Polymer-Modified Single-Walled Carbon Nanotubes Affect Photosystem II Photochemistry, Intersystem Electron Transport Carriers and Photosystem I End Acceptors in Pea Plants
Abstract
:1. Introduction
2. Results
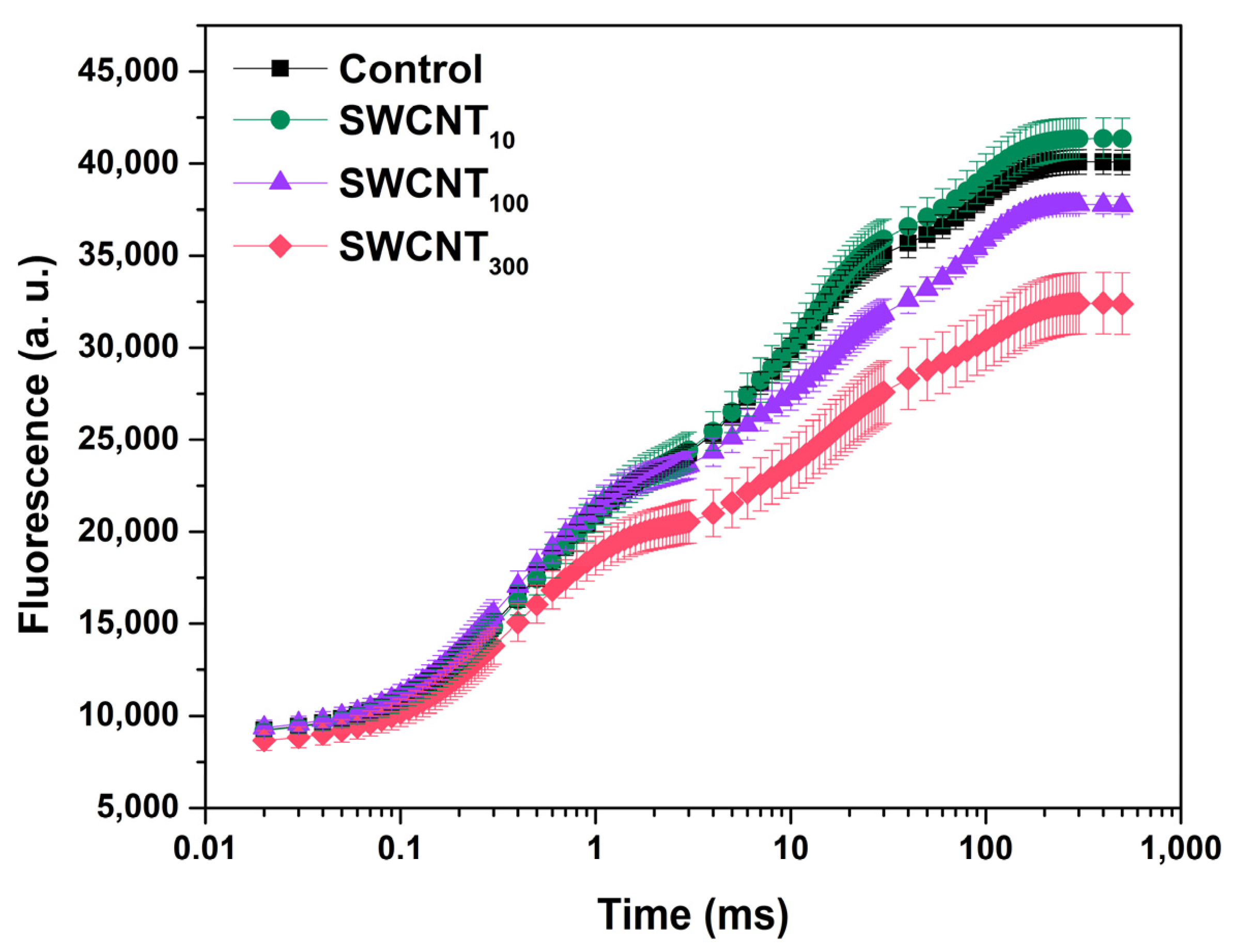
2.1. Prompt Chlorophyll Fluorescence Induction Curves
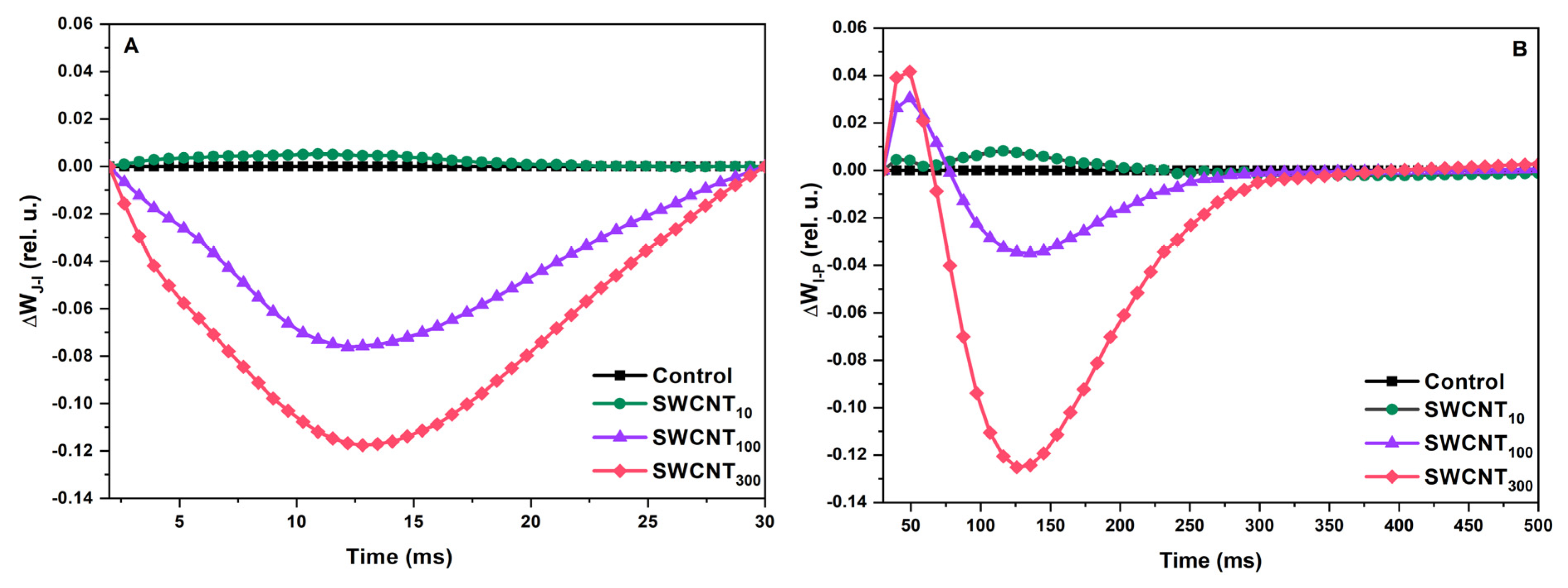
2.2. Differential Curves—Variable Chlorophyll Fluorescence Differences during J-I and I-P Induction Phases
2.3. JIP Test
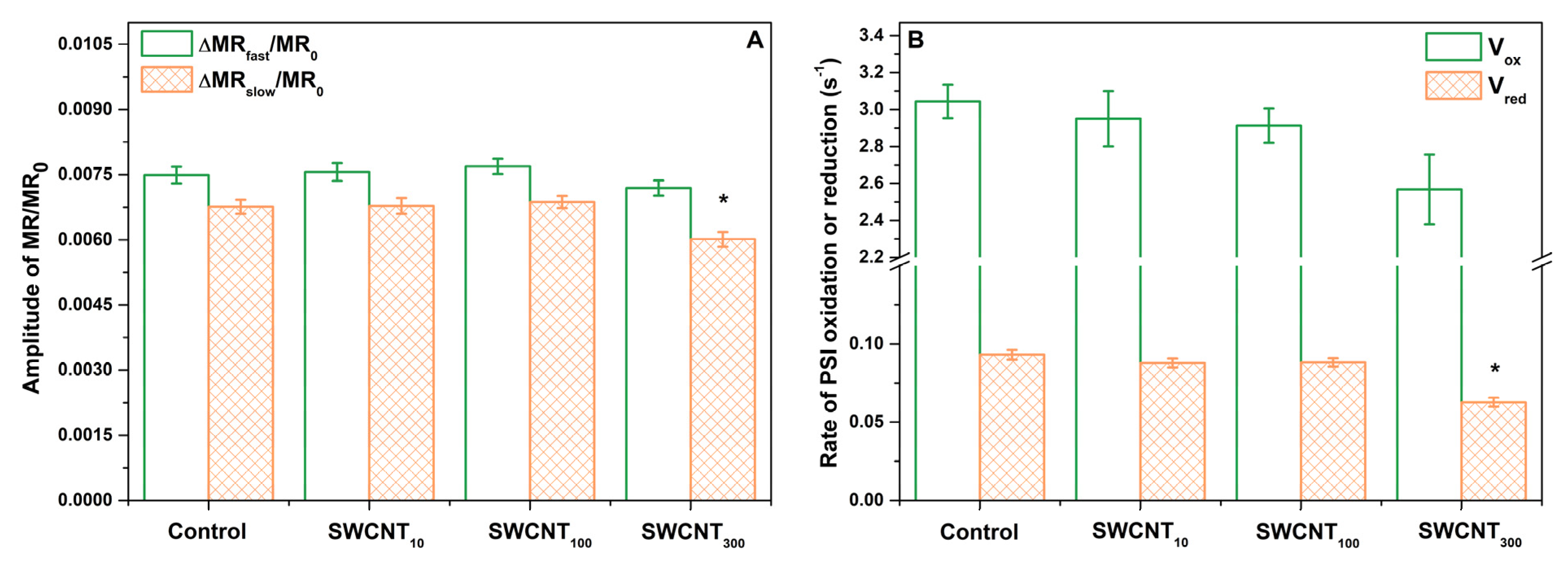
2.4. PSI Activity
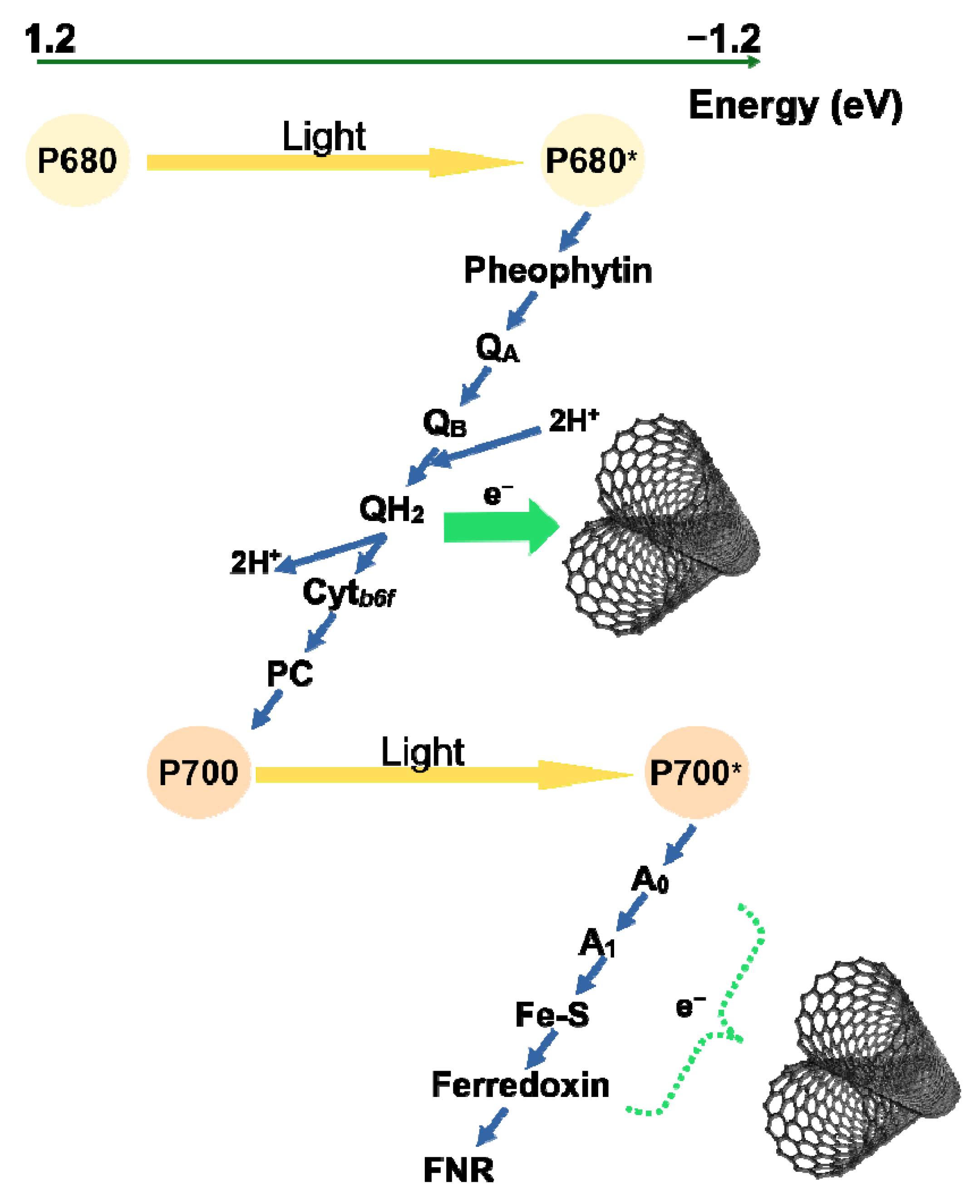
3. Discussion
4. Materials and Methods
4.1. SWCNT Preparation
4.2. Plant Material
4.3. Photosynthetic Performance
4.4. Analysis of Chl Fluorescence Induction Curves
4.5. Analysis of Modulated Reflection at 820 nm
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wong, M.H.; Giraldo, J.P.; Kwak, S.-Y.; Koman, V.B.; Sinclair, R.; Lew, T.T.S.; Bisker, G.; Liu, P.; Strano, M.S. Nitroaromatic detection and infrared communication from wild-type plants using plant nanobionics. Nat. Mater. 2017, 16, 264–272. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Lew, T.T.S.; Sweeney, C.J.; Koman, V.B.; Wong, M.H.; Bohmert-Tatarev, K.; Snell, K.D.; Seo, J.S.; Chua, N.-H.; Strano, M.S. Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nat. Nanotechnol. 2019, 14, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Odom, T.W.; Huang, J.-L.; Kim, P.; Lieber, C.M. Atomic structure and electronic properties of single-walled carbon nanotubes. Nature 1998, 391, 62–64. [Google Scholar] [CrossRef]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaniber, S.M.; Brandstetter, M.; Simmel, F.C.; Carmeli, I.; Holleitner, A.W. On-chip functionalization of carbon nanotubes with photosystem I. J. Am. Chem. Soc. 2010, 132, 2872–2873. [Google Scholar] [CrossRef]
- Nii, D.; Miyachi, M.; Shimada, Y.; Nozawa, Y.; Ito, M.; Homma, Y.; Ikehira, S.; Yamanoi, Y.; Nishihara, H.; Tomo, T. Conjugates between photosystem I and a carbon nanotube for a photoresponse device. Photosynth. Res. 2017, 133, 155–162. [Google Scholar] [CrossRef]
- Dorogi, M.; Bálint, Z.; Mikó, C.; Vileno, B.; Milas, M.; Hernádi, K.; Forró, L.; Váró, G.; Nagy, L. Stabilization effect of single-walled carbon nanotubes on the functioning of photosynthetic reaction centers. J. Phys. Chem. B 2006, 110, 21473–21479. [Google Scholar] [CrossRef]
- Yuan, H.; Hu, S.; Huang, P.; Song, H.; Wang, K.; Ruan, J.; He, R.; Cui, D. Single walled carbon nanotubes exhibit dual-phase regulation to exposed Arabidopsis mesophyll cell. Nanoscale Res. Lett. 2011, 6, 44. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.X.; Zhang, Q.F.; Li, J.; Bi, F.C.; Yao, N. Induction of programmed cell death in Arabidopsis and rice by single-wall carbon nanotubes. Am. J. Bot. 2010, 97, 1602–1609. [Google Scholar] [CrossRef]
- Zhang, H.; Yue, M.; Zheng, X.; Xie, C.; Zhou, H.; Li, L. Physiological effects of single-and multi-walled carbon nanotubes on rice seedlings. IEEE Trans. NanoBioscience 2017, 16, 563–570. [Google Scholar] [CrossRef]
- Hatami, M.; Hadian, J.; Ghorbanpour, M. Mechanisms underlying toxicity and stimulatory role of single-walled carbon nanotubes in Hyoscyamus niger during drought stress simulated by polyethylene glycol. J. Hazard. Mater. 2017, 324, 306–320. [Google Scholar] [CrossRef]
- Velikova, V.; Petrova, N.; Kovács, L.; Petrova, A.; Koleva, D.; Tsonev, T.; Taneva, S.; Petrov, P.; Krumova, S. Single-walled carbon nanotubes modify leaf micromorphology, chloroplast ultrastructure and photosynthetic activity of pea plants. Int. J. Mol. Sci. 2021, 22, 4878. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Kalaji, H.; Baczewska, A.; Pawluśkiewicz, B.; Mastalerczuk, G.; Borawska-Jarmułowicz, B.; Paunov, M.; Goltsev, V. Delayed chlorophyll a fluorescence, MR 820, and gas exchange changes in perennial ryegrass under salt stress. J. Lumin. 2017, 183, 322–333. [Google Scholar] [CrossRef]
- Goltsev, V.; Kalaji, H.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Kociel, H.; Allakhverdiev, S. Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus. Russ. J. Plant Physiol. 2016, 63, 869–893. [Google Scholar] [CrossRef]
- Kalaji, H.; Rastogi, A.; Živčák, M.; Brestic, M.; Daszkowska-Golec, A.; Sitko, K.; Alsharafa, K.; Lotfi, R.; Stypiński, P.; Samborska, I. Prompt chlorophyll fluorescence as a tool for crop phenotyping: An example of barley landraces exposed to various abiotic stress factors. Photosynthetica 2018, 56, 953–961. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef] [Green Version]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Li, P.; Ma, F.; Goltsev, V. Photosynthetic performance during leaf expansion in Malus micromalus probed by chlorophyll a fluorescence and modulated 820 nm reflection. J. Photochem. Photobiol. B Biol. 2014, 137, 144–150. [Google Scholar] [CrossRef]
- Petrov, P.D.; Georgiev, G.L. Fabrication of super-macroporous nanocomposites by deposition of carbon nanotubes onto polymer cryogels. Eur. Polym. J. 2012, 48, 1366–1373. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll Fluorescence; Papageorgiou, G.C., Ed.; Springer: Dorbrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Tóth, S.Z.; Schansker, G.; Strasser, R.J. A non-invasive assay of the plastoquinone pool redox state based on the OJIP-transient. Photosynth. Res. 2007, 93, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Schansker, G.; Tóth, S.Z.; Strasser, R.J. Dark recovery of the Chl a fluorescence transient (OJIP) after light adaptation: The qT-component of non-photochemical quenching is related to an activated photosystem I acceptor side. Biochim. Biophys. Acta Bioenerg. 2010, 1757, 787–797. [Google Scholar] [CrossRef] [Green Version]
- Tóth, S.Z.; Schansker, G.; Garab, G.; Strasser, R.J. Photosynthetic electron transport activity in heat-treated barley leaves: The role of internal alternative electron donors to photosystem II. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Tsimilli-Michael, M. Revisiting JIP-test: An educative review on concepts, assumptions, approximations, definitions and terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Lu, Y.; Goltsev, V.; Strasser, R.J.; Kalaji, H.M.; Wang, H.; Wang, X.; Chen, S.; Qiang, S. Comparative effect of tenuazonic acid, diuron, bentazone, dibromothymoquinone and methyl viologen on the kinetics of Chl a fluorescence rise OJIP and the MR820 signal. Plant Physiol. Biochem. 2020, 156, 39–48. [Google Scholar] [CrossRef]
- Schansker, G.; Srivastava, A.; Strasser, R.J. Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct. Plant Biol. 2003, 30, 785–796. [Google Scholar] [CrossRef] [Green Version]
- Schansker, G.; Tóth, S.Z.; Strasser, R.J. Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim. Biophys. Acta Bioenerg. 2005, 1706, 250–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shikanai, T. Central role of cyclic electron transport around photosystem I in the regulation of photosynthesis. Curr. Opin. Biotechnol. 2014, 26, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Essemine, J.; Xiao, Y.; Qu, M.; Mi, H.; Zhu, X.-G. Cyclic electron flow may provide some protection against PSII photoinhibition in rice (Oryza sativa L.) leaves under heat stress. J. Plant Physiol. 2017, 211, 138–146. [Google Scholar] [CrossRef]
- Zhou, R.; Kan, X.; Chen, J.; Hua, H.; Li, Y.; Ren, J.; Feng, K.; Liu, H.; Deng, D.; Yin, Z. Drought-induced changes in photosynthetic electron transport in maize probed by prompt fluorescence, delayed fluorescence, P700 and cyclic electron flow signals. Environ. Exp. Bot. 2019, 158, 51–62. [Google Scholar] [CrossRef]
- Petrov, P.; Georgiev, G.; Momekova, D.; Momekov, G.; Tsvetanov, C.B. UV-assisted grafting of polymers: A method towards biocompatible carbon nanotubes. Polymer 2010, 51, 2465–2471. [Google Scholar] [CrossRef]
- Petrova, N.; Todinova, S.; Paunov, M.; Kovács, L.; Taneva, S.; Krumova, S. Thylakoid membrane unstacking increases LHCII thermal stability and lipid phase fluidity. J. Bioenerg. Biomembr. 2018, 50, 425–435. [Google Scholar] [CrossRef] [PubMed]
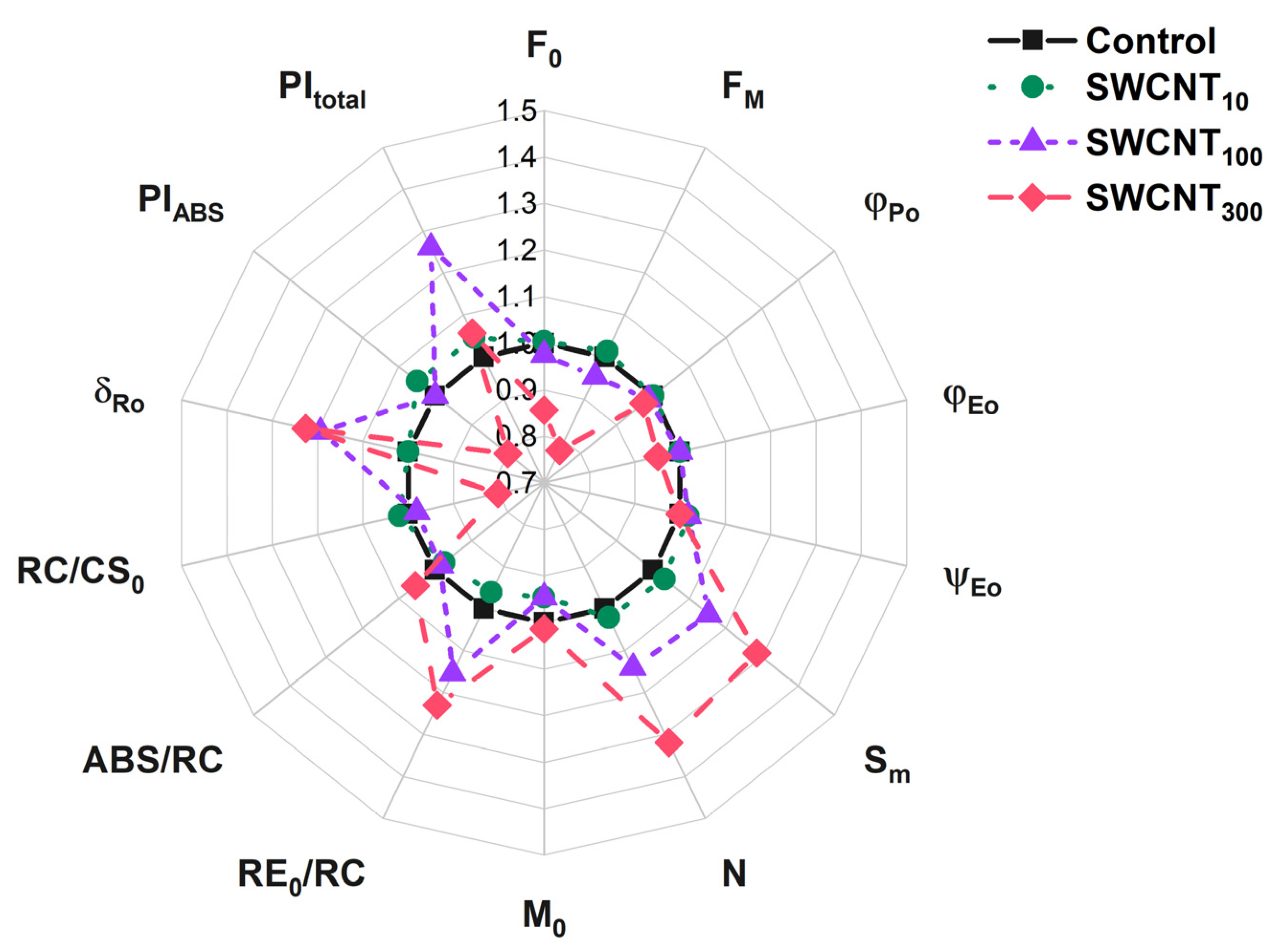

| Control | SWCNT10 | SWCNT100 | SWCNT300 | |
|---|---|---|---|---|
| F0 | 9231 ± 109 | 9267 ± 794 | 9000 ± 542 | 7900 ± 507 * |
| FM | 40108 ± 599 | 40671 ± 360 | 38262 ± 269 | 31169 ± 2711 * |
| φPo | 0.77 ± 0.00 | 0.77 ± 0.02 | 0.76 ± 0.01 | 0.75 ± 0.01 * |
| φEo | 0.41 ± 0.03 | 0.41 ± 0.01 | 0.41 ± 0.01 | 0.39 ± 0.01 |
| ψEo | 0.53 ± 0.04 | 0.54 ± 0.01 | 0.54 ± 0.02 | 0.53 ± 0.02 |
| Sm | 18.9 ± 1.3 | 19.5 ± 1.7 | 21.8 ± 1.6 | 24.3 ± 1.7 * |
| N | 29.1 ± 0.1 | 29.7 ± 3.5 | 33.2 ± 3.3 | 38.4 ± 1.4 * |
| M0 | 0.74 ± 0.11 | 0.7 ± 0.03 | 0.7 ± 0.05 | 0.75 ± 0.05 |
| RE0/RC | 0.26 ± 0.01 | 0.25 ± 0.02 | 0.3 ± 0.03 * | 0.32 ± 0.01 * |
| ABS/RC | 2.02 ± 0.13 | 1.97 ± 0.12 | 1.99 ± 0.12 | 2.13 ± 0.07 |
| RC/CS0 | 4608 ± 235 | 4698 ± 191 | 4520 ± 166 | 3695 ± 124 * |
| δRo | 0.31 ± 0.02 | 0.31 ± 0.01 | 0.37 ± 0.02 * | 0.38 ± 0.01 * |
| PIABS | 2.0 ± 0.4 | 2.1 ± 0.3 | 2.0 ± 0.3 | 1.6 ± 0.1 |
| PItotal | 0.88 ± 0.12 | 0.92 ± 0.09 | 1.11 ± 0.06 | 0.93 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrova, N.; Paunov, M.; Petrov, P.; Velikova, V.; Goltsev, V.; Krumova, S. Polymer-Modified Single-Walled Carbon Nanotubes Affect Photosystem II Photochemistry, Intersystem Electron Transport Carriers and Photosystem I End Acceptors in Pea Plants. Molecules 2021, 26, 5958. https://doi.org/10.3390/molecules26195958
Petrova N, Paunov M, Petrov P, Velikova V, Goltsev V, Krumova S. Polymer-Modified Single-Walled Carbon Nanotubes Affect Photosystem II Photochemistry, Intersystem Electron Transport Carriers and Photosystem I End Acceptors in Pea Plants. Molecules. 2021; 26(19):5958. https://doi.org/10.3390/molecules26195958
Chicago/Turabian StylePetrova, Nia, Momchil Paunov, Petar Petrov, Violeta Velikova, Vasilij Goltsev, and Sashka Krumova. 2021. "Polymer-Modified Single-Walled Carbon Nanotubes Affect Photosystem II Photochemistry, Intersystem Electron Transport Carriers and Photosystem I End Acceptors in Pea Plants" Molecules 26, no. 19: 5958. https://doi.org/10.3390/molecules26195958
APA StylePetrova, N., Paunov, M., Petrov, P., Velikova, V., Goltsev, V., & Krumova, S. (2021). Polymer-Modified Single-Walled Carbon Nanotubes Affect Photosystem II Photochemistry, Intersystem Electron Transport Carriers and Photosystem I End Acceptors in Pea Plants. Molecules, 26(19), 5958. https://doi.org/10.3390/molecules26195958