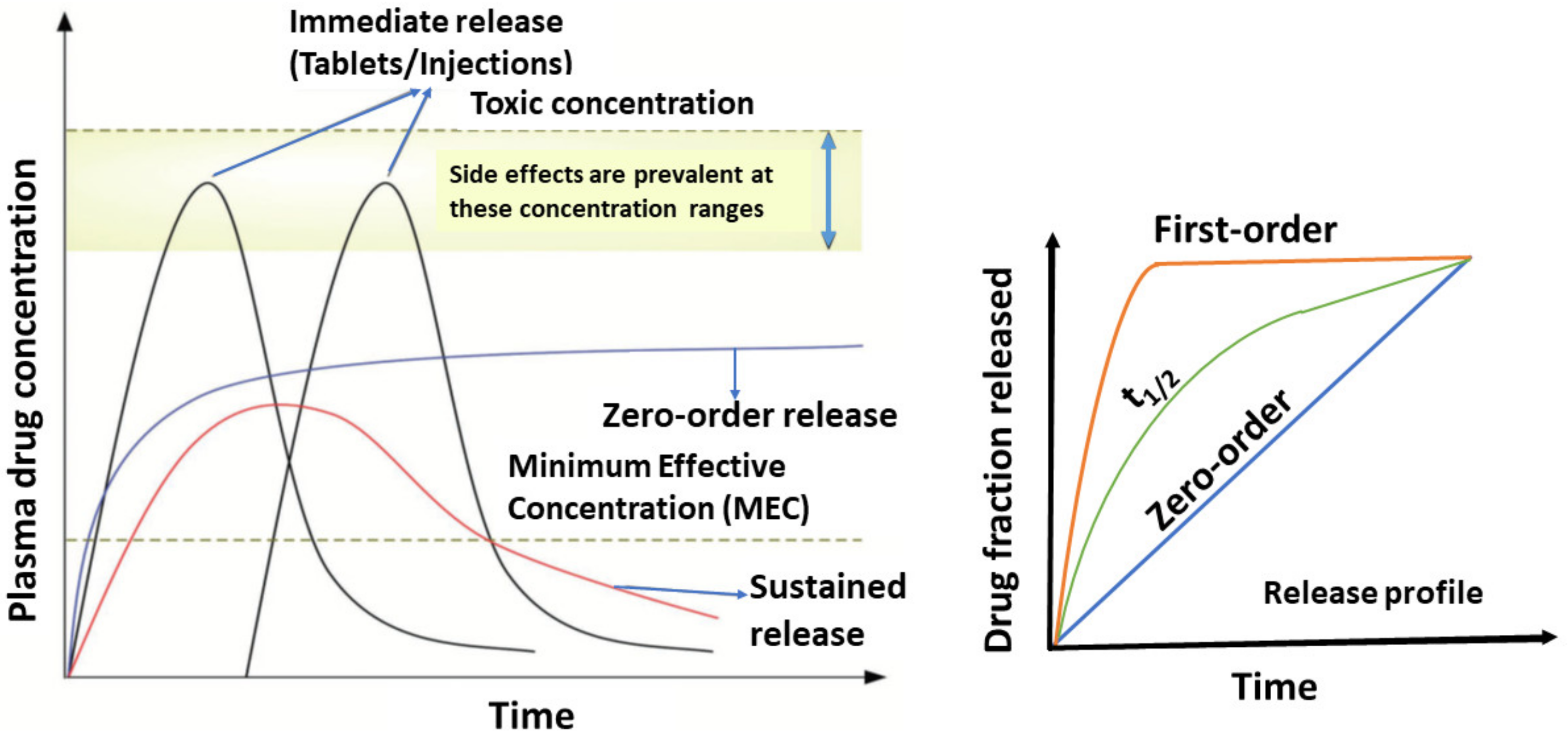
4. Drug Release Kinetics Basic Concepts
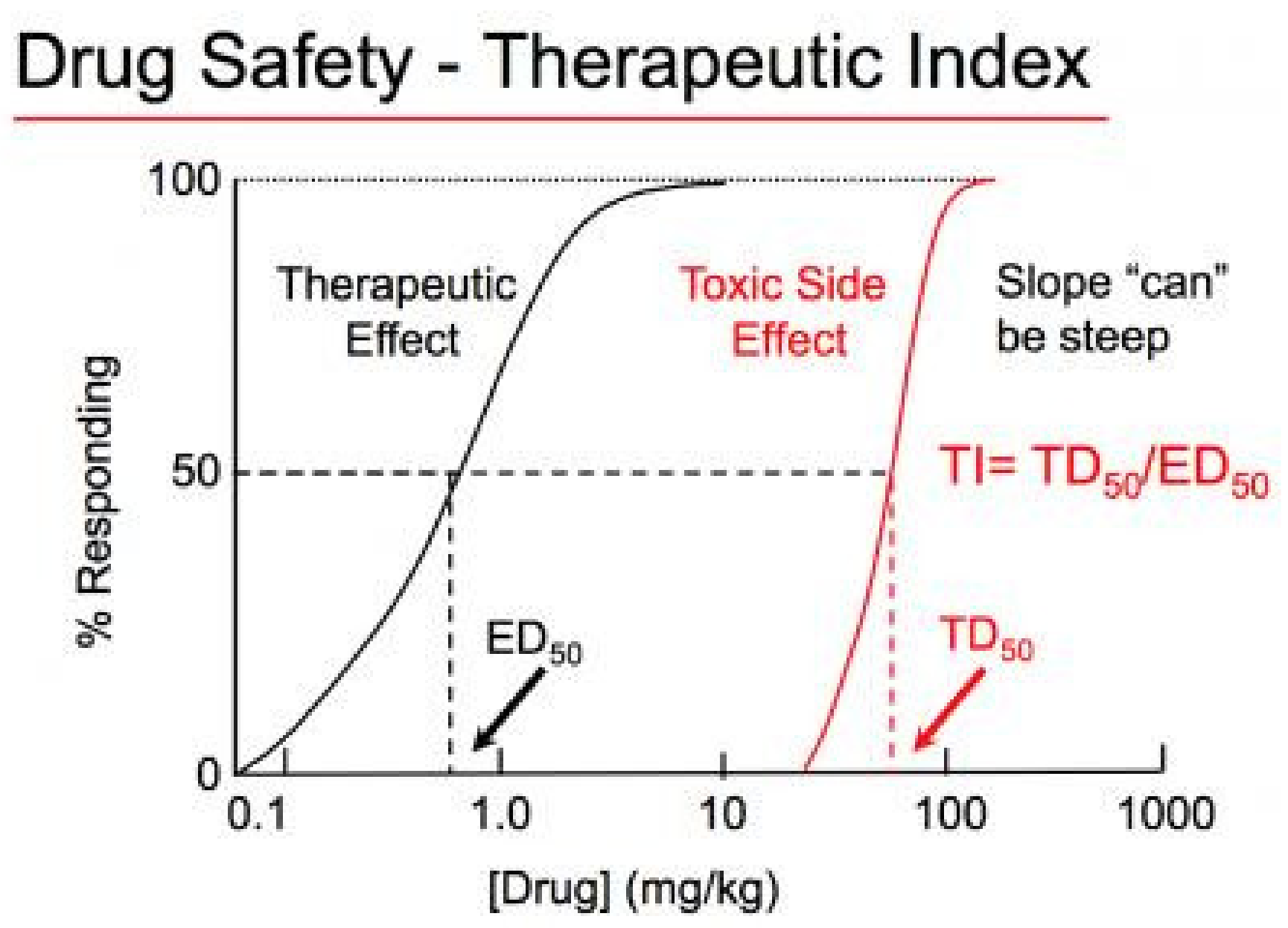
Therapeutic Index (TI) and Therapeutic Window
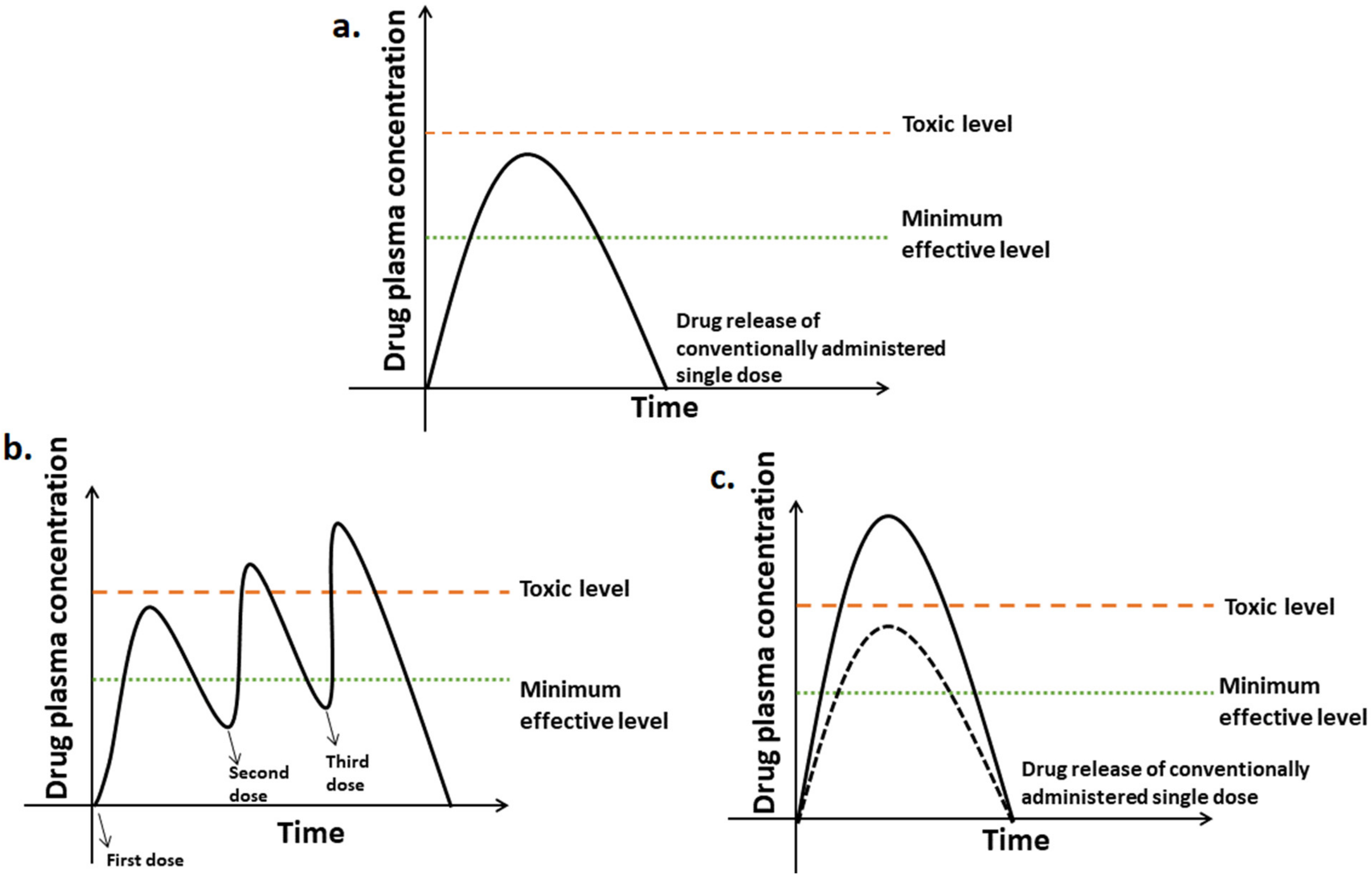
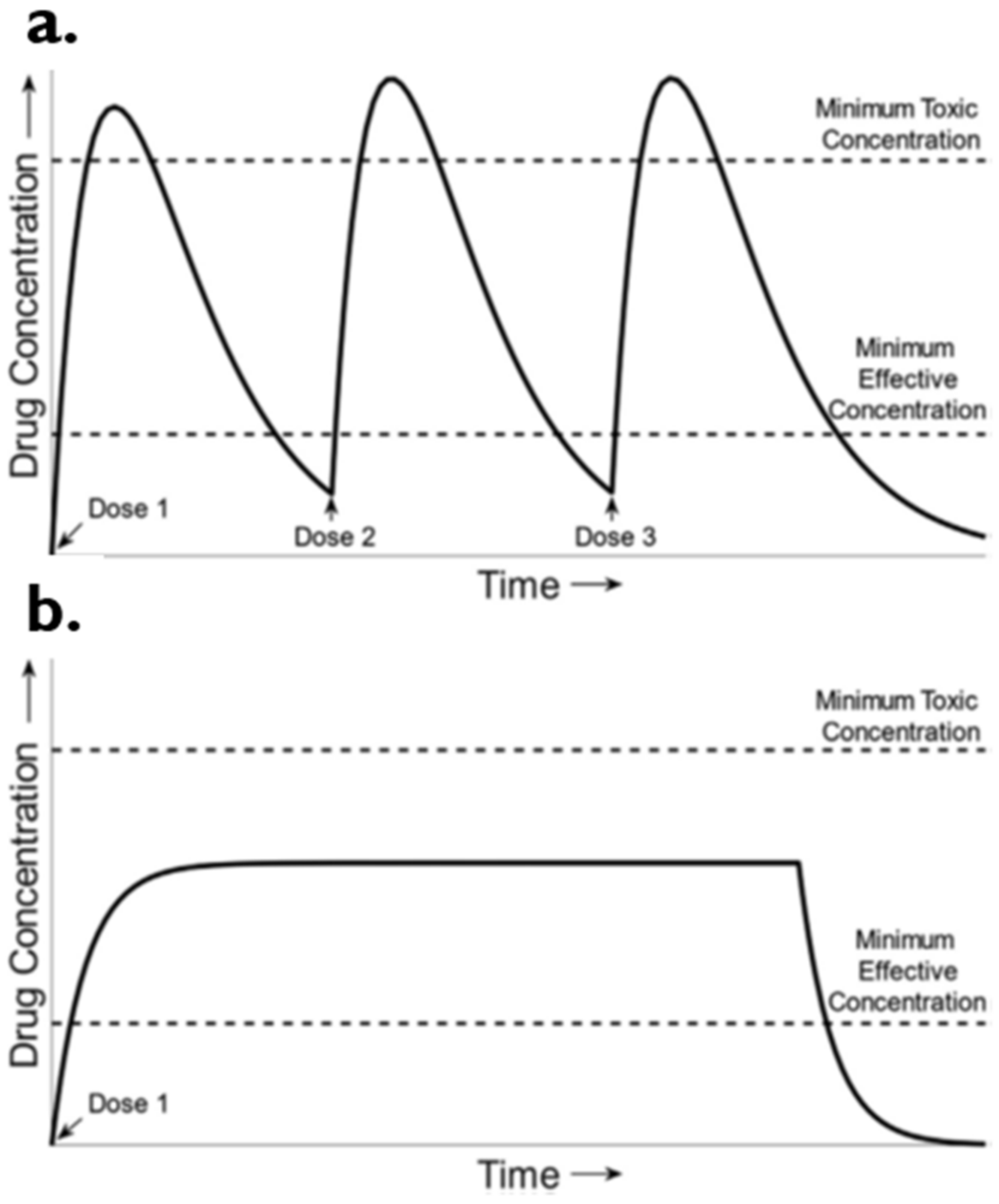
5. Conventional vs. Controlled Drug Delivery Systems
Sustained Drug Delivery System
6. Controlled Drug Delivery Systems
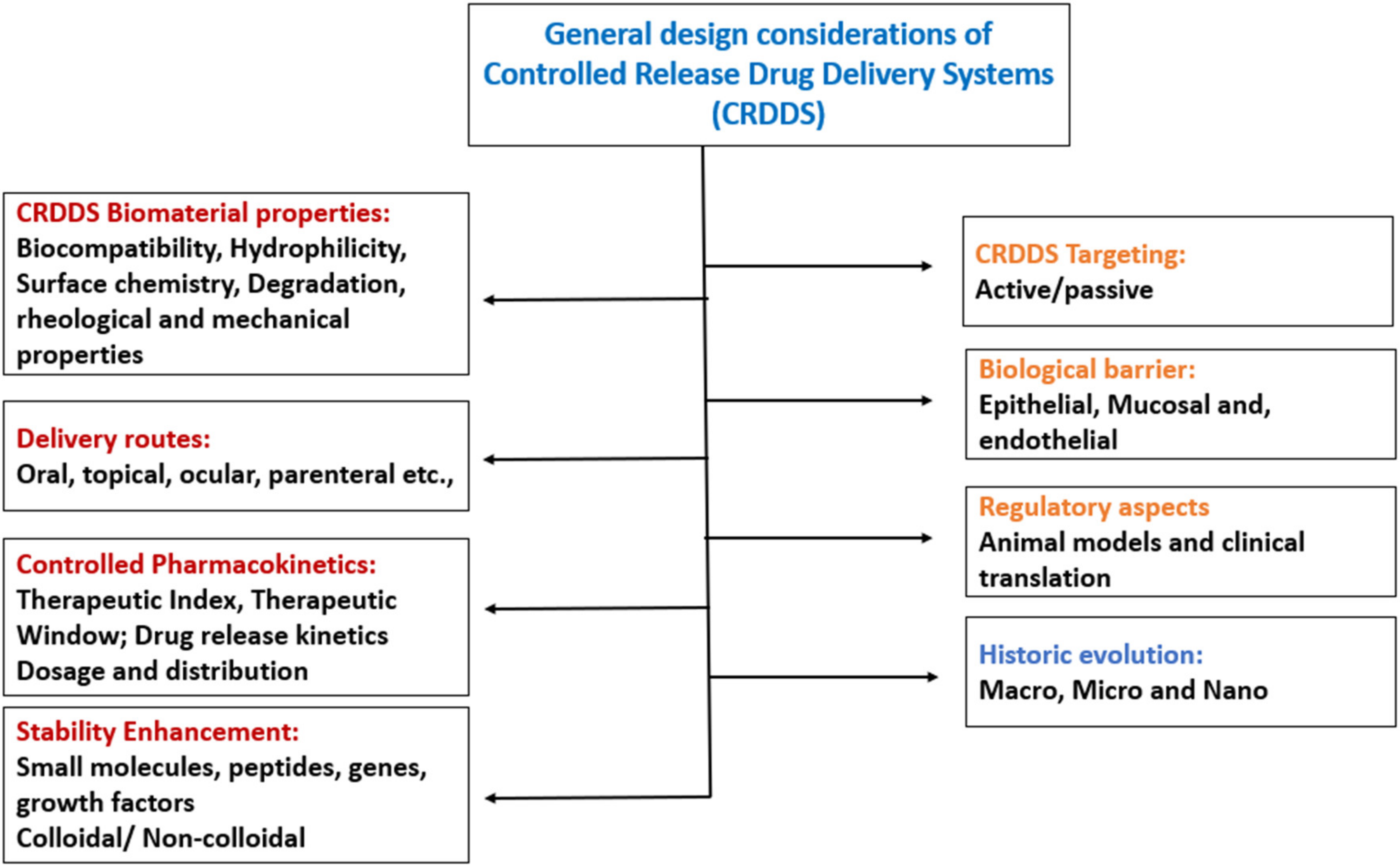
6.1. Design Considerations of Controlled Release Drug Delivery Systems
6.2. Classification of Controlled Release Drug Delivery Systems
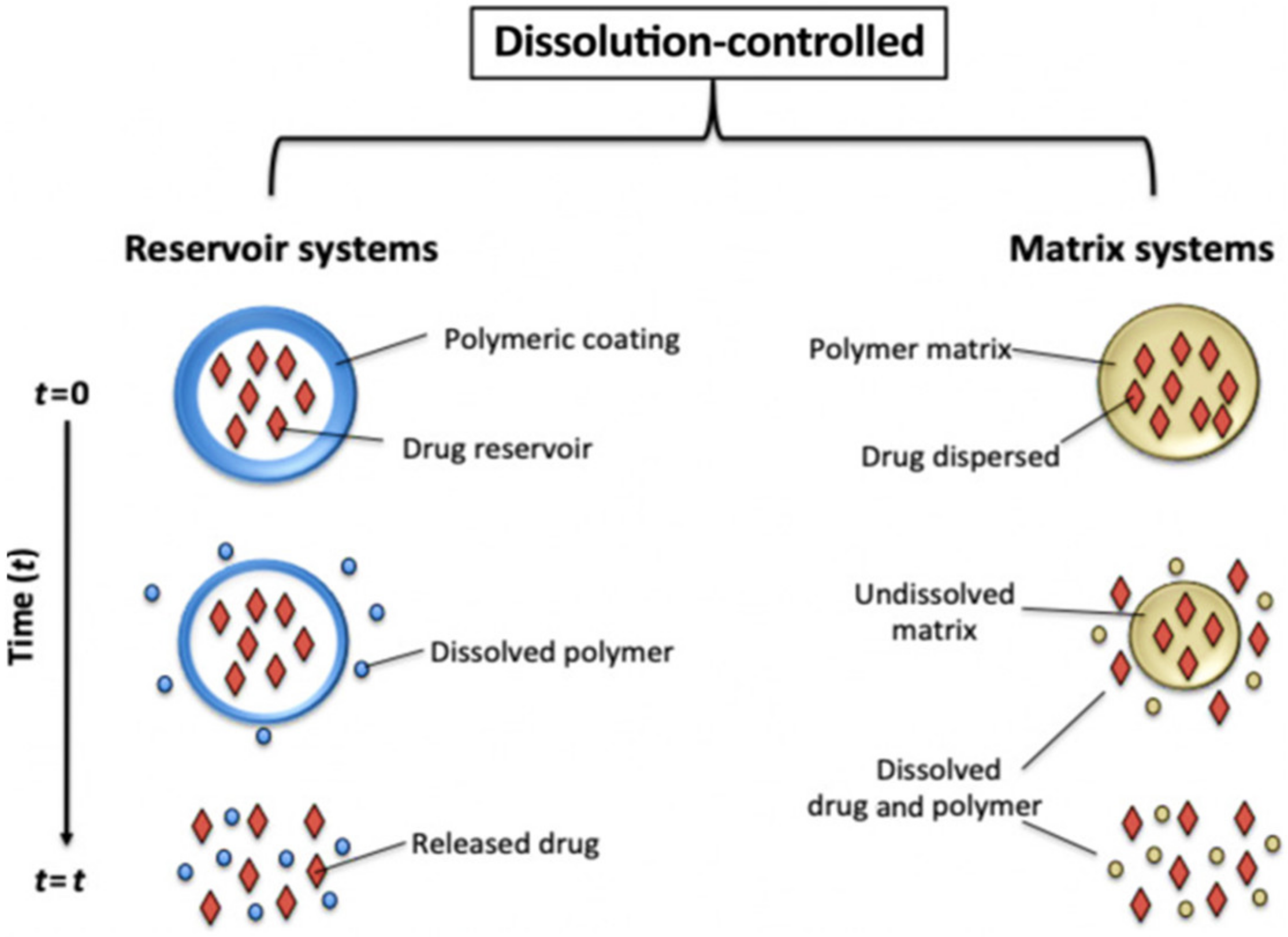
6.2.1. Dissolution Controlled Drug Delivery Systems
6.2.2. Diffusion-Controlled Drug Delivery Systems
6.2.3. Water Penetration-Controlled Drug Delivery Systems
Osmotic Controlled Drug Delivery Systems
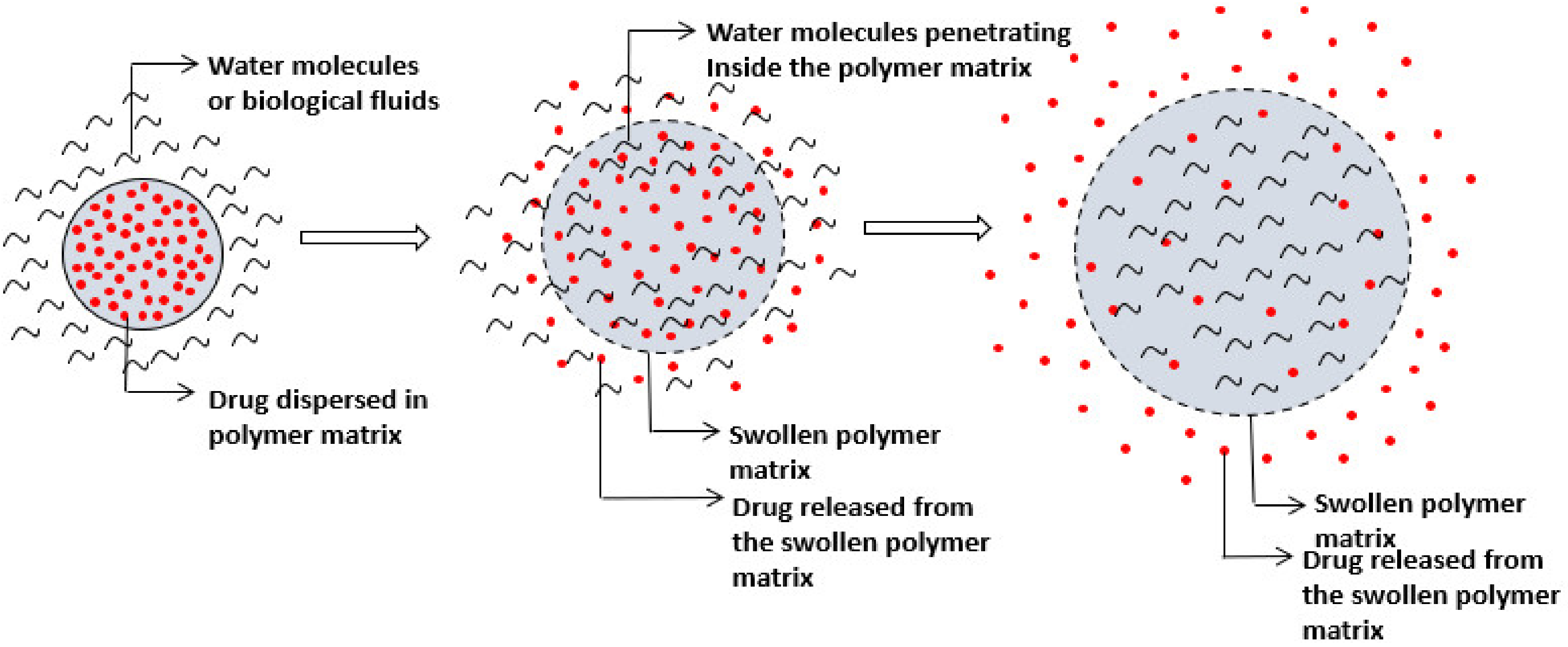
Swelling-Controlled Drug Delivery Systems
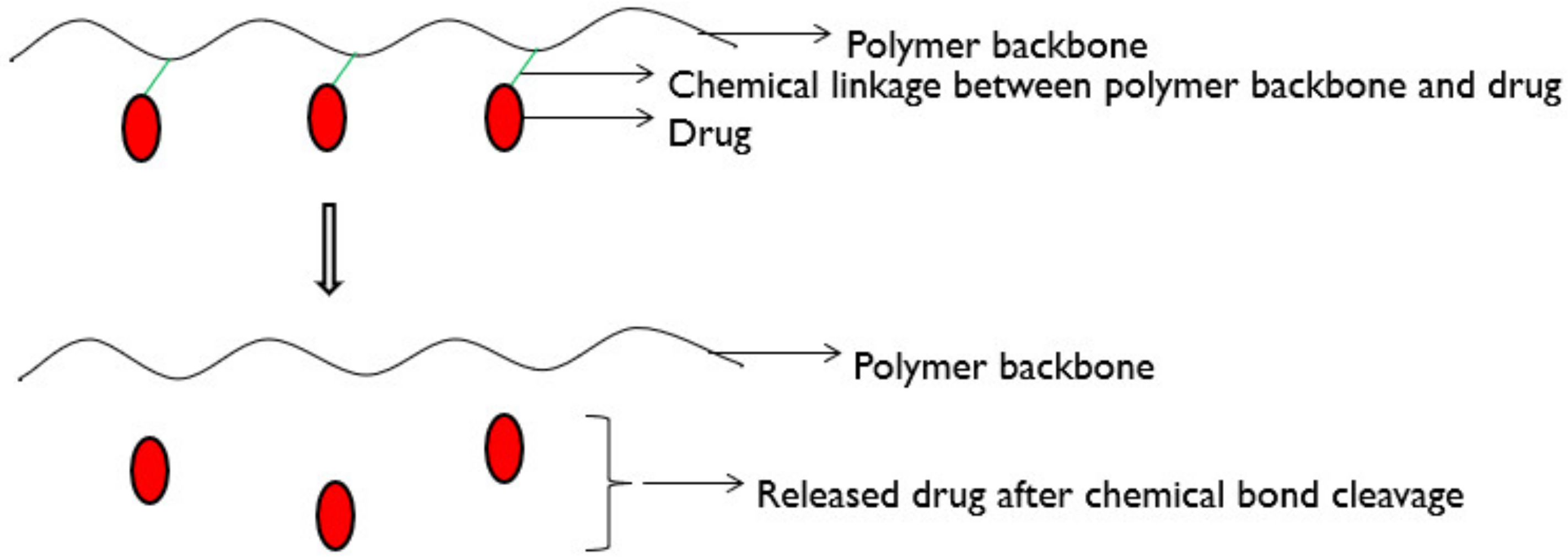
6.2.4. Chemically Controlled Drug Delivery Systems
7. Controlled Release Dosage Form Design: Practical Considerations
Evolution of the Controlled Release Dosage Forms
8. Concept of Biomaterials in Controlled Drug Delivery
Stimuli-Responsive Biomaterials
9. Nanocarriers in Controlled and Targeted Drug Delivery
9.1. Need for Targeted Drug Delivery
9.2. Active and Passive Targeting
9.3. Nanocarriers in Controlled Drug Delivery
9.3.1. Liposomes
9.3.2. Dendrimers
9.3.3. Exosomes
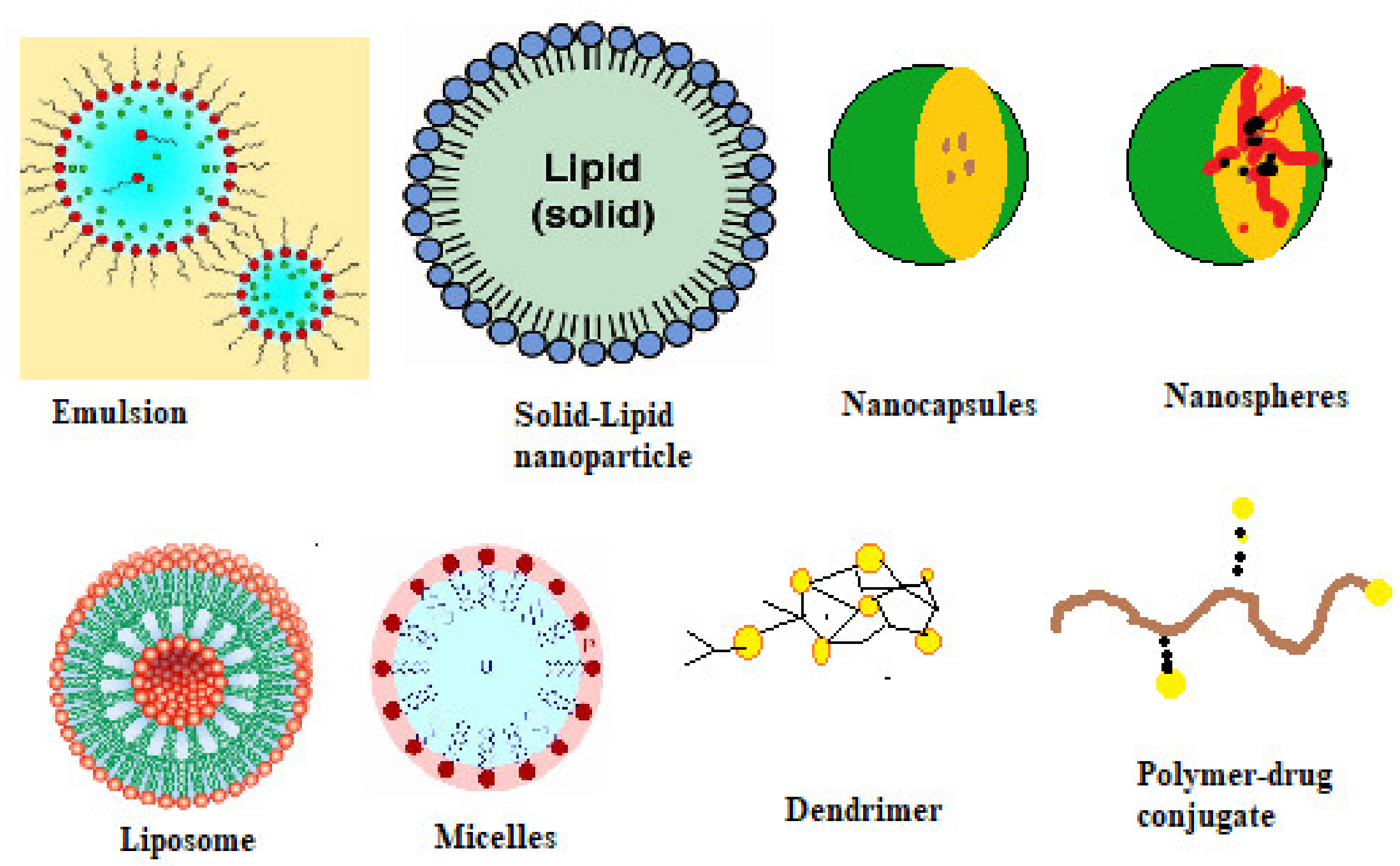
9.3.4. Nanoparticles
9.3.5. Nanosphere or Nanocapsule
9.3.6. Solid-Lipid Nanoparticles
9.3.7. Nanofibers
9.3.8. Polymersomes
9.3.9. Self-Assembled Polymeric Micelles
9.3.10. Carbon Nanotubes
9.3.11. Nanoemulsions
9.3.12. Hydrogels
10. Stimuli-Responsive Drug Delivery Systems Using Smart Biomaterials
10.1. Chemical Stimuli-Responsive Biomaterials
10.1.1. pH-Responsive
10.1.2. Redox Responsive
10.1.3. Enzyme Responsive
10.2. Physical Stimuli-Responsive Biomaterials
10.2.1. Light Responsive
10.2.2. Thermo-Responsive
10.2.3. Electric Responsive
10.2.4. Magnetic Responsive
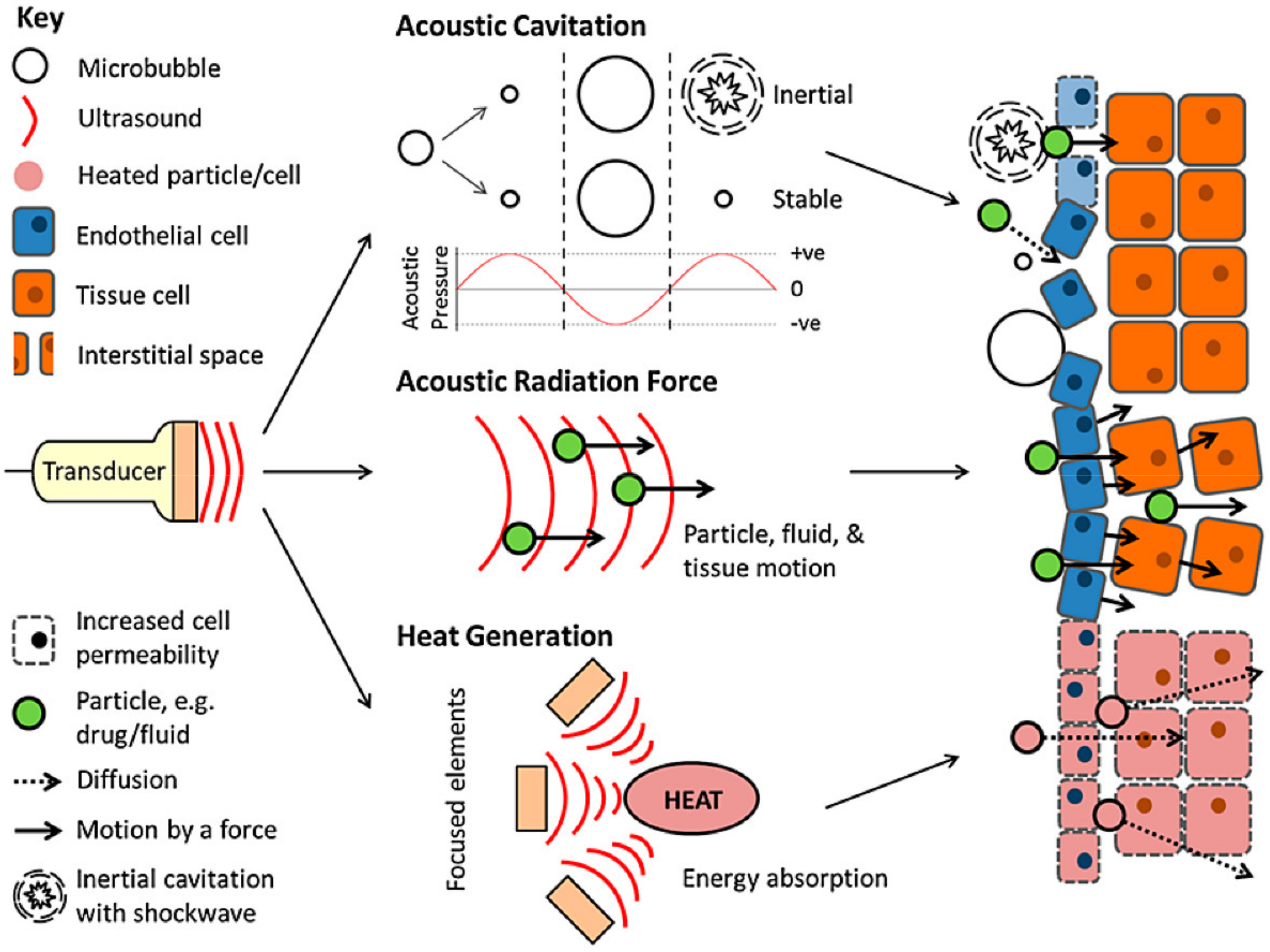
10.2.5. Ultrasound Responsive
11. Challenges and Future Directions
11.1. Nanomedicine Challenges and Improvements
11.2. Microfluidics in Controlled Drug Delivery
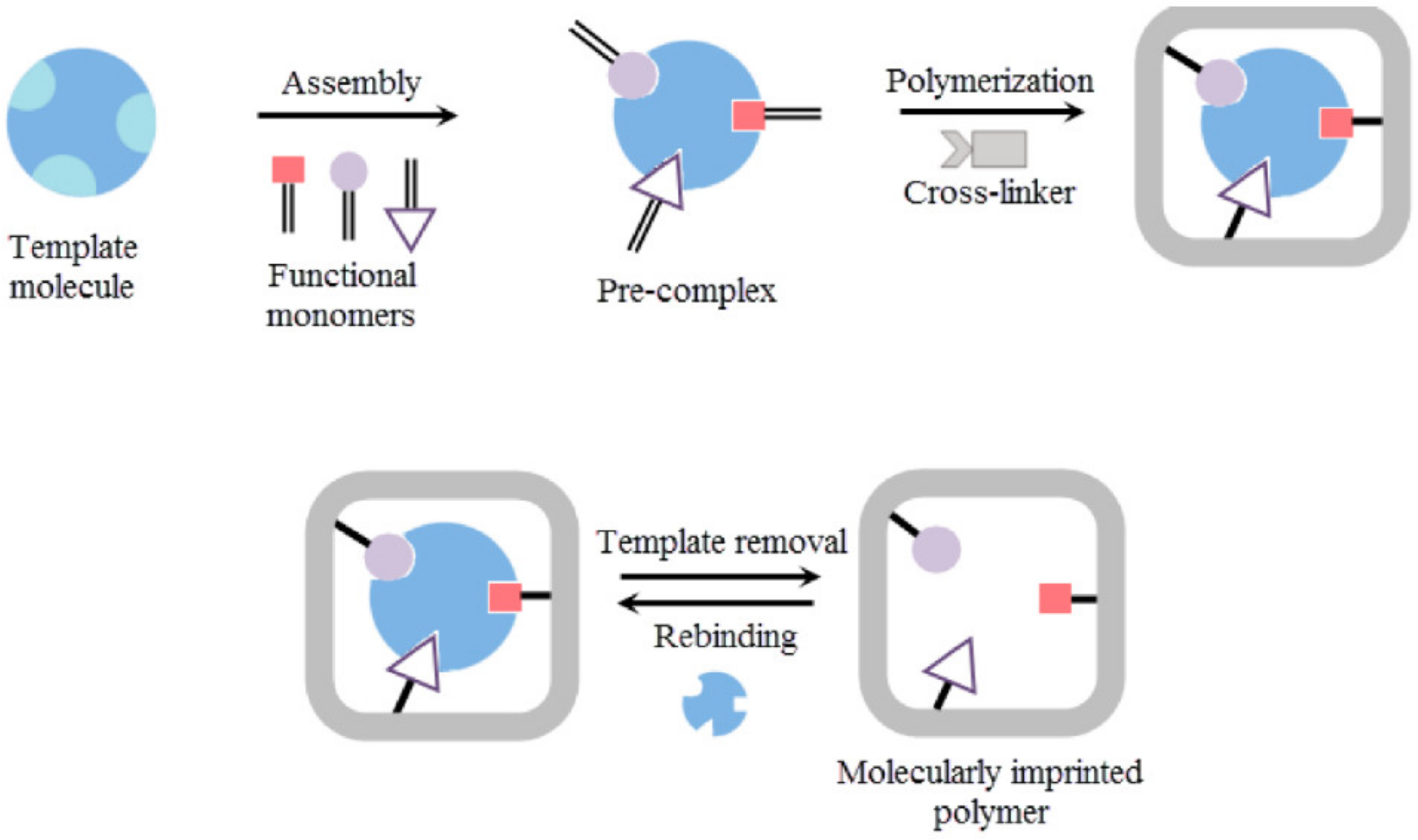
11.3. Molecularly Imprinted Polymers (MIPs)
11.4. Intelligent Biomaterials
11.5. CRISPR CAS9 Based Systems
11.6. Quantum Sensing Drug Delivery
11.7. Three-Dimensional Printing in Drug Delivery
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full form |
| API | Active Pharmaceutical Ingredient |
| FDA | Food and Drug Administration |
| BCS | Biopharmaceutics Classification System |
| BBB | Blood Brain Barrier |
| IVIVC | In vitro In vivo Co-relationship |
| CYP450 | Cytochrome P450 |
| t1/2 | Biological half-life |
| TI | Therapeutic Index |
| TW | Therapeutic Window |
| MEC | Minimum effective concentration |
| PK | Pharmacokinetics |
| ED50 | Effective dose in 50% of subjects |
| TD50 | Toxic dose in 50% of subjects |
| DDS | Drug delivery systems |
| CRDDS | Controlled release drug delivery systems |
| EPR | Enhanced permeability and retention |
| PLK 1 | Serine/threonine-protein kinase |
| siRNA | Small interfering Ribose Nucleic acid |
| CNTs | Carbon nanotubes |
| SWCNTs | Single-walled carbon nanotubes |
| MWCNTs | Multiwalled carbon nanotubes |
| MMP | Matrix metallo proteinases |
| LCST | Lower critical solution temperature |
| MIP | Molecularly Imprinted Polymers |
| CRISPR Cas9 | Clustered regularly interspaced short palindromic repeats |
| sgRNA | Single guide RNA |
| LOC | Lab-on-a-chip |
| QD | Quantun dots |
| RES | Reticulo Endothelial system |
References
- Langer, R. Drug delivery and targeting. Nature 1998, 392, 5–10. [Google Scholar]
- Benoit, D.S.; Overby, C.T.; Sims, K.R., Jr.; Ackun-Farmmer, M.A. Drug delivery systems. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1237–1266. [Google Scholar]
- Langer, R. New methods of drug delivery. Science 1990, 249, 1527–1533. [Google Scholar] [CrossRef]
- Chaudhari, S.P.; Patil, P.S. Pharmaceutical excipients: A review. IJAPBC 2012, 1, 21–34. [Google Scholar]
- Jain, K.K. An overview of drug delivery systems. Drug Deliv. Syst. 2020, 2059, 1–54. [Google Scholar]
- Patel, H.; Shah, V.; Upadhyay, U. New pharmaceutical excipients in solid dosage forms-A review. Int. J. Pharm. Life Sci. 2011, 2, 1006–1019. [Google Scholar]
- Kalasz, H.; Antal, I. Drug excipients. Curr. Med. Chem. 2006, 13, 2535–2563. [Google Scholar] [CrossRef] [PubMed]
- Ku, M.S. Use of the biopharmaceutical classification system in early drug development. AAPS J. 2008, 10, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Thakur, A.; Deshmukh, K.; Jha, A.; Verma, S. Research. Routes of drug administration. Int. J. Pharm. Stud. Res. 2010, 1, 54–59. [Google Scholar]
- Augsburger, L.L.; Hoag, S.W. Pharmaceutical Dosage Forms-Tablets; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Qiu, Y.; Chen, Y.; Zhang, G.G.; Yu, L.; Mantri, R.V. Developing Solid Oral Dosage Forms: Pharmaceutical Theory and Practice; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Mahato, R.I.; Narang, A.S. Pharmaceutical Dosage Forms and Drug Delivery: Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Bora, A.; Deshmukh, S.; Swain, K. Recent advances in semisolid dosage form. Int. J. Pharm. Sci. Res. 2014, 5, 3596. [Google Scholar]
- Niazi, S.K. Handbook of Pharmaceutical Manufacturing Formulations: Volume Four, Semisolid Products; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Mahalingam, R.; Li, X.; Jasti, B.R. Semisolid dosages: Ointments, creams, and gels. Pharm. Manuf. Handb. 2008, 1, 267–312. [Google Scholar]
- Allen, L.V., Jr. Basics of compounding: Tips and hints, Part 3: Compounding with ointments, creams, pastes, gels, and gel-creams. Int. J. Pharm. Compd. 2014, 18, 228–230. [Google Scholar]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Al Hanbali, O.A.; Khan, H.M.S.; Sarfraz, M.; Arafat, M.; Ijaz, S.; Hameed, A. Transdermal patches: Design and current approaches to painless drug delivery. Acta Pharm. 2019, 69, 197–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhiman, S.; Singh, T.G.; Rehni, A.K. Transdermal patches: A recent approach to new drug delivery system. Int. J. Pharm. Pharm. Sci. 2011, 3, 26–34. [Google Scholar]
- Perrigo. Transderm-Scop, Scopalamine Transdermal Patch. Available online: https://investor.perrigo.com/2019-10-03-Perrigo-Announces-the-Relaunch-of-the-AB-Rated-Generic-Version-of-Transderm-Scop-R-1-5-MG (accessed on 15 August 2021).
- Gad, S.C. Semisolid dosages: Ointments, creams and gels. In Pharmaceutical Manufacturing Handbook: Production and Processes; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 5, pp. 267–312. [Google Scholar]
- Rubio-Bonilla, M.V.; Londono, R.; Rubio, A. Liquid dosage forms. In Pharmaceutical Manufacturing Handbook: Production and Processes; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 5, pp. 313–344. [Google Scholar]
- Kumar, R.S.; Yagnesh, T.N.S. Pharmaceutical suspensions: Patient compliance oral dosage forms. World J. Pharm. Pharm. Sci. 2016, 5, 1471–1537. [Google Scholar]
- Kalantzi, L.; Reppas, C.; Dressman, J.; Amidon, G.; Junginger, H.; Midha, K.; Shah, V.; Stavchansky, S.; Barends, D.M. Biowaiver monographs for immediate release solid oral dosage forms: Acetaminophen (paracetamol). J. Pharm. Sci. 2006, 95, 4–14. [Google Scholar] [CrossRef] [Green Version]
- Sears, W. Linctuses. Practitioner 1951, 166, 91–92. [Google Scholar]
- Payne, K.; Roelofse, J.; Shipton, E. Pharmacokinetics of oral tramadol drops for postoperative pain relief in children aged 4 to 7 years—A pilot study. Anesth. Prog. 2002, 49, 109. [Google Scholar]
- Van Schoor, J. Using gargles and mouthwashes: Medicine cupboard. SA Pharm. Assist. 2011, 11, 26. [Google Scholar]
- Shargel, L.; Andrew, B.; Wu-Pong, S. Applied Biopharmaceutics & Pharmacokinetics; Appleton & Lange Stamford: Stamford, UK, 1999; Volume 264. [Google Scholar]
- Jambhekar, S.S.; Breen, P.J. Basic Pharmacokinetics; Pharmaceutical Press: London, UK, 2009; Volume 76. [Google Scholar]
- Fleisher, D.; Li, C.; Zhou, Y.; Pao, L.-H.; Karim, A. Drug, meal and formulation interactions influencing drug absorption after oral administration. Clin. Pharmacokinet. 1999, 36, 233–254. [Google Scholar] [CrossRef]
- Obata, K.; Sugano, K.; Saitoh, R.; Higashida, A.; Nabuchi, Y.; Machida, M.; Aso, Y. Prediction of oral drug absorption in humans by theoretical passive absorption model. Int. J. Pharm. 2005, 293, 183–192. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Openstax, R.U. 1.1 The Science of Biology. Available online: http://cnx.org/contents/GFy_h8cu@10.53:rZudN6XP@2/Introduction (accessed on 12 August 2021).
- Quizlet. 2.1.5 Biological Membranes. Available online: https://quizlet.com/gb/377924943/215-biological-membranes-diagram/ (accessed on 12 August 2021).
- Seydel, J.K.; Wiese, M. Drug-Membrane Interactions: Analysis, Drug Distribution, Modeling; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 15. [Google Scholar]
- Gillette, J.R. Factors affecting drug metabolism. Ann. N. Y. Acad. Sci. 1971, 179, 43–66. [Google Scholar] [CrossRef]
- Ekins, S.; Ring, B.J.; Grace, J.; McRobie-Belle, D.J.; Wrighton, S.A. Present and future in vitro approaches for drug metabolism. J. Pharmacol. Toxicol. Methods 2000, 44, 313–324. [Google Scholar] [CrossRef]
- Taft, D.R. Drug excretion. In Pharmacology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 175–199. [Google Scholar]
- Reinberg, A.E. Concepts of circadian chronopharmacology. Ann. N. Y. Acad. Sci. 1991, 618, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Prabu, S.L.; Suriyaprakash, T.; Ruckmani, K.; Thirumurugan, R. Biopharmaceutics and Pharmacokinetics. In Basic Pharmacokinetic Concepts and Some Clinical Applications; IntechOpen: London, UK, 2015. [Google Scholar]
- Hallare, J.; Gerriets, V. Half Life; StatPearls Publishing LLC: Treasure Island, FL, USA, 2020. [Google Scholar]
- Hardenia, A.; Maheshwari, N.; Hardenia, S.S.; Dwivedi, S.K.; Maheshwari, R.; Tekade, R.K. Scientific rationale for designing controlled drug delivery systems. In Basic Fundamentals of Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–28. [Google Scholar]
- Paarakh, M.P.; Jose, P.A.; Setty, C.; Christoper, G.P. Release kinetics–concepts and applications. Int. J. Pharm. Res. Technol. 2018, 8, 12–20. [Google Scholar]
- Habet, S. Narrow Therapeutic Index drugs: Clinical pharmacology perspective. J. Pharm. Pharmacol. 2021, 73, 1285–1291. [Google Scholar] [CrossRef]
- Lowe, E.S.; BALIS, F.M. Dose-effect and concentration-effect analysis. In Principles of Clinical Pharmacology; Elsevier: Amsterdam, The Netherlands, 2007; pp. 289–300. [Google Scholar]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in biomaterials for drug delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Ther. 2019, 4, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, B.P.; Thakur, N.; Jain, N.P.; Banweer, J.; Jain, S. Osmotically controlled drug delivery system with associated drugs. J. Pharm. Pharm. Sci. 2010, 13, 571–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Shmeis, R.A. Dissolution controlled drug delivery systems. Des. Control. Release Drug Deliv. Systems. 2006, 139–172. [Google Scholar]
- Siepmann, J.; Siegel, R.A.; Siepmann, F. Diffusion controlled drug delivery systems. In Fundamentals and Applications of Controlled Release Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2012; pp. 127–152. [Google Scholar]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef]
- Srikonda, S.; Kotamraj, P.; Barclay, B. Osmotic controlled drug delivery systems. Des. Control. Release Drug Deliv. Syst. 2006, 1, 203. [Google Scholar]
- Patil, P.B.; Uphade, K.B.; Saudagar, R.B. A review: Osmotic drug delivery system. Pharma Sci. Monit. 2018, 9, 2. [Google Scholar]
- Kumar, P.; Mishra, B. An overview of recent patents on oral osmotic drug delivery systems. Recent Pat. Drug Deliv. Formul. 2007, 1, 236–255. [Google Scholar] [CrossRef] [PubMed]
- Pfizer Laboratories Div Pfizer Inc. Procardia XL. Osmotic Pump. Available online: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=8ebcb33c-f43b-4b36-9f94-9774b2a59e06 (accessed on 15 August 2021).
- Sudafed Osmotic Pump. Available online: https://www.sudafed.com/products/sudafed-sinus-congestion (accessed on 15 August 2021).
- Inc., B. Viadur (Leuprolide Acetate Implantable Osmotic Pump). Available online: http://usrf.org/breakingnews/viadur_implant.html (accessed on 15 August 2021).
- Siepmann, J.; Siepmann, F. Swelling controlled drug delivery systems. In Fundamentals and Applications of Controlled Release Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2012; pp. 153–170. [Google Scholar]
- Kopeček, J. Polymer–drug conjugates: Origins, progress to date and future directions. Adv. Drug Deliv. Rev. 2013, 65, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Yun, Y.H.; Lee, B.K.; Park, K. Controlled drug delivery: Historical perspective for the next generation. J. Control. Release 2015, 219, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.X. Dissolution Testing: In Vitro Characterization of Oral Controlled Release Dosage Forms; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Adeosun, S.O.; Ilomuanya, M.O.; Gbenebor, O.P.; Dada, M.O.; Odili, C.C. Biomaterials for Drug Delivery: Sources, Classification, Synthesis, Processing, and Applications. In Advanced Functional Materials; IntechOpen: London, UK, 2020. [Google Scholar]
- Neuse, E.W. Synthetic polymers as drug-delivery vehicles in medicine. Met. Based Drugs 2008, 2008, 469531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, A.; Yadav, T.; Sharma, S.; Nayak, A.; Kumari, A.A.; Mishra, N. Polymers in drug delivery. J. Biosci. Med. 2015, 4, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Mogoşanu, G.D.; Grumezescu, A.M.; Bejenaru, L.E.; Bejenaru, C. Chapter 8—Natural and synthetic polymers for drug delivery and targeting. In Nanobiomaterials in Drug Delivery; Grumezescu, A.M., Ed.; William Andrew Publishing: Amsterdam, The Netherlands, 2016; pp. 229–284. [Google Scholar]
- Bawa, P.; Pillay, V.; Choonara, Y.E.; du Toit, L.C. Stimuli-responsive polymers and their applications in drug delivery. Biomed. Mater. 2009, 4, 022001. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, X.; Shen, H.; He, Q.; Wu, Z.; Liao, W.; Yuan, M. Application of the Nano-Drug Delivery System in Treatment of Cardiovascular Diseases. Front. Bioeng. Biotechnol. 2020, 7, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natarajan, J.V.; Nugraha, C.; Ng, X.W.; Venkatraman, S. Sustained-release from nanocarriers: A review. J. Control. Release 2014, 193, 122–138. [Google Scholar] [CrossRef]
- Chamundeeswari, M.; Jeslin, J.; Verma, M.L. Nanocarriers for drug delivery applications. Environ. Chem. Lett. 2019, 17, 849–865. [Google Scholar] [CrossRef]
- Yadav, K.S.; Mishra, D.K.; Deshpande, A.; Pethe, A.M. Chapter 7—Levels of Drug Targeting. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 269–305. [Google Scholar]
- Naz, F.; Siddique, Y.H.J.C.; Targets, N.D.-D. Nanotechnology: Its application in treating neurodegenerative diseases. CNS Neurol. Disord. Drug Targets 2021, 20, 34–53. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, A.; Aftab, S.; Nisar, J.; Ashiq, M.N.; Iftikhar, F.J. Nanocarriers for targeted drug delivery. J. Drug Deliv. Sci. Technol. 2021, 13, 102426. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Dimov, N.; Kastner, E.; Hussain, M.; Perrie, Y.; Szita, N. Formation and purification of tailored liposomes for drug delivery using a module-based micro continuous-flow system. Sci. Rep. 2017, 7, 12045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, D.; Sandeep, K.; Pandey, D.; Dutta, R.K. Liposomes for drug delivery. Drugs 2017, 7, 15–28. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Turrin, C.-O. Dendrimers for drug delivery. J. Mater. Chem. B 2014, 2, 4055–4066. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef] [Green Version]
- Le, N.T.T.; Nguyen, T.N.Q.; Cao, V.D.; Hoang, D.T.; Ngo, V.C.; Hoang Thi, T.T. Recent progress and advances of multi-stimuli-responsive dendrimers in drug delivery for cancer treatment. Pharmaceutics 2019, 11, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G.J. Recent advancements in the use of exosomes as drug delivery systems. J. Nanobiotechnology 2018, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnology 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Adepu, S.; Luo, H.; Ramakrishna, S. Heparin-Tagged PLA-PEG Copolymer-Encapsulated Biochanin A-Loaded (Mg/Al) LDH Nanoparticles Recommended for Non-Thrombogenic and Anti-Proliferative Stent Coating. Int. J. Mol. Sci. 2021, 22, 5433. [Google Scholar] [CrossRef]
- Adepu, S.; Khandelwal, M. Broad-spectrum antimicrobial activity of bacterial cellulose silver nanocomposites with sustained release. Int. J. Mol. Sci. 2018, 53, 1596–1609. [Google Scholar] [CrossRef]
- Adepu, S.; Khandelwal, M.J.M. Bacterial cellulose with microencapsulated antifungal essential oils: A novel double barrier release system. Materialia 2020, 9, 100585. [Google Scholar] [CrossRef]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 319. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Siva Kumar, N.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Kajdič, S.; Planinšek, O.; Gašperlin, M.; Kocbek, P. Electrospun nanofibers for customized drug-delivery systems. J. Drug Deliv. Sci. Technol. 2019, 51, 672–681. [Google Scholar] [CrossRef]
- Zare, M.; Ramakrishna, S. Current Progress of Electrospun Nanocarriers for Drug Delivery Applications. Proceedings 2020, 4, 8790. [Google Scholar]
- Adepu, S.; Gaydhane, M.K.; Kakunuri, M.; Sharma, C.S.; Khandelwal, M.; Eichhorn, S.J. Effect of micropatterning induced surface hydrophobicity on drug release from electrospun cellulose acetate nanofibers. Appl. Surf. Sci. 2017, 426, 755–762. [Google Scholar] [CrossRef]
- Adepu, S.; Khandelwal, M. Drug release behaviour and mechanism from unmodified and in situ modified bacterial cellulose. Proc. Indian Natl. Sci. Acad. 2021, 87, 110–120. [Google Scholar] [CrossRef]
- Adepu, S.; Kalyani, P.; Khandelwal, M. Bacterial Cellulose-Based Drug Delivery System for Dual Mode Drug Release. Trans. Indian Natl. Acad. Eng. 2021, 6, 265–271. [Google Scholar] [CrossRef]
- Adepu, S.; Khandelwal, M. Ex-situ modification of bacterial cellulose for immediate and sustained drug release with insights into release mechanism. Carbohydr. Polym. 2020, 249, 116816. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Reis, R.L.; Ramakrishna, S.; Kundu, S.C. Functionalized silk fibroin nanofibers as drug carriers: Advantages and challenges. J. Control. Release 2020, 321, 324–347. [Google Scholar] [CrossRef] [PubMed]
- Torres-Martinez, E.J.; Cornejo Bravo, J.M.; Serrano Medina, A.; Pérez González, G.L.; Villarreal Gómez, L.J. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, K. Structural properties of self-assembled polymeric micelles. Curr. Opin. Colloid Interface Sci. 1998, 3, 12–19. [Google Scholar] [CrossRef]
- Alex, M.A.; Nehate, C.; Veeranarayanan, S.; Kumar, D.S.; Kulshreshtha, R.; Koul, V. Self assembled dual responsive micelles stabilized with protein for co-delivery of drug and siRNA in cancer therapy. Biomaterials 2017, 133, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Saleemi, M.A.; Kong, Y.L.; Yong, P.V.C.; Wong, E.H. An overview of recent development in therapeutic drug carrier system using carbon nanotubes. J. Drug Deliv. Sci. Technol. 2020, 59, 101855. [Google Scholar] [CrossRef]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef] [PubMed]
- Madani, S.Y.; Naderi, N.; Dissanayake, O.; Tan, A.; Seifalian, A.M. A new era of cancer treatment: Carbon nanotubes as drug delivery tools. Int. J. Nanomed. 2011, 6, 2963–2979. [Google Scholar] [CrossRef] [Green Version]
- Kaur, J.; Gill, G.S.; Jeet, K. Chapter 5 - Applications of Carbon Nanotubes in Drug Delivery A Comprehensive Review. In Characterization and Biology of Nanomaterials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–135. [Google Scholar]
- Li, Z.; de Barros, A.L.B.; Soares, D.C.F.; Moss, S.N.; Alisaraie, L. Functionalized single-walled carbon nanotubes: Cellular uptake, biodistribution and applications in drug delivery. Int. J. Pharm. 2017, 524, 41–54. [Google Scholar] [CrossRef]
- Tayeb, H.H.; Sainsbury, F. Nanoemulsions in drug delivery: Formulation to medical application. Nanomedicine 2018, 13, 2507–2525. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Mi, F.-L.; Kuan, C.-Y.; Shyu, S.-S.; Lee, S.-T.; Chang, S.-F. The study of gelation kinetics and chain-relaxation properties of glutaraldehyde-cross-linked chitosan gel and their effects on microspheres preparation and drug release. Carbohydr. Polym. 2000, 41, 389–396. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Li, J.; Qi, X.; Zhong, Y.; Zuo, G.; Pan, X.; Su, T.; Zhang, J.; Dong, W. Synthesis and characterization of a multi-sensitive polysaccharide hydrogel for drug delivery. Carbohydr. Polym. 2017, 177, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, C.; Dong, Y.; Liu, D. Supramolecular hydrogels: Mechanical strengthening with dynamics. Polymer 2020, 210, 122993. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Zou, Z.; Zhang, B.; Nie, X.; Cheng, Y.; Hu, Z.; Liao, M.; Li, S. A sodium alginate-based sustained-release IPN hydrogel and its applications. RSC Adv. 2020, 10, 39722–39730. [Google Scholar] [CrossRef]
- Shubhika, K. Nanotechnology and medicine-The upside and the downside. Int. J. Drug Dev. Res. 2012, 5, 1–10. [Google Scholar]
- Manzoor, A.A.; Lindner, L.H.; Landon, C.D.; Park, J.-Y.; Simnick, A.J.; Dreher, M.R.; Das, S.; Hanna, G.; Park, W.; Chilkoti, A. Overcoming limitations in nanoparticle drug delivery: Triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012, 72, 5566–5575. [Google Scholar] [CrossRef] [Green Version]
- Barenholz, Y. Liposome application: Problems and prospects. Curr. Opin. Colloid Interface Sci. 2001, 6, 66–77. [Google Scholar] [CrossRef]
- Lin, M.; Wang, R.; Qi, X.-R. Quality Evaluation of Drug-Loaded Liposomes. Liposome-Based Drug Deliv. Syst. 2021, 123–140. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G. Applications and limitations of dendrimers in biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Jain, S.; Pathak, K.; Pathak, D. Dendrimers: Nanosized multifunctional platform for drug delivery. Drug Deliv. Lett. 2018, 8, 3–19. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Agarwal, V.; Agarwal, M.; Singh, M. Exosomes: Structure, biogenesis, types and application in diagnosis and gene and Drug delivery. Curr. Gene Ther. 2020, 20, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Momin, M.; Khan, T.; Gharat, S.; Ningthoujam, R.S.; Omri, A. Metallic nanoparticles as drug delivery system for the treatment of cancer. Expert Opin. Drug Deliv. 2021, 18, 1–30. [Google Scholar]
- Alyassin, Y.; Sayed, E.G.; Mehta, P.; Ruparelia, K.; Arshad, M.S.; Rasekh, M.; Shepherd, J.; Kucuk, I.; Wilson, P.B.; Singh, N. Application of mesoporous silica nanoparticles as drug delivery carriers for chemotherapeutic agents. Drug Discov. Today 2020, 25, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anık, Ü.; Timur, S.; Dursun, Z. Recent pros and cons of nanomaterials in drug delivery systems. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1090–1100. [Google Scholar] [CrossRef]
- Erdoğar, N.; Akkın, S.; Bilensoy, E. Nanocapsules for drug delivery: An updated review of the last decade. Recent Pat. Drug Deliv. Formul. 2018, 12, 252–266. [Google Scholar] [CrossRef]
- Thassu, D.; Pathak, Y.; Deleers, M. Nanoparticulate Drug-Delivery Systems: An Overview; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Ghaderi, S.; Ramesh, B.; Seifalian, A.M. Fluorescence nanoparticles “quantum dots” as drug delivery system and their toxicity: A review. J. Drug Target. 2011, 19, 475–486. [Google Scholar] [CrossRef]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomed. 2017, 12, 5421. [Google Scholar] [CrossRef] [Green Version]
- Barroso, M.M. Quantum dots in cell biology. J. Histochem. Cytochem. 2011, 59, 237–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahu, D.K.; Ghosh, G.; Rath, G. Nanofibers in Drug Delivery. Nanopharmaceutical Adv. Deliv. Syst. 2021, 99–123. [Google Scholar] [CrossRef]
- Zare, M.; Dziemidowicz, K.; Williams, G.R.; Ramakrishna, S. Encapsulation of pharmaceutical and nutraceutical active ingredients using electrospinning processes. Nanomaterials 2021, 11, 1968. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. CHAPTER 1 Fundamentals of Stimuli-responsive Drug and Gene Delivery Systems. In Stimuli-Responsive Drug Delivery Systems; The Royal Society of Chemistry: London, UK, 2018; pp. 1–32. [Google Scholar] [CrossRef]
- Dastidar, D.G.; Chakrabarti, G. Chapter 6—Thermoresponsive Drug Delivery Systems, Characterization and Application. In Applications of Targeted Nano Drugs and Delivery Systems; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 133–155. [Google Scholar]
- Palanikumar, L.; Al-Hosani, S.; Kalmouni, M.; Nguyen, V.P.; Ali, L.; Pasricha, R.; Barrera, F.N.; Magzoub, M. pH-responsive high stability polymeric nanoparticles for targeted delivery of anticancer therapeutics. Commun. Biol. 2020, 3, 95. [Google Scholar] [CrossRef]
- Datta, S.; Jutková, A.; Šrámková, P.; Lenkavská, L.; Huntošová, V.; Chorvát, D.; Miškovský, P.; Jancura, D.; Kronek, J. Unravelling the Excellent Chemical Stability and Bioavailability of Solvent Responsive Curcumin-Loaded 2-Ethyl-2-oxazoline-grad-2-(4-dodecyloxyphenyl)-2-oxazoline Copolymer Nanoparticles for Drug Delivery. Biomacromolecules 2018, 19, 2459–2471. [Google Scholar] [CrossRef]
- Cai, X.; Jiang, Y.; Lin, M.; Zhang, J.; Guo, H.; Yang, F.; Leung, W.; Xu, C. Ultrasound-Responsive Materials for Drug/Gene Delivery. Front. Pharmacol. 2020, 10, 1650. [Google Scholar] [CrossRef] [PubMed]
- Kolosnjaj-Tabi, J.; Gibot, L.; Fourquaux, I.; Golzio, M.; Rols, M.-P. Electric field-responsive nanoparticles and electric fields: Physical, chemical, biological mechanisms and therapeutic prospects. Adv. Drug Deliv. Rev. 2019, 138, 56–67. [Google Scholar] [CrossRef]
- Price, P.M.; Mahmoud, W.E.; Al-Ghamdi, A.A.; Bronstein, L.M. Magnetic Drug Delivery: Where the Field Is Going. Front. Chem. 2018, 6, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amukarimi, S.; Ramakrishna, S.; Mozafari, M. Smart biomaterials-A proposed definition and overview of the field. Curr. Opin. Biomed. Eng. 2021, 19, 100311. [Google Scholar] [CrossRef]
- Zhuo, S.; Zhang, F.; Yu, J.; Zhang, X.; Yang, G.; Liu, X. pH-Sensitive Biomaterials for Drug Delivery. Molecules 2020, 25, 5649. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.; Yadav, A.K.; Jain, N.K.; Agrawal, G.P. Eudragit-coated dextran microspheres of 5-fluorouracil for site-specific delivery to colon. Drug Deliv. 2016, 23, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.R.; Such, G.K. pH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 2019, 40, 1800917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Wang, Z.; Peng, Y.; Ding, J.; Zhou, W. A Smart pH-Sensitive Delivery System for Enhanced Anticancer Efficacy via Paclitaxel Endosomal Escape. Front. Pharmacol. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
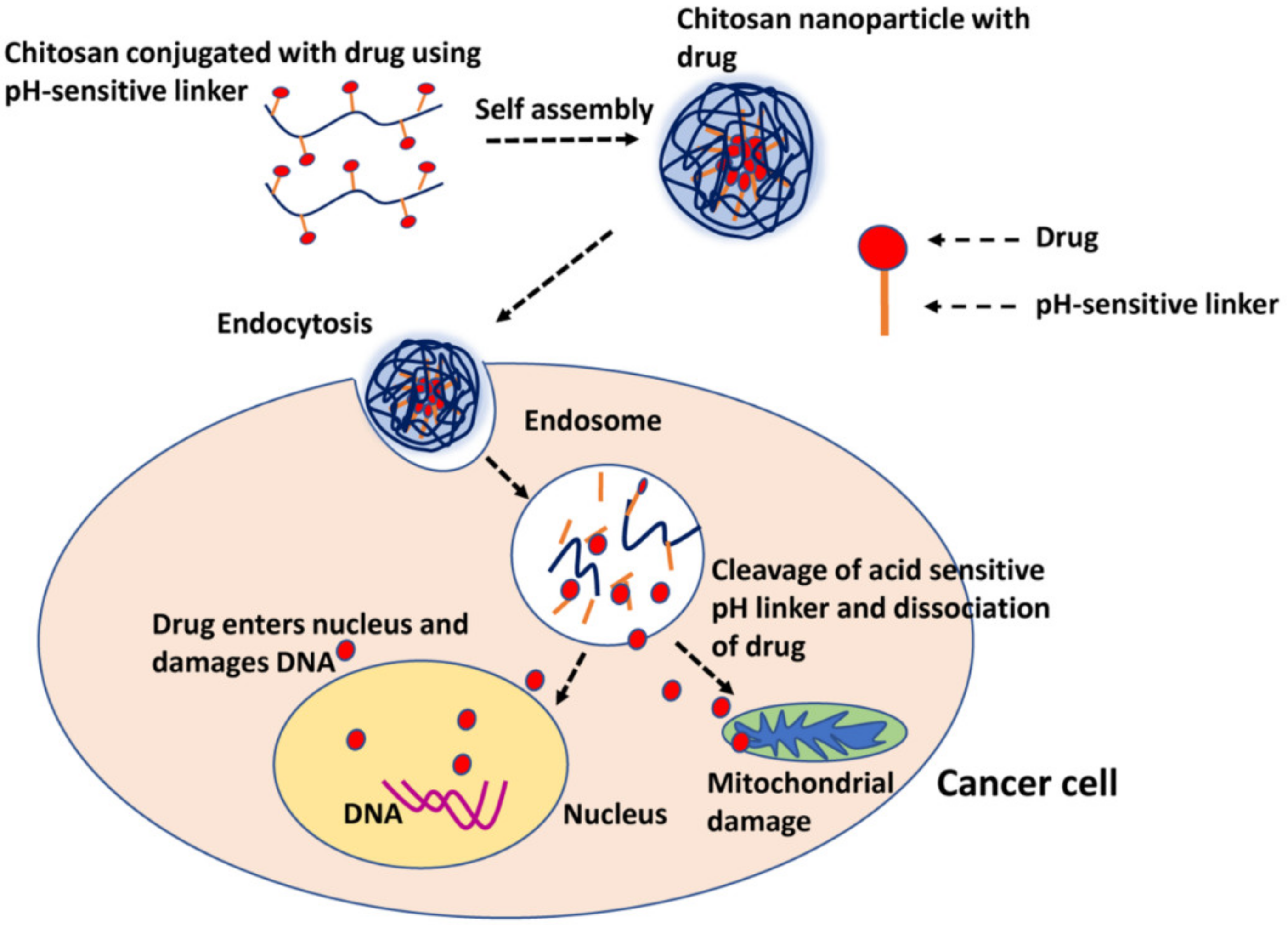
- Vivek, R.; Babu, V.N.; Thangam, R.; Subramanian, K.; Kannan, S.J.C.; Biointerfaces, S.B. pH-responsive drug delivery of chitosan nanoparticles as Tamoxifen carriers for effective anti-tumor activity in breast cancer cells. Colloids Surf. B Biointerfaces 2013, 111, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.; Ramesh, R. Multifaceted applications of chitosan in cancer drug delivery and therapy. Mar. Drugs 2017, 15, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, P.F.; Travanut, A.; Conte, C.; Alexander, C. Reduction-responsive polymers for drug delivery in cancer therapy—Is there anything new to discover? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2021, 13, e1678. [Google Scholar] [CrossRef]
- Geiselhart, C.M.; Xue, W.; Barner-Kowollik, C.; Mutlu, H. Degradable Redox-Responsive Polyolefins. Macromolecules 2021, 54, 1775–1782. [Google Scholar] [CrossRef]
- Shahriari, M.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Enzyme responsive drug delivery systems in cancer treatment. J. Control. Release 2019, 308, 172–189. [Google Scholar] [CrossRef]
- Lee, S.; Song, S.J.; Lee, J.; Ha, T.H.; Choi, J.S. Cathepsin B-Responsive Liposomes for Controlled Anticancer Drug Delivery in Hep G2 Cells. Pharmaceutics 2020, 12, 876. [Google Scholar] [CrossRef]
- Linsley, C.S.; Wu, B.M. Recent advances in light-responsive on-demand drug-delivery systems. Ther. Deliv. 2017, 8, 89–107. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Zhao, Y.; Wang, Q.; Liu, T.; Sun, J.; Zhang, R. Remote Light-Responsive Nanocarriers for Controlled Drug Delivery: Advances and Perspectives. Small 2019, 15, 1903060. [Google Scholar] [CrossRef] [PubMed]
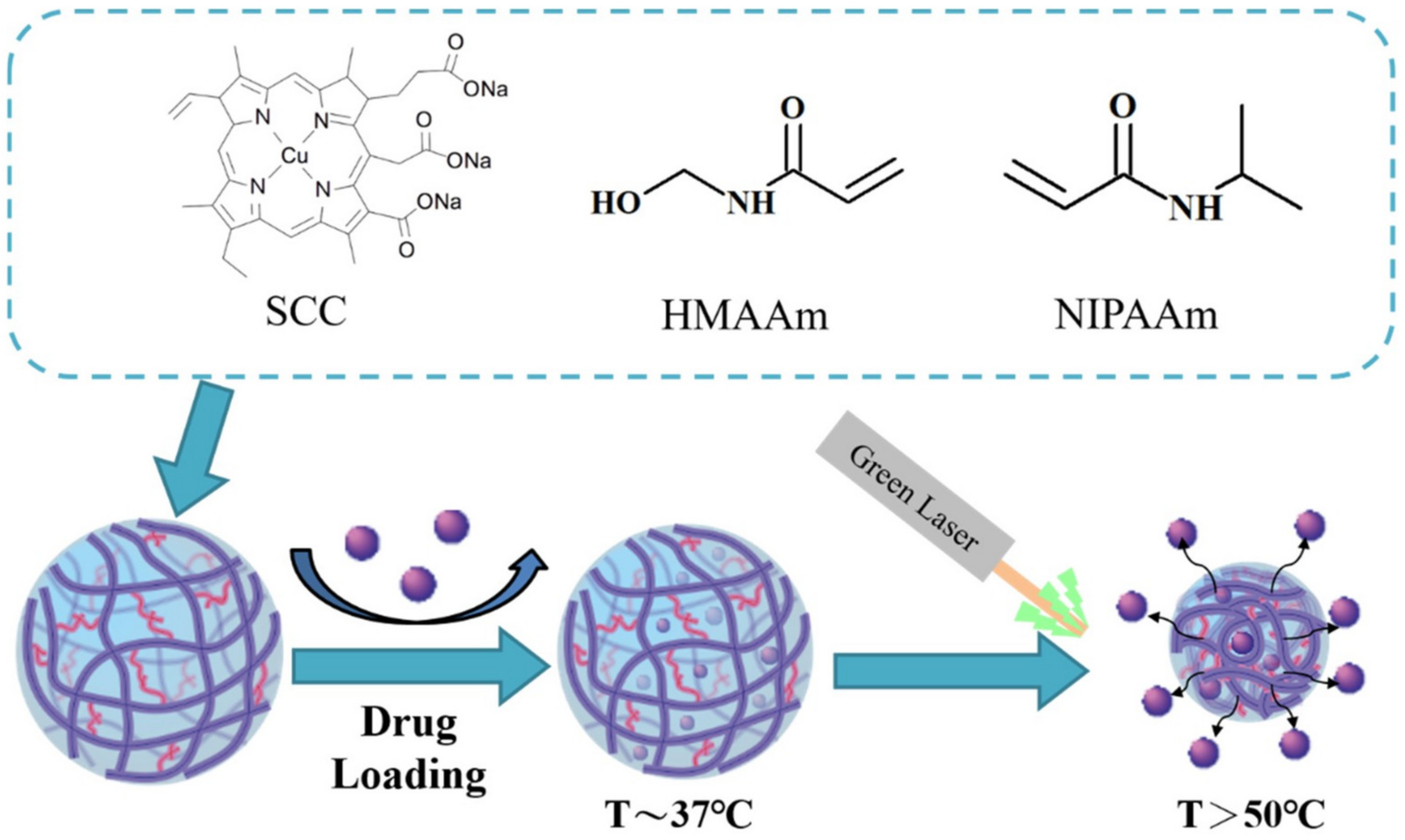
- Chang, R.; Tsai, W.-B. Photo-induced thermal-responsive nanogels for controlled drug release. Front. Bioeng. Biotechnol. 2016, 4. [Google Scholar] [CrossRef]
- Singh, S.P.; Alvi, S.B.; Pemmaraju, D.B.; Singh, A.D.; Manda, S.V.; Srivastava, R.; Rengan, A.K. NIR triggered liposome gold nanoparticles entrapping curcumin as in situ adjuvant for photothermal treatment of skin cancer. Int. J. Biol. Macromol. 2018, 110, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Tsai, W.-B. Fabrication of photothermo-responsive drug-loaded nanogel for synergetic cancer therapy. Polymers 2018, 10, 1098. [Google Scholar] [CrossRef] [Green Version]
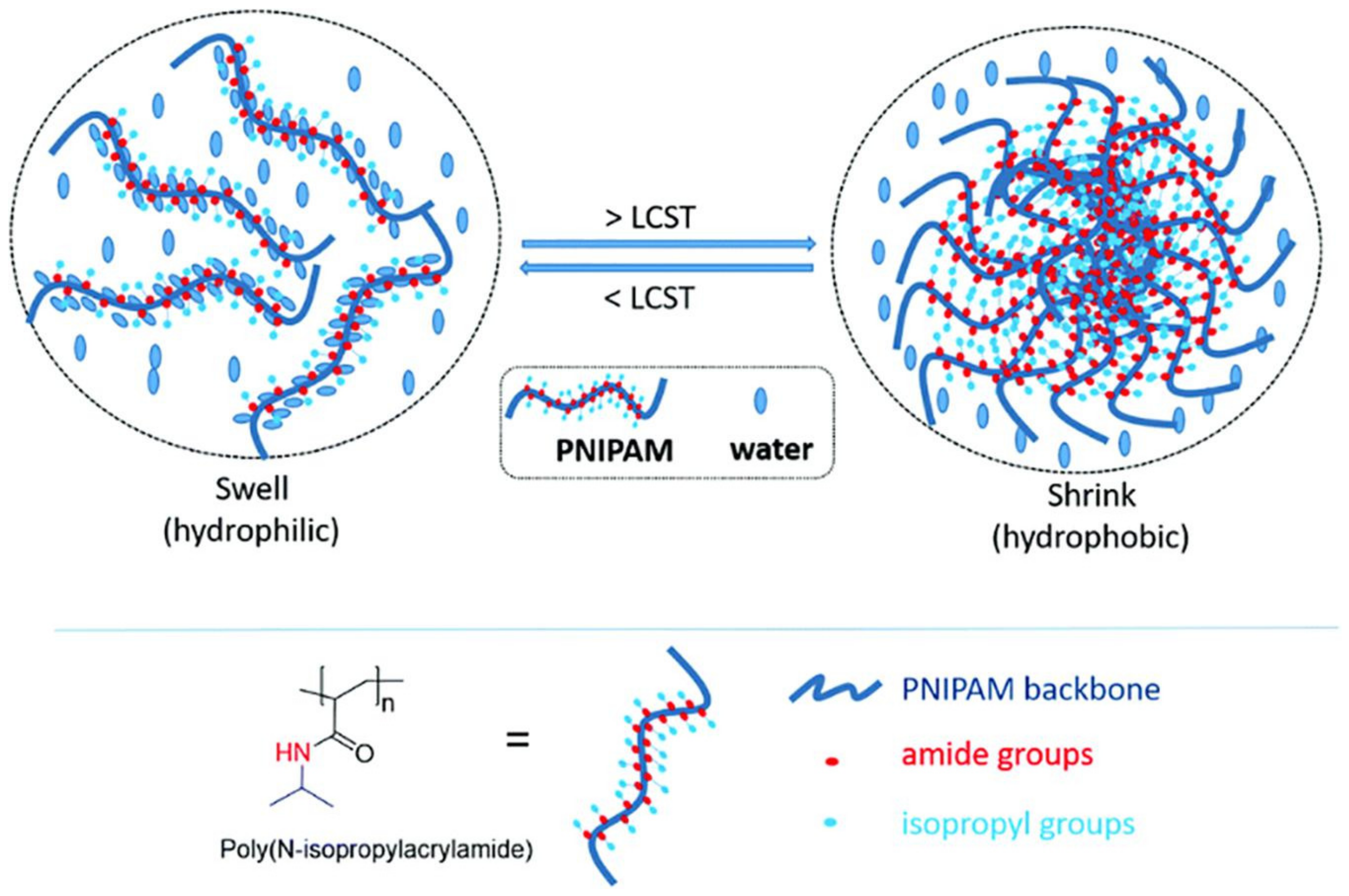
- Jain, K.; Vedarajan, R.; Watanabe, M.; Ishikiriyama, M.; Matsumi, N. Tunable LCST behavior of poly(N-isopropylacrylamide/ionic liquid) copolymers. Polym. Chem. 2015, 6, 6819–6825. [Google Scholar] [CrossRef]
- Cao, M.; Wang, Y.; Hu, X.; Gong, H.; Li, R.; Cox, H.; Zhang, J.; Waigh, T.A.; Xu, H.; Lu, J.R. Reversible thermoresponsive peptide–PNIPAM hydrogels for controlled drug delivery. Biomacromolecules 2019, 20, 3601–3610. [Google Scholar] [CrossRef]
- Tu, Y.; Chen, N.; Li, C.; Liu, H.; Zhu, R.; Chen, S.; Xiao, Q.; Liu, J.; Ramakrishna, S.; He, L. Advances in injectable self-healing biomedical hydrogels. Acta Biomater. 2019, 90, 1–20. [Google Scholar] [CrossRef]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive polymers and their biomedical application in tissue engineering–A review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef]
- Ge, J.; Neofytou, E.; Cahill, T.J., 3rd; Beygui, R.E.; Zare, R.N. Drug release from electric-field-responsive nanoparticles. ACS Nano 2012, 6, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tavares, A.C.; Gauthier, M.A. Nano-engineered electro-responsive drug delivery systems. J. Mater. Chem. B 2016, 4, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Lim, K.-T. Graphene Oxide-Based Stimuli-Responsive Platforms for Biomedical Applications. Molecules 2021, 26, 2797. [Google Scholar] [CrossRef]
- Hou, H.-L.; Cardo, L.; Mancino, D.; Arnaiz, B.; Criado, A.; Prato, M. Electrochemically controlled cleavage of imine bonds on a graphene platform: Towards new electro-responsive hybrids for drug release. Nanoscale 2020, 12, 23824–23830. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Du, L.N.; Lu, C.T.; Jin, Y.G.; Ge, S.P. Potential and problems in ultrasound-responsive drug delivery systems. Int. J. Nanomed. 2013, 8, 1621–1633. [Google Scholar] [CrossRef] [Green Version]
- Browning, R.J.; Stride, E. Microbubble-mediated delivery for cancer therapy. Fluids 2018, 3, 74. [Google Scholar] [CrossRef] [Green Version]
- Bartucci, R.; Paramanandana, A.; Boersma, Y.L.; Olinga, P.; Salvati, A. Comparative study of nanoparticle uptake and impact in murine lung, liver and kidney tissue slices. Nanotoxicology 2020, 14, 847–865. [Google Scholar] [CrossRef]
- Lu, L.; Qi, S.; Chen, Y.; Luo, H.; Huang, S.; Yu, X.; Luo, Q.; Zhang, Z. Targeted immunomodulation of inflammatory monocytes across the blood-brain barrier by curcumin-loaded nanoparticles delays the progression of experimental autoimmune encephalomyelitis. Biomaterials 2020, 245, 119987. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous silica nanoparticles for drug delivery: Current insights. Molecules 2018, 23, 47. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.; Zhang, Y.S. Chapter 10—Microfluidic technologies for local drug delivery. In Microfluidics for Pharmaceutical Applications; Santos, H.A., Liu, D., Zhang, H., Eds.; William Andrew Publishing: Amsterdam, The Netherlands, 2019; pp. 281–305. [Google Scholar]
- Simutis, K.; Stonyte, G.; Mažutis, L. Chapter 12—Antibody discovery using microfluidic systems. In Microfluidics for Pharmaceutical Applications; Santos, H.A., Liu, D., Zhang, H., Eds.; William Andrew Publishing: Amsterdam, The Netherlands, 2019; pp. 337–351. [Google Scholar]
- Chen, R.; Sun, Z.; Chen, D. Chapter 11—Droplet-based microfluidics for cell encapsulation and delivery. In Microfluidics for Pharmaceutical Applications; Santos, H.A., Liu, D., Zhang, H., Eds.; William Andrew Publishing: Amsterdam, The Netherlands, 2019; pp. 307–335. [Google Scholar]
- Zaidi, S.A. Molecular imprinting: A useful approach for drug delivery. Mater. Sci. Energy Technol. 2020, 3, 72–77. [Google Scholar] [CrossRef]
- Saylan, Y.; Akgönüllü, S.; Yavuz, H.; Ünal, S.; Denizli, A. Molecularly imprinted polymer based sensors for medical applications. Sensors 2019, 19, 1279. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.; Cheng, Q.; Min, Y.-L.; Olson, E.N.; Siegwart, D.J. Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing. Nat. Commun. 2020, 11, 3232. [Google Scholar] [CrossRef] [PubMed]
- Subham Jain, N.; Preeti, S.; Amit, B.P. Application of Quantum Dots in Drug Delivery. Nanosci. Nanotechnol. Asia 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Beg, S.; Almalki, W.H.; Malik, A.; Farhan, M.; Aatif, M.; Alharbi, K.S.; Alruwaili, N.K.; Alrobaian, M.; Tarique, M.; Rahman, M. 3D printing for drug delivery and biomedical applications. Drug Discov. Today 2020, 25, 1668–1681. [Google Scholar] [CrossRef] [PubMed]
- Trenfield, S.J.; Awad, A.; Madla, C.M.; Hatton, G.B.; Firth, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Shaping the future: Recent advances of 3D printing in drug delivery and healthcare. Expert Opin. Drug Deliv. 2019, 16, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
















| Oral | Swallowed by Mouth as a Tablet, Capsule, Lozenge, or Liquid |
|---|---|
| Buccal | Held inside the cheek |
| Sub-lingual | Placed below the tongue |
| Enteral | Delivered directly into the stomach or intestine |
| Inhalable | Breathed in through a tube or mask |
| Nasal | Given into the nose by spray or pump |
| Ophthalmic | Given into the eye by drops, gel, or ointment |
| Otic | Given by drops into the ear |
| Rectal | Inserted into the rectum |
| Vaginal | Inserted into the vagina |
| Topical | Applied to the skin |
| Transdermal | Given through a patch placed on the skin |
| Infused | Injected into a vein with an IV line and slowly dripped in over time |
| Intramuscular | Injected into the muscle with a syringe |
| Intravenous | Injected into a vein or an IV line |
| Subcutaneous | Injected just under the skin |
| Ointment | Cream | Paste | Gel |
|---|---|---|---|
| Hydrocarbon based greasy semisolid | Mostly water-based where drugs are loaded in O/W or W/O emulsion | It is basically an ointment where a high percentage of insoluble solids are added | The liquid phase is trapped within a three-dimensional polymeric matrix |
| Translucent to opaque | Opaque | Opaque | Transparent |
| Greasy | Less greasy | Less greasy | Non-greasy |
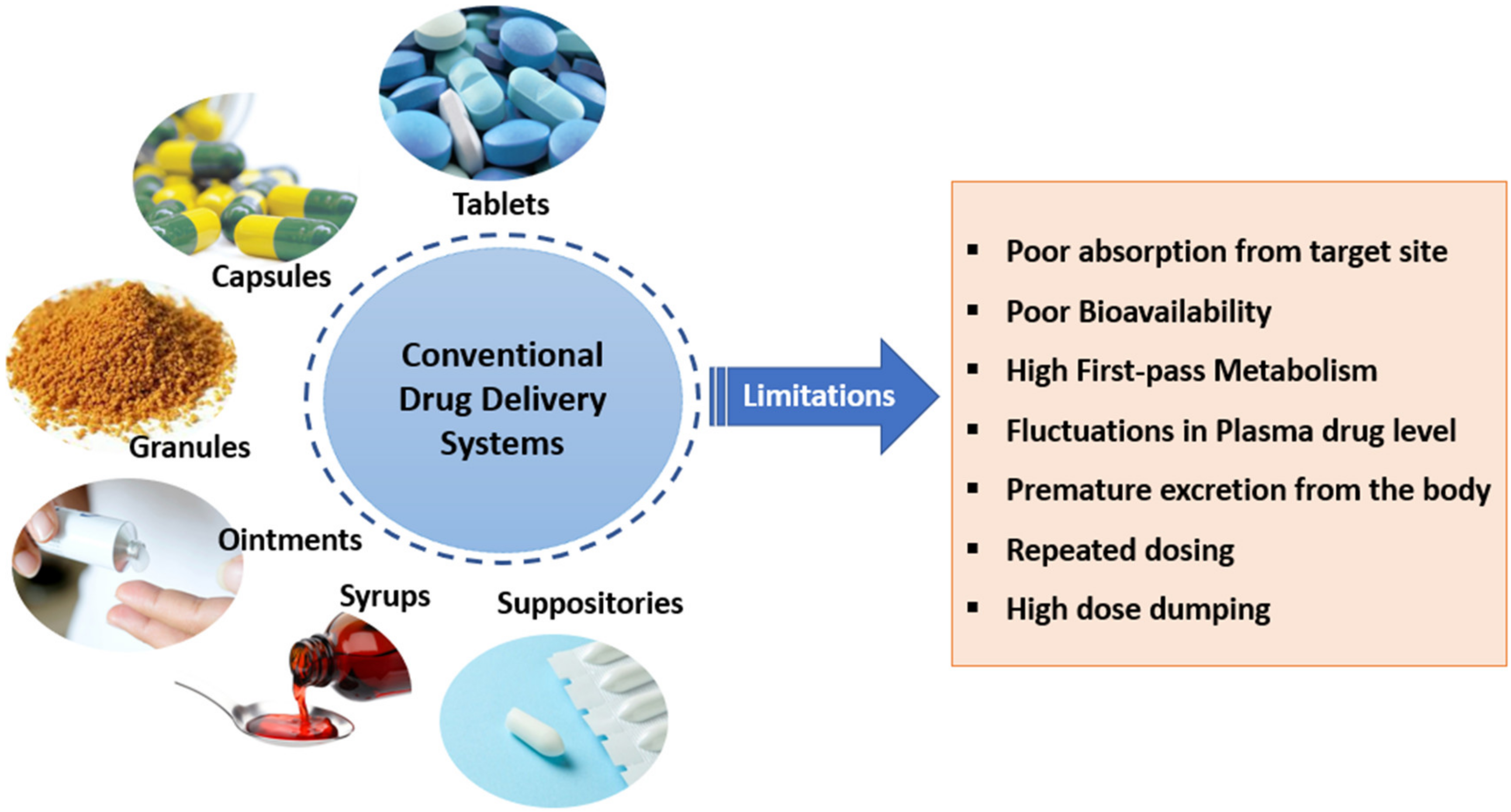
| Advantages of Conventional DDS | Disadvantages of Conventional DDS |
|---|---|
| Convenience in administration | Poor absorption from site of administration |
| Non-invasive and better IVIVC | No target specificity |
| Accurate and measured unit dosage form | Premature excretion from the body |
| Higher shelf-life | Premature metabolism of the drug |
| Accommodate patient variation | Poor bioavailability |
| Flexibility for physician to dose adjustment | Repeated dosing |
| Low cost | Poor patient compliance |
| Advantages of Controlled DDS | Disadvantages of Controlled DDS |
|---|---|
| Controlled or defined drug release | Possible toxicity of materials used |
| Target specificity | Dose dumping |
| Long residence of drug | Invasive procedure to implant or remove the system |
| Protection from metabolism by enzymes/chemicals | Uptake by RES reduces efficacy |
| Improved bioavailability | Poorer IVIVC |
| Low dosing frequency | Limited standards |
| Better patient compliance | Higher manufacturing cost |
| Diffusion Controlled Reservoir Systems | Diffusion Controlled Monolithic/Matrix Systems |
|---|---|
| Easier to achieve zero order | Difficult to achieve zero order |
| Degradable systems may be difficult to design | Suitable for degradable and non-degradable systems |
| Rupture can result in dose dumping | No danger of dose dumping |
| Drug inactivation by contact with the polymeric matrix can be avoided | Not all drugs can be blended with a given polymeric matrix |
| Sr. No | Molecule/Drug | Marketed CR Formulation | Manufacturing Company |
|---|---|---|---|
| 1 | Zolpidem Extended-Release Tablets | Ambien CR | SANOFI AVENTIS |
| 2 | Cyanocobalamin Ferrous Fumarate Folic Acid | Fericap CR | Raptakos, Brett & Co. Ltd. |
| 3 | Fluvoxamine Extended-Release | Luvox CR | JAZZ PHARMS |
| 4 | disopyramide | Norpace CR | Pfizer Laboratories |
| 5 | carbidopa 25 mg, levodopa 100 mg | Sinemet CR | Sun Pharmaceuticals |
| 6 | Paroxetine Hydrochloride Hemihydrate 12.5 mg | Paxil CR | GSK |
| Drug Delivery System Size Scale | Macroscale | Microscale and Nanoscale | Nanoscale (Targeted Delivery) |
|---|---|---|---|
| Implants (e.g., Subcutaneous or intramuscular | Reservoir DDS (e.g., Oral tablets, drug-eluting stents, catheters) | Injected nanocarrier DDS (e.g., PEGylated drugs, PEGylated liposomes, PEGylated polymeric micelles, polymer-drug conjugates | |
| Inserts (e.g., Vaginal, ophthalmic) | Injected matrix or monolith depots (e.g., Degradable microparticles and phase separation) | ||
| Ingested DDS (e.g., Osmotic pumps, hydrogels) | Early nanoparticles and PEGylation DDS (e.g., Polymeric micelles and liposomes) | ||
| Topical DDS (e.g., Skin patches) | |||
| 1st Generation | 2nd Generation | 3rd Generation | |
| Drug Delivery System Technologies | Basics of Controlled Release (1950–1980) | Smart Delivery System (1980–2010) | Modulated Delivery System (2010–2040) |
| Oral delivery | Zero-order release | On-Off insulin release | |
| Transdermal delivery | Smart polymers and hydrogels | Targeted delivery | |
| Drug release mechanism | Peptide and protein delivery | Long term delivery system | |
| Nanoparticles | In-vitro and in-vivo correlation |
| Synthetic Polymers [66,67] | Natural Polymers [67,68] | Stimuli-Responsive Polymers [69] |
|---|---|---|
| Polyhydroxy ethyl methacrylate poly (2-hydroxyethyl methacrylate) Ethyl cellulose Hydroxypropyl methyl cellulose (HPMC) Eudragits Polylactic acid (PLA) Polylactic-co-glycolic acid (PLGA) Polycaprolactone Polyvinyl Pyrrolidone (PVP) Poly methyl methacrylate (PMMA) Poly-(N-Isopropyl acrylamide) (PNIPAM) Poly(ethylenimine) Cyclodextrin (α, β, γ) Carbomers | Alginates Starches Dextrans Cellulose Gums (Acacia, Tragacanth, Guar gum) Chitosan Collagen Gelatine Microbial polymers (Polyhydroxy butyrate) Arginine derivatives | pH-responsive: Polyacids (PLA, Polymethacrylate, Poly aspartate, alginates, polystyrene sulphonic acid) Polybases (Chitosan, poly-L-Lysine, Polyallylamine, Poly ethylene amine, Poly amidoamine dendrimer) Thermoresponsive: Poly-(N-Isopropyl acrylamide) (PNIPAM) Poly-(N-Vinylcaprolactam) Poly(N,N-dimethyl acrylamide) Poly (methyl vinyl ether) Electric responsive: Sulfonated polystyrenes Poly(thiophene)s Poly(ethyl oxazoline)s Ultrasound responsive: Ethylene-vinyl acetate Light responsive: Modified poly(acrylamide)s |
| Advantages | Disadvantages |
|---|---|
| Specificity and targeted delivery of drugs can be achieved | Unintended penetration and translocation of nanocarriers to the blood–brain barrier, lungs results in toxicity |
| Improved tumour penetration for anticancer drugs | Nanocarriers can change in shape and size resulting in varied physicochemical interactions and activity |
| Enhanced Permeability and Retention can permit the passive accumulation | Suboptimal delivery due to heterogeneities of nanocarriers in vascular permeability |
| Enhanced bioavailability and efficacy | Uptake by RES can reduce the efficacy |
| Controlled delivery of drugs with low dose | Limited availability of animal models |
| Nanocarrier | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| Liposomes | Less cytotoxic Amphiphilic and Self-assembly capability Can load both hydrophilic and lipophilic drugs High payload Longer duration of action | Could crystallize during long term storage Poor control over the drug release rate Lack of means to prevail biological barriers Sufficient loading of drugs for which pH and ion gradients do not apply Leakage and fusion of loaded drug Phospholipids may undergo oxidation and hydrolysis | [117,118] |
| Dendrimers | Uniformity in molecular weight, size, shape and branch length A high degree of branching results in a high surface area Availability of internal cavities with Polyvalency offer high loading and targetting High water solubility Biocompatibility and absence of immunogenicity | Complex synthesis process Possibility of incomplete reactions with terminal groups Steric hindrance to the core molecule and dendrons obstructs the formation of high generation dendrimer | [119,120] |
| Exosomes | Cell targetting anad gene delivery Ability to loading both hydrophilic and lipophilic drugs Exosomes membranes possess many proteins thus show very high organotropism Immunocompatible if derived autologous | Rapid clearance from the blood Current methods available suffer low drug loading and retention Purification and large scale extraction is a hassle | [121,122] |
| Metal Nanoparticles | Tunable sizes and shapes (spherical, triangular, cubic, rods, starts, etc.) Possibilities of easy functionalization Size-dependent activity | RES uptake might result in low biocompatibility and cytotoxicity Instability of nanoparticles | [88,123] |
| Mesoporous silica nanoparticles | Ordered porous structure High surface area Tunable pore size and functionalization Poorly water-soluble drugs and gene delivery | More studies are needed on cytotoxicity The presence of high surface silanol groups interacts with the phospholipids of the red blood cell membranes leads to hemolysis | [124,125] |
| Carbon nanotubes | High surface area, enhanced conductivity and strength Vast functionalization sites Optical properties For targeted delivery | High immunogenicity, carcinogenicity and cytotoxicity Non-biodegradable Poor aqueous solubility and poor absorption | [103,126] |
| Nanocapsules/nanospheres | Efficient drug accumulation at the target site Controlled release of drug over weeks | Non-degradable polymers accumulate in tissues In vivo metabolism and elimination, routes are not elucidated | [127,128] |
| Quantum dots | Semiconductor nanocrystals with broad excitation spectra, narrow emission spectra, tunable emission peaks Possess long fluorescence lifetimes and negligible photobleaching Ability to conjugate with proteins and multiple molecular targets simultaneously | Quantum dot degradation result in the leaching of heavy metals such as Cadmium which generates reactive oxygen species (ROS) High cytotoxicity | [129,130,131] |
| Nanofibers | High specific surface area Multiple drugs with high loading capacity Tunable physicochemical properties Good Spatio-temporal distribution of drugs Great choice of polymers that are biodegradable and biocompatible Designed for various routes of administration for both hydrophilic and hydrophobic drugs | Scalability is an issue Poor control over nanofiber dimensions Need to optimize the solvent system for each polymer in the electrospinning process | [96,132,133,134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. https://doi.org/10.3390/molecules26195905
Adepu S, Ramakrishna S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules. 2021; 26(19):5905. https://doi.org/10.3390/molecules26195905
Chicago/Turabian StyleAdepu, Shivakalyani, and Seeram Ramakrishna. 2021. "Controlled Drug Delivery Systems: Current Status and Future Directions" Molecules 26, no. 19: 5905. https://doi.org/10.3390/molecules26195905
APA StyleAdepu, S., & Ramakrishna, S. (2021). Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules, 26(19), 5905. https://doi.org/10.3390/molecules26195905







