Peroxide-Induced Synthesis of Maleic Anhydride-Grafted Poly(butylene succinate) and Its Compatibilizing Effect on Poly(butylene succinate)/Pistachio Shell Flour Composites
Abstract
:1. Introduction
2. Results
2.1. Morphology of PSF
2.2. Optical Properies of PBS/PSF Composites
2.3. Mechanical Properties of PBS/PSF Composites
2.4. Morphology of PBS/PSF Composites
2.5. Spectroscopic Properties of PBS/PSF Composites
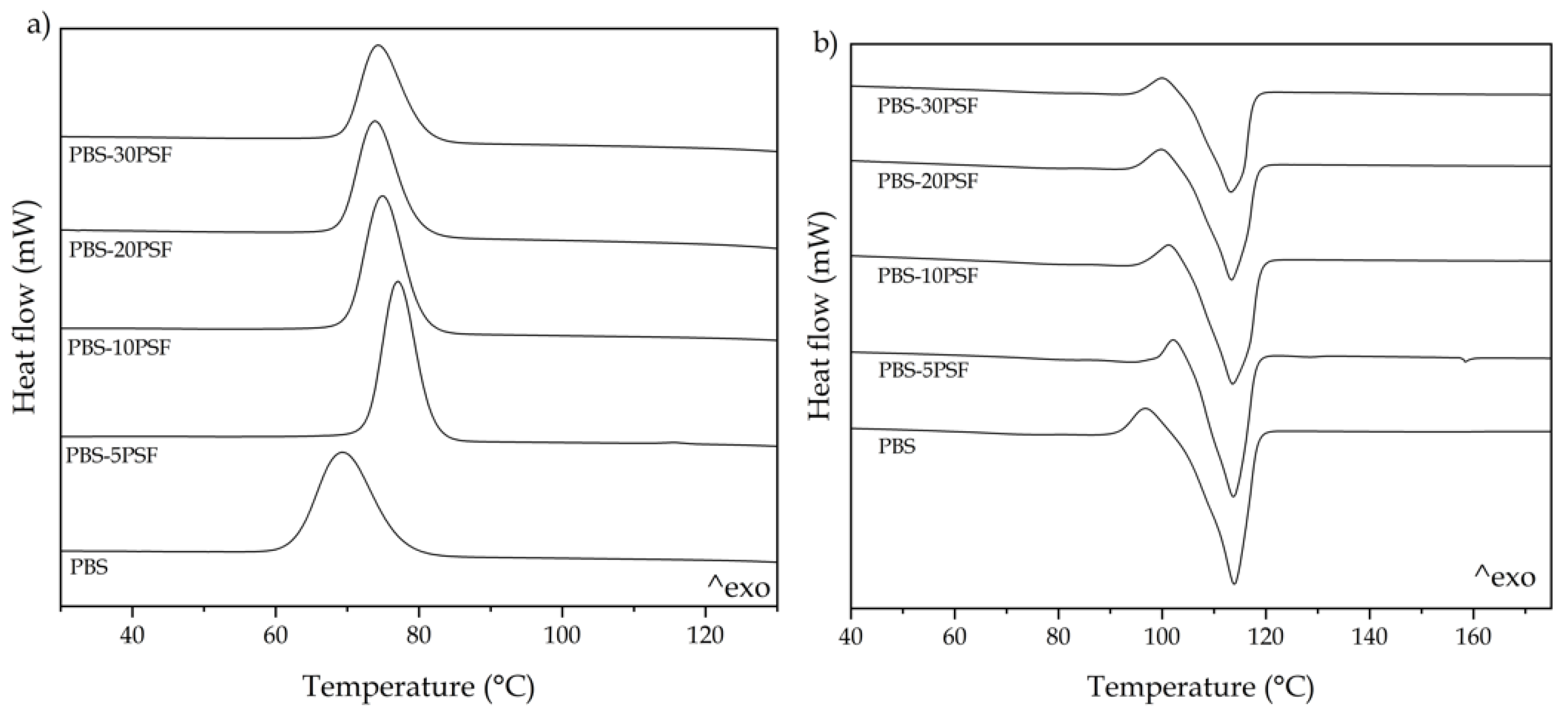
2.6. Thermal Properties of PBS/PSF Composites
2.7. Thermomechanical Properties of PBS/PSF Composites
2.8. Water Uptake of PBS/PSF Composites
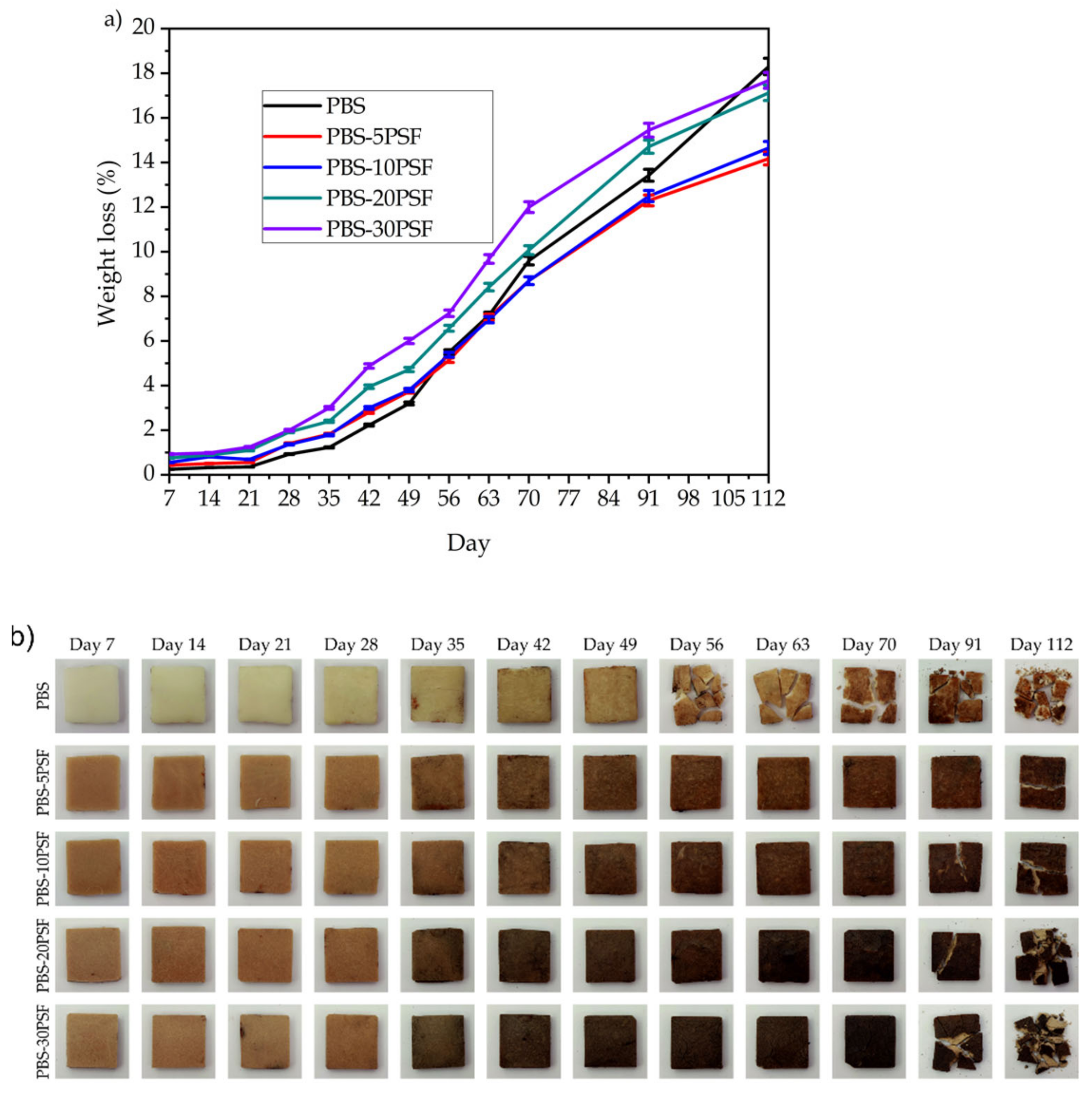
2.9. Disintegration in Controlled Compost Soil of PBS/PSF Composites
3. Discussion
4. Materials and Methods
4.1. Materials
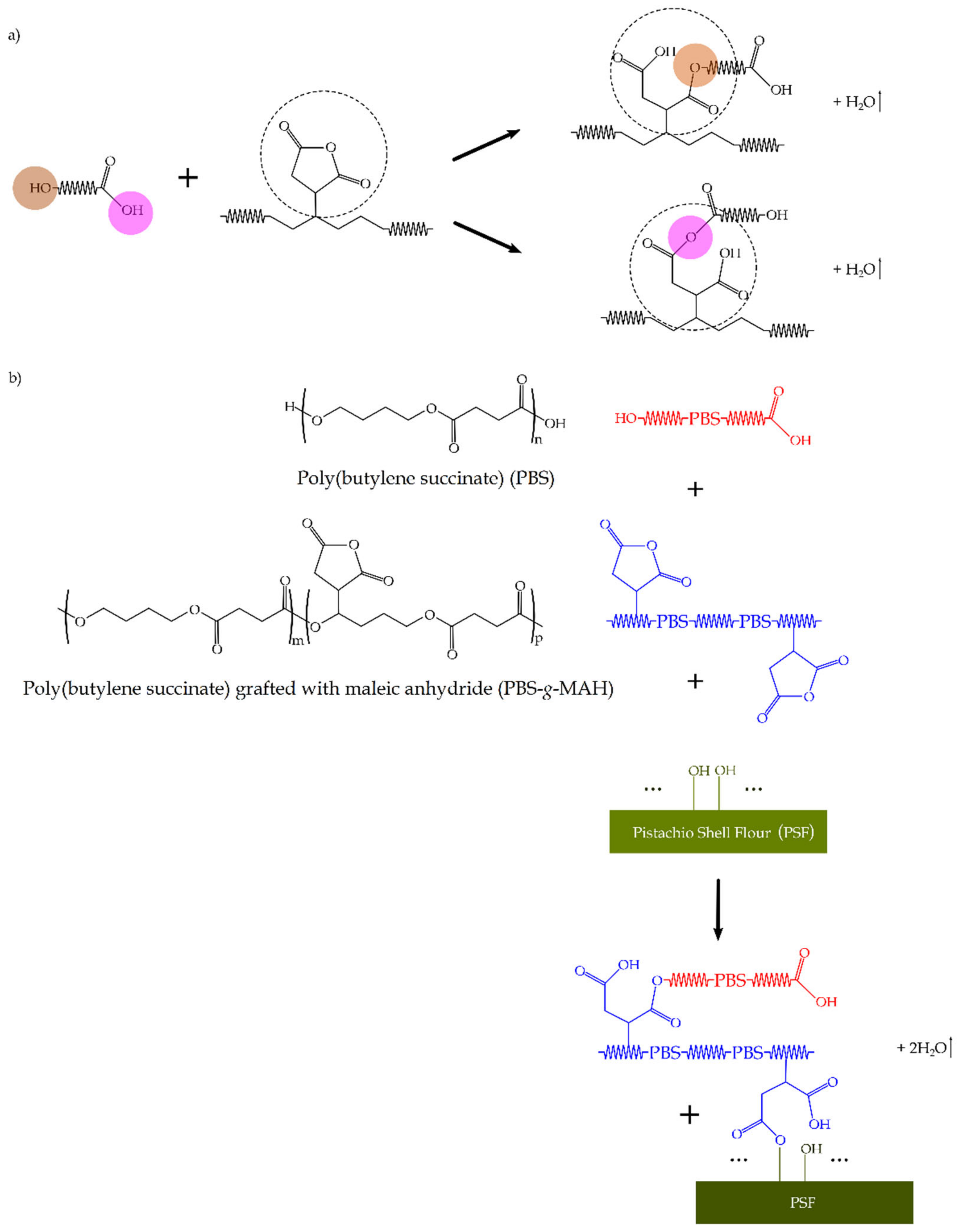
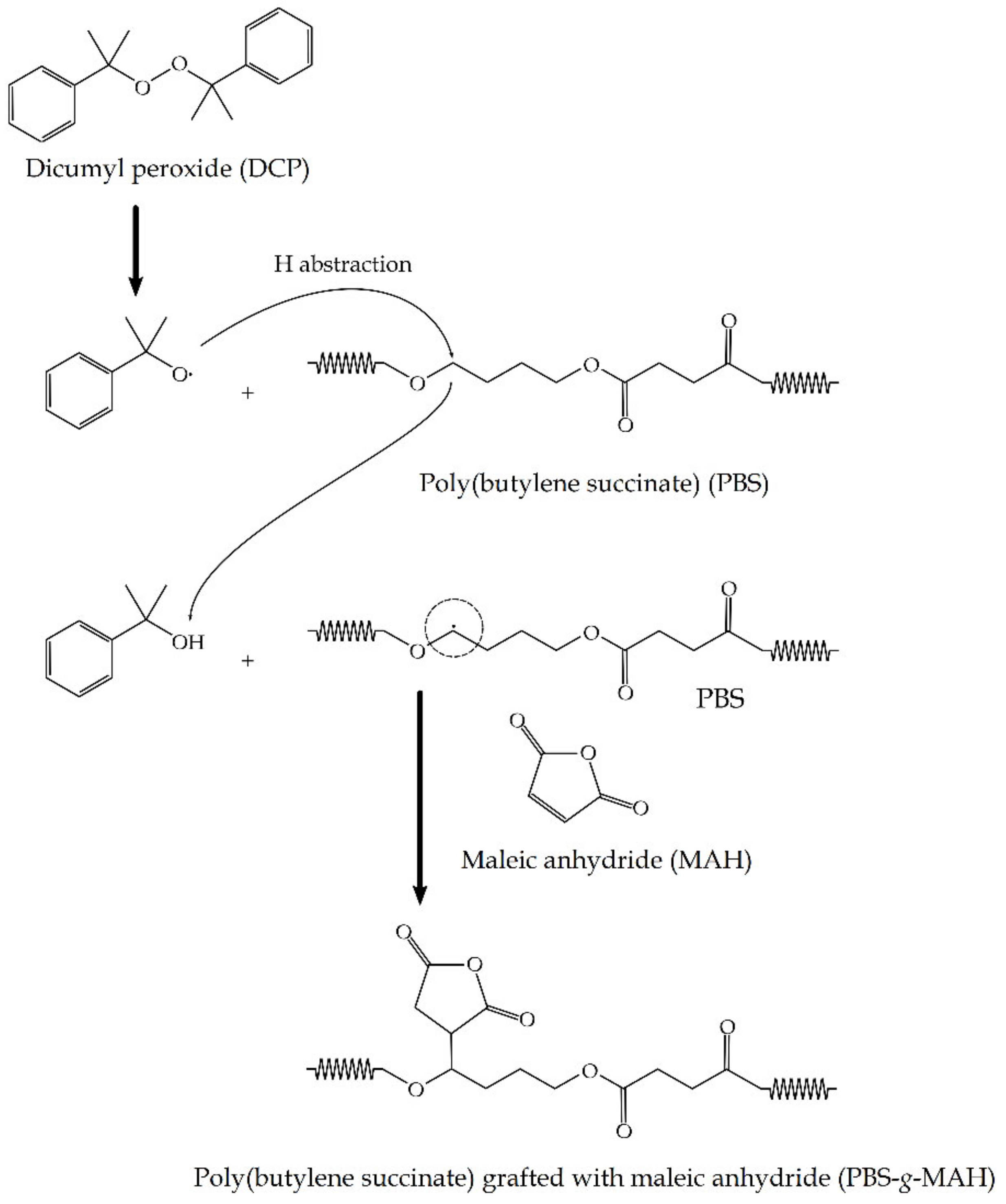
4.2. Grafting Procedure
4.3. Preparation of PBS/PSF Composites
4.4. Characterization of PBS/PSF Composite Pieces
4.4.1. Morphological Characterization
4.4.2. Color Characterization
4.4.3. Mechanical Characterization
4.4.4. Infrared Spectroscopy
4.4.5. Thermal Analysis
4.4.6. Thermomechanical Characterization
4.4.7. Water Absorption Test
4.4.8. Disintegration Test
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Torres-Giner, S.; Figueroa-Lopez, K.J.; Melendez-Rodriguez, B.; Prieto, C.; Pardo-Figuerez, M.; Lagaron, J.M. Emerging Trends in Biopolymers for Food Packaging. In Sustainable Food Packaging Technology; Athanassiou, A., Ed.; Wiley-VCH GmbH: Weinheim, Germany, 2021; pp. 1–33. [Google Scholar] [CrossRef]
- Bajpai, P. Biobased Polymers: Properties and Applications in Packaging; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–231. [Google Scholar] [CrossRef]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Signori, F.; Pelagaggi, M.; Bronco, S.; Righetti, M.C. Amorphous/crystal and polymer/filler interphases in biocomposites from poly (butylene succinate). Thermochim. Acta 2012, 543, 74–81. [Google Scholar] [CrossRef]
- Bhatia, A.; Gupta, R.K.; Bhattacharya, S.N.; Choi, H. Compatibility of biodegradable poly(lactic acid) (PLA) and poly(butylene succinate) (PBS) blends for packaging application. Korea-Aust. Rheol. J. 2007, 19, 125–131. [Google Scholar]
- Jacquel, N.; Freyermouth, F.; Fenouillot, F.; Rousseau, A.; Pascault, J.P.; Fuertes, P.; Saint-Loup, R. Synthesis and properties of poly(butylene succinate): Efficiency of different transesterification catalysts. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 5301–5312. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.H. Microbial succinic acid, its polymer poly(butylene succinate), and applications. In Plastics from Bacteria; Chen, G.G.-Q., Ed.; Springer: Berlin, Germany, 2010; pp. 347–388. [Google Scholar] [CrossRef]
- Siracusa, V.; Lotti, N.; Munari, A.; Dalla Rosa, M. Poly(butylene succinate) and poly(butylene succinate-co-adipate) for food packaging applications: Gas barrier properties after stressed treatments. Polym. Degrad. Stab. 2015, 119, 35–45. [Google Scholar] [CrossRef]
- Cheng, H.H.; Xiong, J.; Xie, Z.N.; Zhu, Y.T.; Liu, Y.M.; Wu, Z.Y.; Yu, J.; Guo, Z.X. Thrombin-Loaded Poly (butylene succinate)-Based Electrospun Membranes for Rapid Hemostatic Application. Macromol. Mater. Eng. 2018, 303, 1700395. [Google Scholar] [CrossRef]
- Gigli, M.; Fabbri, M.; Lotti, N.; Gamberini, R.; Rimini, B.; Munari, A. Poly(butylene succinate)-based polyesters for biomedical applications: A review. Eur. Polym. J. 2016, 75, 431–460. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.; Zuhri, M.; Ilyas, R.; Syafiq, R.; Sherwani, S. Nanocellulose reinforced thermoplastic starch (TPS), polylactic acid (PLA), and polybutylene succinate (PBS) for food packaging applications. Front. Chem. 2020, 8. [Google Scholar] [CrossRef]
- Liu, R.; Peng, Y.; Cao, J.; Chen, Y.J.C.S. Comparison on properties of lignocellulosic flour/polymer composites by using wood, cellulose, and lignin flours as fillers. Compos. Sci. Technol. 2014, 103, 1–7. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Sammon, C.; Balart, R.; Torres-Giner, S. Compatibilization of highly sustainable polylactide/almond shell flour composites by reactive extrusion with maleinized linseed oil. Ind. Crop. Prod. 2018, 111, 878–888. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Lagaron, J.M.; Balart, R.; Torres-Giner, S. On the use of acrylated epoxidized soybean oil as a reactive compatibilizer in injection-molded compostable pieces consisting of polylactide filled with orange peel flour. Polym. Int. 2018, 67, 1341–1351. [Google Scholar] [CrossRef]
- Montava-Jordà, S.; Quiles-Carrillo, L.; Richart, N.; Torres-Giner, S.; Montanes, N. Enhanced Interfacial Adhesion of Polylactide/Poly(ε-caprolactone)/Walnut Shell Flour Composites by Reactive Extrusion with Maleinized Linseed Oil. Polymers 2019, 11, 758. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Barajas, D.L.; Arévalo-Prada, J.A.; Fenollar, O.; Rueda-Ordóñez, Y.J.; Torres-Giner, S. Torrefaction of Coffee Husk Flour for the Development of Injection-Molded Green Composite Pieces of Polylactide with High Sustainability. Appl. Sci. 2020, 10, 6468. [Google Scholar] [CrossRef]
- Sutivisedsak, N.; Cheng, H.N.; Burks, C.S.; Johnson, J.A.; Siegel, J.P.; Civerolo, E.L.; Biswas, A. Use of Nutshells as Fillers in Polymer Composites. J. Polym. Environ. 2012, 20, 305–314. [Google Scholar] [CrossRef]
- Tonbul, Y. Pyrolysis of pistachio shell as a biomass. J. Therm. Anal. Calorim. 2008, 91, 641–647. [Google Scholar] [CrossRef]
- Kasiri, N.; Fathi, M. Production of cellulose nanocrystals from pistachio shells and their application for stabilizing Pickering emulsions. Int. J. Biol. Macromol. 2018, 106, 1023–1031. [Google Scholar] [CrossRef]
- Taghizadeh-Alisaraei, A.; Assar, H.A.; Ghobadian, B.; Motevali, A. Potential of biofuel production from pistachio waste in Iran. Renew. Sustain. Energy Rev. 2017, 72, 510–522. [Google Scholar] [CrossRef]
- Ling, B.; Zhang, B.; Li, R.; Wang, S. Nutritional quality, functional properties, bioactivity, and microstructure of defatted pistachio kernel flour. J. Am. Oil Chem. Soc. 2016, 93, 689–699. [Google Scholar] [CrossRef]
- Cardullo, N.; Leanza, M.; Muccilli, V.; Tringali, C. Valorization of Agri-Food Waste from Pistachio Hard Shells: Extraction of Polyphenols as Natural Antioxidants. Resources 2021, 10, 45. [Google Scholar] [CrossRef]
- Kashaninejad, M.; Tabil, L. Pistachio (Pistacia vera L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Elsevier: Cambridge, UK, 2011; pp. 218e–247e. [Google Scholar] [CrossRef]
- Apaydin-Varol, E.; Pütün, E.; Pütün, A.E. Slow pyrolysis of pistachio shell. Fuel 2007, 86, 1892–1899. [Google Scholar] [CrossRef]
- Okutucu, C.; Duman, G.; Ucar, S.; Yasa, I.; Yanik, J. Production of fungicidal oil and activated carbon from pistachio shell. J. Anal. Appl. Pyrolysis 2011, 91, 140–146. [Google Scholar] [CrossRef]
- Açıkalın, K.; Karaca, F.; Bolat, E. Pyrolysis of pistachio shell: Effects of pyrolysis conditions and analysis of products. Fuel 2012, 95, 169–177. [Google Scholar] [CrossRef]
- Hesam, F.; Tarzi, B.G.; Honarvar, M.; Jahadi, M. Pistachio (Pistacia vera) shell as a new candidate for enzymatic production of xylooligosaccharides. J. Food Meas. Charact. 2021, 15, 33–45. [Google Scholar] [CrossRef]
- Gürü, M.; Şahin, M.; Tekeli, S.; Tokgöz, H. Production of polymer matrix composite particleboard from pistachio shells and improvement of its fire resistance by fly ash. High Temp. Mater. Process. 2009, 28, 191–195. [Google Scholar] [CrossRef]
- Alsaadi, M.; Erkliğ, A.; Albu-khaleefah, K. Effect of pistachio shell particle content on the mechanical properties of polymer composite. Arab. J. Sci. Eng. 2018, 43, 4689–4696. [Google Scholar] [CrossRef]
- Najafabadi, M.A.; Khorasani, S.N.; Esfahani, J.M. High density polyethylene/pistachio shell flour/nanoclay composites-effect of accelerated weathering conditions on mechanical properties, relative brightness and total colour change. Polym. Polym. Compos. 2017, 25, 299–308. [Google Scholar] [CrossRef]
- Karaağaç, B. Use of ground pistachio shell as alternative filler in natural rubber/styrene–butadiene rubber-based rubber compounds. Polym. Compos. 2014, 35, 245–252. [Google Scholar] [CrossRef]
- Lascano, D.; Guillen-Pineda, R.; Quiles-Carrillo, L.; Ivorra-Martínez, J.; Balart, R.; Montanes, N.; Boronat, T. Manufacturing and Characterization of Highly Environmentally Friendly Sandwich Composites from Polylactide Cores and Flax-Polylactide Faces. Polymers 2021, 13, 342. [Google Scholar] [CrossRef]
- Agüero, Á.; Garcia-Sanoguera, D.; Lascano, D.; Rojas-Lema, S.; Ivorra-Martinez, J.; Fenollar, O.; Torres-Giner, S. Evaluation of Different Compatibilization Strategies to Improve the Performance of Injection-Molded Green Composite Pieces Made of Polylactide Reinforced with Short Flaxseed Fibers. Polymers 2020, 12, 821. [Google Scholar] [CrossRef] [Green Version]
- Carbonell-Verdú, A.; García-García, D.; Jordá, A.; Samper, M.; Balart, R. Development of slate fiber reinforced high density polyethylene composites for injection molding. Compos. Part B Eng. 2015, 69, 460–466. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Boronat, T.; Montanes, N.; Balart, R.; Torres-Giner, S. Injection-molded parts of fully bio-based polyamide 1010 strengthened with waste derived slate fibers pretreated with glycidyl- and amino-silane coupling agents. Polym. Test. 2019, 77, 105875. [Google Scholar] [CrossRef]
- Gharbi, A.; Hassen, R.B.; Boufi, S. Composite materials from unsaturated polyester resin and olive nuts residue: The effect of silane treatment. Ind. Crop. Prod. 2014, 62, 491–498. [Google Scholar] [CrossRef]
- Acha, B.A.; Aranguren, M.I.; Marcovich, N.E.; Reboredo, M.M. Composites from PMMA modified thermosets and chemically treated woodflour. Polym. Eng. Sci. 2003, 43, 999–1010. [Google Scholar] [CrossRef]
- Mohammed, M.H.; Dauda, B. Unsaturated polyester resin reinforced with chemically modified natural fibre. IOSR J. Polym. Text. Eng. 2014, 1, 31–38. [Google Scholar]
- Yuan, X.; Jayaraman, K.; Bhattacharyya, D. Effects of plasma treatment in enhancing the performance of woodfibre-polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2004, 35, 1363–1374. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Torres-Giner, S.; Aldureid, A.; Cabedo, L.; Lagaron, J.M. Reactive Melt Mixing of Poly(3-Hydroxybutyrate)/Rice Husk Flour Composites with Purified Biosustainably Produced Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate). Materials 2019, 12, 2152. [Google Scholar] [CrossRef] [Green Version]
- Torres-Giner, S.; Montanes, N.; Boronat, T.; Quiles-Carrillo, L.; Balart, R. Melt grafting of sepiolite nanoclay onto poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by reactive extrusion with multi-functional epoxy-based styrene-acrylic oligomer. Eur. Polym. J. 2016, 84, 693–707. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable compatibilized polymer blends for packaging applications: A literature review. J. Appl. Polym. Sci. 2018, 135, 45726. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Rong, C.; Lin, T.; Chen, Y.; Wu, J.; You, J.; Wang, H.; Li, Y. Stable Co-Continuous PLA/PBAT Blends Compatibilized by Interfacial Stereocomplex Crystallites: Toward Full Biodegradable Polymer Blends with Simultaneously Enhanced Mechanical Properties and Crystallization Rates. Macromolecules 2021, 54, 2852–2861. [Google Scholar] [CrossRef]
- Wang, H.; Wei, B.; Gu, X.; Lin, T.; Li, Y. Determining the optimal molecular architecture for reactive splicing compatibilization: Toward a better understanding of reactive polymer processing. Polymer 2020, 208, 122948. [Google Scholar] [CrossRef]
- Tserki, V.; Matzinos, P.; Panayiotou, C. Novel biodegradable composites based on treated lignocellulosic waste flour as filler. Part II. Development of biodegradable composites using treated and compatibilized waste flour. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1231–1238. [Google Scholar] [CrossRef]
- Kennouche, S.; Le Moigne, N.; Kaci, M.; Quantin, J.-C.; Caro-Bretelle, A.-S.; Delaite, C.; Lopez-Cuesta, J.-M. Morphological characterization and thermal properties of compatibilized poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)/poly (butylene succinate) (PBS)/halloysite ternary nanocomposites. Eur. Polym. J. 2016, 75, 142–162. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biocomposite consisting of miscanthus fiber and biodegradable binary blend matrix: Compatibilization and performance evaluation. RSC Adv. 2017, 7, 27538–27548. [Google Scholar] [CrossRef] [Green Version]
- da Silva, J.C.G.; Alves, J.L.F.; Galdino, W.V.d.A.; Moreira, R.d.F.P.M.; José, H.J.; de Sena, R.F.; Andersen, S.L.F. Combustion of pistachio shell: Physicochemical characterization and evaluation of kinetic parameters. Environ. Sci. Pollut. Res. 2018, 25, 21420–21429. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Lua, A.C. Characteristics of activated carbons prepared from pistachio-nut shells by physical activation. J. Colloid Interface Sci. 2003, 267, 408–417. [Google Scholar] [CrossRef]
- Kadhim, N.N.; Hamad, Q.A.; Oleiwi, J.K. Tensile and Morphological Properties of PMMA Composite Reinforced by Pistachio Shell Powder Used in Denture Applications. In Proceedings of the 2nd International Conference on Materials Engineering and Science (IConMEAS 2019), Baghdad, Iraq, 25–26 September 2019. [Google Scholar]
- Agüero, A.; Morcillo, M.C.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Lascano, D.; Torres-Giner, S.; Fenollar, O. Study of the influence of the reprocessing cycles on the final properties of polylactide pieces obtained by injection molding. Polymers 2019, 11, 1908. [Google Scholar] [CrossRef] [Green Version]
- Barcík, Š.; Gašparík, M.; Razumov, E.Y. Effect of temperature on the color changes of wood during thermal modification. Cellul. Chem. Technol. 2015, 49, 789–798. [Google Scholar]
- Liminana, P.; Garcia-Sanoguera, D.; Quiles-Carrillo, L.; Balart, R.; Montanes, N. Development and characterization of environmentally friendly composites from poly (butylene succinate) (PBS) and almond shell flour with different compatibilizers. Compos. Part B Eng. 2018, 144, 153–162. [Google Scholar] [CrossRef]
- Jin, T.x.; Zhou, M.; Hu, S.d.; Chen, F.; Fu, Q.; Fu, Y. Effect of molecular weight on the properties of poly(butylene succinate). Chin. J. Polym. Sci. 2014, 32, 953–960. [Google Scholar] [CrossRef]
- Huang, H.X.; Zhang, J.J. Effects of filler–filler and polymer–filler interactions on rheological and mechanical properties of HDPE–wood composites. J. Appl. Polym. Sci. 2009, 111, 2806–2812. [Google Scholar] [CrossRef]
- Chun, K.S.; Husseinsyah, S.; Osman, H. Mechanical and thermal properties of coconut shell powder filled polylactic acid biocomposites: Effects of the filler content and silane coupling agent. J. Polym. Res. 2012, 19, 1–8. [Google Scholar] [CrossRef]
- Kajaks, J.; Kalnins, K.; Naburgs, R. Wood plastic composites (WPC) based on high-density polyethylene and birch wood plywood production residues. Int. Wood Prod. J. 2018, 9, 15–21. [Google Scholar] [CrossRef]
- Ivorra-Martinez, J.; Manuel-Mañogil, J.; Boronat, T.; Sanchez-Nacher, L.; Balart, R.; Quiles-Carrillo, L. Development and characterization of sustainable composites from bacterial polyester poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) and almond shell flour by reactive extrusion with oligomers of lactic acid. Polymers 2020, 12, 1097. [Google Scholar] [CrossRef]
- Ferri, J.M.; Garcia-Garcia, D.; Montanes, N.; Fenollar, O.; Balart, R. The effect of maleinized linseed oil as biobased plasticizer in poly(lactic acid)-based formulations. Polym. Int. 2017, 66, 882–891. [Google Scholar] [CrossRef]
- Liminana, P.; Quiles-Carrillo, L.; Boronat, T.; Balart, R.; Montanes, N. The Effect of Varying Almond Shell Flour (ASF) Loading in Composites with Poly(Butylene Succinate (PBS) Matrix Compatibilized with Maleinized Linseed Oil (MLO). Materials 2018, 11, 2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phua, Y.; Chow, W.; Mohd Ishak, Z. Reactive processing of maleic anhydride-grafted poly(butylene succinate) and the compatibilizing effect on poly(butylene succinate) nanocomposites. Express Polym. Lett. 2013, 7. [Google Scholar] [CrossRef]
- El-Nabarawy, T.; Petro, N.S.; Abdel-Aziz, S. Adsorption Characteristics of Coal-based Activated Carbons. II. Adsorption of Water Vapour, Pyridine and Benzene. Adsorpt. Sci. Technol. 1997, 15, 47–57. [Google Scholar] [CrossRef]
- Çelik, Y.H.; Yalcin, R.; Topkaya, T.; Başaran, E.; Kilickap, E. Characterization of Hazelnut, Pistachio, and Apricot Kernel Shell Particles and Analysis of Their Composite Properties. J. Nat. Fibers 2021, 18, 1054–1068. [Google Scholar] [CrossRef]
- Yao, S.-F.; Chen, X.-T.; Ye, H.-M. Investigation of Structure and Crystallization Behavior of Poly(butylene succinate) by Fourier Transform Infrared Spectroscopy. J. Phys. Chem. B 2017, 121, 9476–9485. [Google Scholar] [CrossRef]
- John, J.; Tang, J.; Yang, Z.; Bhattacharya, M. Synthesis and characterization of anhydride-functional polycaprolactone. J. Polym. Sci. Part. A Polym. Chem. 1997, 35, 1139–1148. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradation of maleated linear low-density polyethylene and starch blends. Polym. Degrad. Stab. 1997, 56, 185–202. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, D.; Yang, C.; Zhang, H.; Qin, C.; Huang, B. Structure and properties of maleated high-density polyethylene. J. Appl. Polym. Sci. 1994, 52, 1411–1417. [Google Scholar] [CrossRef]
- Wu, C.S.; Liao, H.T.; Lai, S.M. Study on the graft reaction of maleic anhydride onto metallocene-based polyethylene-octene elastomer. Polym.—Plast. Technol. Eng. 2002, 41, 645–661. [Google Scholar] [CrossRef]
- Yu, Z.Z.; Ou, Y.C.; Qi, Z.N.; Hu, G.H. Toughening of nylon 6 with a maleated core-shell impact modifier. J. Polym. Sci. Part. B Polym. Phys. 1998, 36, 1987–1994. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Reihmane, S.; Gassan, J. Properties and modification methods for vegetable fibers for natural fiber composites. J. Appl. Polym. Sci. 1996, 59, 1329–1336. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, H.J.; Lee, J.W.; Choi, I.G. Biodegradability of bio-flour filled biodegradable poly(butylene succinate) bio-composites in natural and compost soil. Polym. Degrad. Stab. 2006, 91, 1117–1127. [Google Scholar] [CrossRef]
- Chen, R.y.; Zou, W.; Zhang, H.c.; Zhang, G.z.; Yang, Z.t.; Jin, G.; Qu, J.p. Thermal behavior, dynamic mechanical properties and rheological properties of poly(butylene succinate) composites filled with nanometer calcium carbonate. Polym. Test. 2015, 42, 160–167. [Google Scholar] [CrossRef]
- Chen, R.; Zou, W.; Zhang, H.; Zhang, G.; Qu, J. Crystallization behavior and thermal stability of poly(butylene succinate)/poly(propylene carbonate) blends prepared by novel vane extruder. AIP Conf. Proc. 2016, 1713, 050002. [Google Scholar] [CrossRef] [Green Version]
- Song, D.K.; Sung, Y.K. Synthesis and characterization of biodegradable poly(1,4-butanediol succinate). J. Appl. Polym. Sci. 1995, 56, 1381–1395. [Google Scholar] [CrossRef]
- Nikolic, M.S.; Djonlagic, J. Synthesis and characterization of biodegradable poly(butylene succinate-co-butylene adipate)s. Polym. Degrad. Stab. 2001, 74, 263–270. [Google Scholar] [CrossRef]
- Luo, X.; Li, J.; Feng, J.; Yang, T.; Lin, X. Mechanical and thermal performance of distillers grains filled poly(butylene succinate) composites. Mater. Des. 2014, 57, 195–200. [Google Scholar] [CrossRef]
- Balart, J.; García-Sanoguera, D.; Balart, R.; Boronat, T.; Sánchez-Nacher, L. Manufacturing and properties of biobased thermoplastic composites from poly(lactid acid) and hazelnut shell wastes. Polym. Compos. 2018, 39, 848–857. [Google Scholar] [CrossRef]
- Liminana, P.; Garcia-Sanoguera, D.; Quiles-Carrillo, L.; Balart, R.; Montanes, N. Optimization of maleinized linseed oil loading as a biobased compatibilizer in poly(butylene succinate) composites with almond shell flour. Materials 2019, 12, 685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendahou, A.; Kaddami, H.; Sautereau, H.; Raihane, M.; Erchiqui, F.; Dufresne, A. Short palm tree fibers polyolefin composites: Effect of filler content and coupling agent on physical properties. Macromol. Mater. Eng. 2008, 293, 140–148. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, G.K.; Mondal, M.K. Thermal degradation characteristics, kinetics, thermodynamic, and reaction mechanism analysis of pistachio shell pyrolysis for its bioenergy potential. Biomass Convers. Biorefin. 2020, 1–15. [Google Scholar] [CrossRef]
- Açıkalın, K. Pyrolytic characteristics and kinetics of pistachio shell by thermogravimetric analysis. J. Therm. Anal. Calorim. 2012, 109, 227–235. [Google Scholar] [CrossRef]
- Lee, S.-H.; Wang, S. Biodegradable polymers/bamboo fiber biocomposite with bio-based coupling agent. Compos. Part. A Appl. Sci. Manuf. 2006, 37, 80–91. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, B.H.; Choi, S.W.; Kim, S.; Kim, H.J. The effect of types of maleic anhydride-grafted polypropylene (MAPP) on the interfacial adhesion properties of bio-flour-filled polypropylene composites. Compos. Part. A Appl. Sci. Manuf. 2007, 38, 1473–1482. [Google Scholar] [CrossRef]
- Montava-Jorda, S.; Lascano, D.; Quiles-Carrillo, L.; Montanes, N.; Boronat, T.; Martinez-Sanz, A.V.; Ferrandiz-Bou, S.; Torres-Giner, S. Mechanical Recycling of Partially Bio-Based and Recycled Polyethylene Terephthalate Blends by Reactive Extrusion with Poly(styrene-co-glycidyl methacrylate). Polymers 2020, 12, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bautista, Y.; Gozalbo, A.; Mestre, S.; Sanz, V. Thermal Degradation Mechanism of a Thermostable Polyester Stabilized with an Open-Cage Oligomeric Silsesquioxane. Materials 2018, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Shen, D.; Gu, S.; Luo, K.; Wang, S.; Fang, M. The pyrolytic degradation of wood-derived lignin from pulping process. Bioresour. Technol. 2010, 101, 6136–6146. [Google Scholar] [CrossRef] [PubMed]
- Hexig, B.; Alata, H.; Asakawa, N.; Inoue, Y. Novel biodegradable poly(butylene succinate)/poly(ethylene oxide) blend film with compositional and spherulite-size gradients. J. Polym. Sci. Part. B Polym. Phys. 2005, 43, 368–377. [Google Scholar] [CrossRef]
- Lin, N.; Fan, D.; Chang, P.R.; Yu, J.; Cheng, X.; Huang, J. Structure and properties of poly(butylene succinate) filled with lignin: A case of lignosulfonate. J. Appl. Polym. Sci. 2011, 121, 1717–1724. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Montanes, N.; Fenollar, O.; García-Sanoguera, D.; Balart, R. Development and optimization of renewable vinyl plastisol/wood flour composites exposed to ultraviolet radiation. Mater. Des. 2016, 108, 648–658. [Google Scholar] [CrossRef]
- Frollini, E.; Bartolucci, N.; Sisti, L.; Celli, A. Poly(butylene succinate) reinforced with different lignocellulosic fibers. Ind. Crop. Prod. 2013, 45, 160–169. [Google Scholar] [CrossRef]
- Kiani, H.; Ashori, A.; Mozaffari, S.A. Water resistance and thermal stability of hybrid lignocellulosic filler-PVC composites. Polym. Bull. 2011, 66, 797–802. [Google Scholar] [CrossRef]
- Burgada, F.; Fages, E.; Quiles-Carrillo, L.; Lascano, D.; Ivorra-Martinez, J.; Arrieta, M.P.; Fenollar, O. Upgrading Recycled Polypropylene from Textile Wastes in Wood Plastic Composites with Short Hemp Fiber. Polymers 2021, 13, 1248. [Google Scholar] [CrossRef]
- Gairola, S.; Gairola, S.; Sharma, H.; Rakesh, P.K. Impact Behavior of Pine Needle Fiber/Pistachio Shell Filler Based Epoxy Composite. In Proceedings of the 2nd International Conference on New Frontiers in Engineering, Science and Technology (NFEST 2019), Kurukshetra, India, 18–22 February 2019. [Google Scholar]
- Quiles-Carrillo, L.; Montanes, N.; Garcia-Garcia, D.; Carbonell-Verdu, A.; Balart, R.; Torres-Giner, S. Effect of different compatibilizers on injection-molded green composite pieces based on polylactide filled with almond shell flour. Compos. Part. B Eng. 2018, 147, 76–85. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Vázquez, A. Influence of fiber chemical modification procedure on the mechanical properties and water absorption of MaterBi-Y/sisal fiber composites. Compos. Part. A Appl. Sci. Manuf. 2006, 37, 1672–1680. [Google Scholar] [CrossRef]
- Tserki, V.; Matzinos, P.; Kokkou, S.; Panayiotou, C. Novel biodegradable composites based on treated lignocellulosic waste flour as filler. Part I. Surface chemical modification and characterization of waste flour. Compos. Part. A Appl. Sci. Manuf. 2005, 36, 965–974. [Google Scholar] [CrossRef]
- Gassan, J.; Bledzki, A.K. The influence of fiber-surface treatment on the mechanical properties of jute-polypropylene composites. Compos. Part. A Appl. Sci. Manuf. 1997, 28, 1001–1005. [Google Scholar] [CrossRef]
- Tolga, S.; Kabasci, S.; Duhme, M. Progress of disintegration of polylactide (PLA)/poly(butylene succinate) (PBS) blends containing talc and chalk inorganic fillers under industrial composting conditions. Polymers 2021, 23, 10. [Google Scholar] [CrossRef]
- Zhao, J.H.; Wang, X.Q.; Zeng, J.; Yang, G.; Shi, F.H.; Yan, Q. Biodegradation of poly(butylene succinate) in compost. J. Appl. Polym. Sci. 2005, 97, 2273–2278. [Google Scholar] [CrossRef]
- Mwaikambo, L.Y.; Martuscelli, E.; Avella, M. Kapok/cotton fabric-polypropylene composites. Polym. Test. 2000, 19, 905–918. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Sain, M. Mechanical properties of biodegradable composites from poly lactic acid (PLA) and microcrystalline cellulose (MCC). J. Appl. Polym. Sci. 2005, 97, 2014–2025. [Google Scholar] [CrossRef]
- Kumar, R.; Yakubu, M.K.; Anandjiwala, R.D. Biodegradation of flax fiber reinforced poly lactic acid. Express Polym. Lett. 2010, 4. [Google Scholar] [CrossRef]
- Puchalski, M.; Szparaga, G.; Biela, T.; Gutowska, A.; Sztajnowski, S.; Krucińska, I. Molecular and Supramolecular Changes in Polybutylene Succinate (PBS) and Polybutylene Succinate Adipate (PBSA) Copolymer during Degradation in Various Environmental Conditions. Polymers 2018, 10, 251. [Google Scholar] [CrossRef] [Green Version]
- Gabara, W.; Porejko, S. Grafting of maleic anhydride on polyethylene. I. Mechanism of grafting in a heterogeneous medium in the presence of radical initiators. J. Polym. Sci. Part. A-1 Polym. Chem. 1967, 5, 1547–1562. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Injection Molded Sustainable Biocomposites From Poly(butylene succinate) Bioplastic and Perennial Grass. ACS Sustain. Chem. Eng. 2015, 3, 2767–2776. [Google Scholar] [CrossRef]

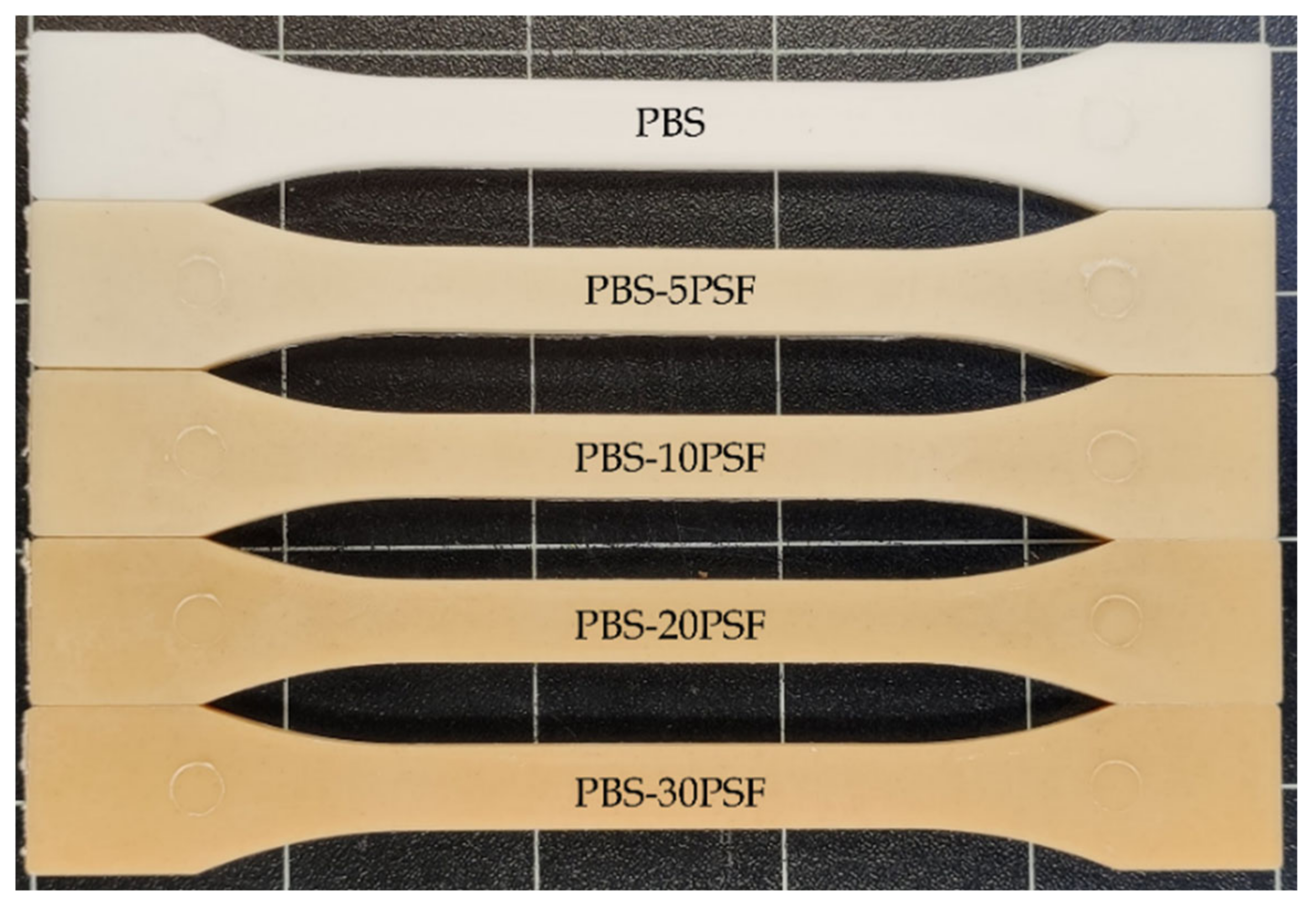
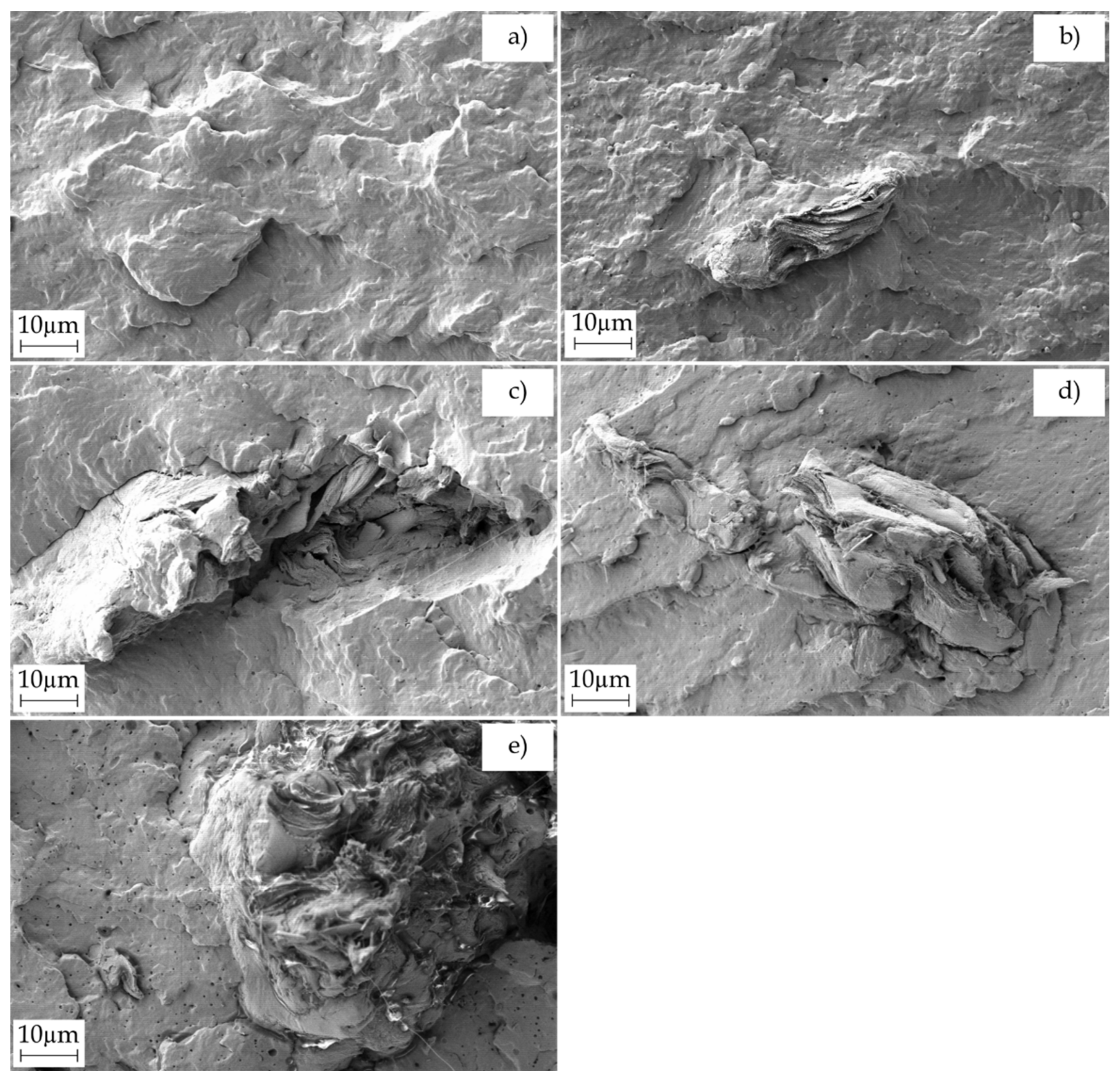
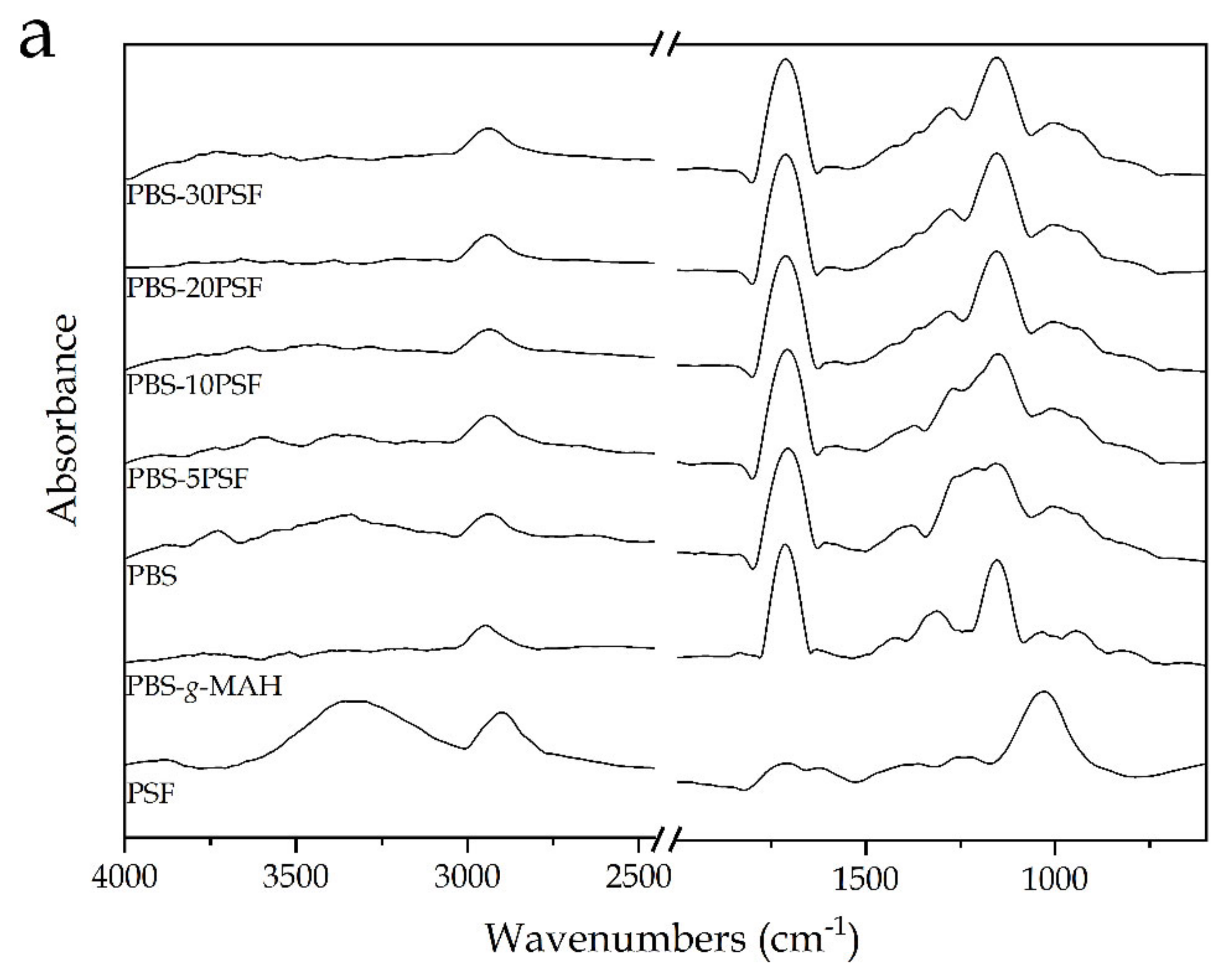
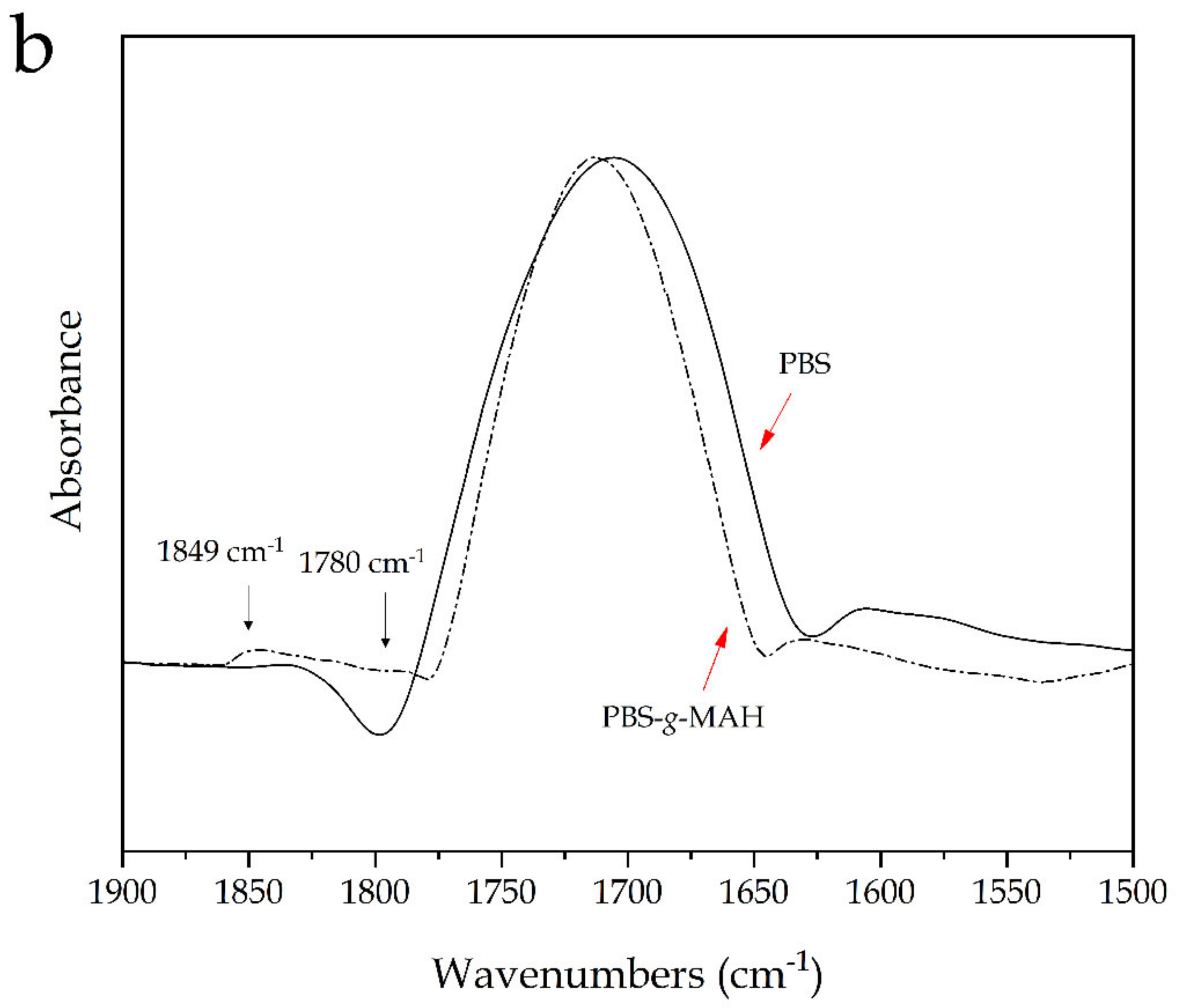




| Piece | L* | a* | b* | |
|---|---|---|---|---|
| PBS | 83.8 ± 0.1 a | −1.8 ± 0.1 a | 1.1 ± 0.2 a | - |
| PBS-5PSF | 71.8 ± 0.2 b | 1.1 ± 0.1 b | 15.8 ± 0.2 b | 19.0 ± 0.5 a |
| PBS-10PSF | 67.6 ± 0.1 c | 2.8 ± 0.2 c | 20.1 ± 0.1 c | 25.0 ± 0.5 b |
| PBS-20PSF | 61.8 ± 0.1 d | 5.1 ± 0.2 d | 23.0 ± 0.1 d | 31.2 ± 0.7 c |
| PBS-30PSF | 57.8 ± 0.2 e | 6.8 ± 0.2 e | 25.1 ± 0.1 e | 35.7 ± 0.6 d |
| Piece | E (MPa) | σmax (MPa) | εb (%) | Shore D Hardness | Impact Strength (kJ/m2) |
|---|---|---|---|---|---|
| PBS | 598.8 ± 13.4 a | 27.8 ± 0.5 a | 225.5 ± 15.2 a | 62.9 ± 0.7 a | 11.4 ± 0.8 a |
| PBS-5PSF | 605.6 ± 15.2 b | 23.1 ± 1.3 b | 42.9 ± 3.5 b | 63.6 ± 0.6 a | 6.8 ± 0.4 b |
| PBS-10PSF | 655.5 ± 18.7 c | 20.1 ± 0.8 c | 27.7 ± 1.7 c | 67.8 ± 0.4 b | 5.6 ± 0.2 c |
| PBS-20PSF | 852.2 ± 28.7 d | 17.0 ± 0.5 d | 15.9 ± 0.7 d | 68.8 ± 0.4 b | 3.3 ± 0.3 d |
| PBS-30PSF | 1039.6 ± 32.5 e | 16.0 ± 1.0 d | 9.7 ± 1.1 e | 69.5 ± 0.5 c | 2.9 ± 0.3 e |
| Piece | Tc (°C) | ΔHc (J/g) | Tcc (°C) | ΔHcc (J/g) | Tm (°C) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|---|---|---|
| PBS | 69.7 ± 0.9 a | 63.8 ± 0.8 a | 96.6 ± 0.4 a | 5.3 ± 0.8 a | 113.4 ± 0.8 a | 62.5 ± 0.9 a | 51.8 ± 1.2 a |
| PBS-5PSF | 77.7 ± 1.1 b | 65.9 ± 0.5 a | 102.0 ± 0.7 b | 3.0 ± 0.4 b | 113.3 ± 0.5 a | 57.3 ± 0.7 b | 52.2 ± 0.9 a |
| PBS-10PSF | 75.5 ± 0.7 c | 64.2 ± 1.0 a | 101.4 ± 0.6 b | 3.5 ± 0.6 c | 113.0 ± 0.7 a | 56.9 ± 1.1 b | 54.9 ± 1.0 b |
| PBS-20PSF | 74.3 ± 0.6 d | 53.8 ± 1.2 b | 99.7 ± 0.4 c | 3.5 ± 0.8 c | 112.8 ± 1.0 a | 56.6 ± 0.8 b | 62.8 ± 1.2 c |
| PBS-30PSF | 74.7 ± 1.1 d | 48.2 ± 0.5 c | 99.8 ± 0.9 c | 2.5 ± 0.5 d | 112.9 ± 0.6 a | 47.5 ± 1.2 c | 62.7 ± 0.7 c |
| Piece | T5% (°C) | Tmax (°C) | Residual Mass (%) |
|---|---|---|---|
| PSF | 224.8 ± 0.9 a | 264.8 ± 0.8 a/293.7 ± 1.1 b | 8.2 ± 0.9 a |
| PBS | 296.6 ± 1.0 b | 398.3 ± 0.7 c | 0.4 ± 0.2 b |
| PBS-5PSF | 341.9 ± 0.8 c | 401.6 ± 1.5 c | 1.4 ± 0.5 c |
| PBS-10PSF | 318.3 ± 0.7 d | 399.0 ± 0.7 c | 2.3 ± 0.7 d |
| PBS-20PSF | 290.9 ± 1.2 e | 397.4 ± 0.8 c | 4.4 ± 0.5 e |
| PBS-30PSF | 279.6 ± 1.0 f | 394.9 ± 1.0 c | 6.6 ± 0.8 f |
| Piece | Storage Modulus (MPa) | tan δ Peak (°C) | ||
|---|---|---|---|---|
| −45 °C | 25 °C | 70 °C | ||
| PBS | 1973.3 ± 30 a | 440 ± 10 a | 214.3 ± 12 a | −23.3 ± 0.2 a |
| PBS-5PSF | 2016.1 ± 35 a,b | 468 ± 21 a | 233.1 ± 20 a,b | −23.0 ± 0.1 b |
| PBS-10PSF | 2056.5 ± 37 a,b | 516 ± 14 b | 279.9 ± 22 b,c | −22.7 ± 0.3 c |
| PBS-20PSF | 2072.2 ± 38 a,b | 585 ± 12 c | 310.3 ± 17 c | −22.3 ± 0.4 d |
| PBS-30PSF | 2161.1 ± 34 b | 705 ± 15 d | 387.0 ± 21 d | −21.8 ± 0.2 e |
| Composition | PBS (wt.%) | PSF (wt.%) | PBS-g-MAH (wt.%) |
|---|---|---|---|
| PBS | 100.00 | 0.00 | 0.00 |
| PBS-5PSF | 94.20 | 5.00 | 0.83 |
| PBS-10PSF | 88.30 | 10.00 | 1.67 |
| PBS-20PSF | 76.60 | 20.00 | 3.30 |
| PBS-30PSF | 65.00 | 30.00 | 5.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Lema, S.; Arevalo, J.; Gomez-Caturla, J.; Garcia-Garcia, D.; Torres-Giner, S. Peroxide-Induced Synthesis of Maleic Anhydride-Grafted Poly(butylene succinate) and Its Compatibilizing Effect on Poly(butylene succinate)/Pistachio Shell Flour Composites. Molecules 2021, 26, 5927. https://doi.org/10.3390/molecules26195927
Rojas-Lema S, Arevalo J, Gomez-Caturla J, Garcia-Garcia D, Torres-Giner S. Peroxide-Induced Synthesis of Maleic Anhydride-Grafted Poly(butylene succinate) and Its Compatibilizing Effect on Poly(butylene succinate)/Pistachio Shell Flour Composites. Molecules. 2021; 26(19):5927. https://doi.org/10.3390/molecules26195927
Chicago/Turabian StyleRojas-Lema, Sandra, Jordi Arevalo, Jaume Gomez-Caturla, Daniel Garcia-Garcia, and Sergio Torres-Giner. 2021. "Peroxide-Induced Synthesis of Maleic Anhydride-Grafted Poly(butylene succinate) and Its Compatibilizing Effect on Poly(butylene succinate)/Pistachio Shell Flour Composites" Molecules 26, no. 19: 5927. https://doi.org/10.3390/molecules26195927
APA StyleRojas-Lema, S., Arevalo, J., Gomez-Caturla, J., Garcia-Garcia, D., & Torres-Giner, S. (2021). Peroxide-Induced Synthesis of Maleic Anhydride-Grafted Poly(butylene succinate) and Its Compatibilizing Effect on Poly(butylene succinate)/Pistachio Shell Flour Composites. Molecules, 26(19), 5927. https://doi.org/10.3390/molecules26195927










