Glyceric Prodrug of Ursodeoxycholic Acid (UDCA): Novozym 435-Catalyzed Synthesis of UDCA-Monoglyceride
Abstract
1. Introduction
2. Results and Discussion
2.1. Preliminary Experiments: Choice of the Suitable Organic Co-Solvent
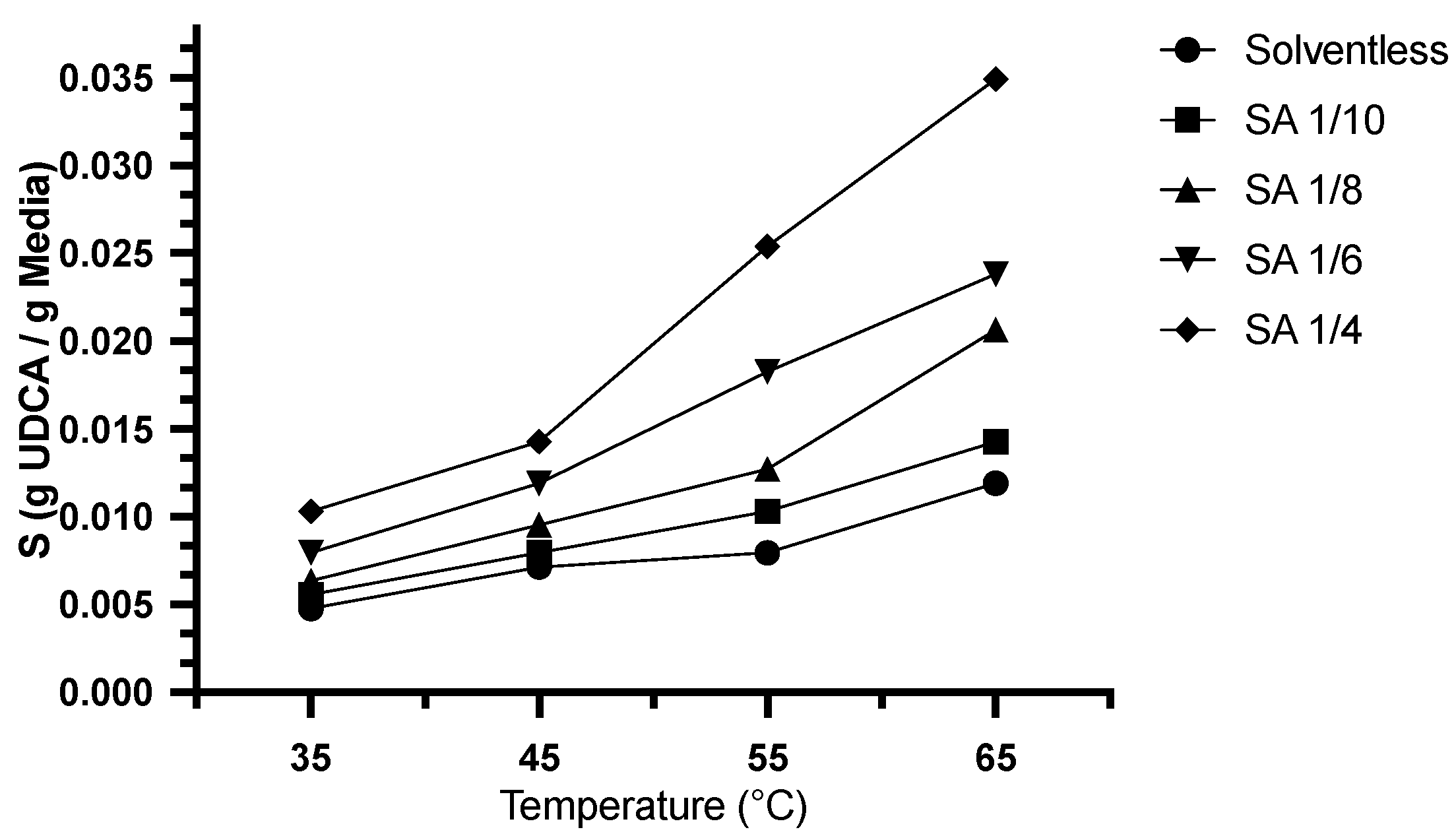
2.2. Solubility of UDCA: Solventless and Solvent-Assisted Media
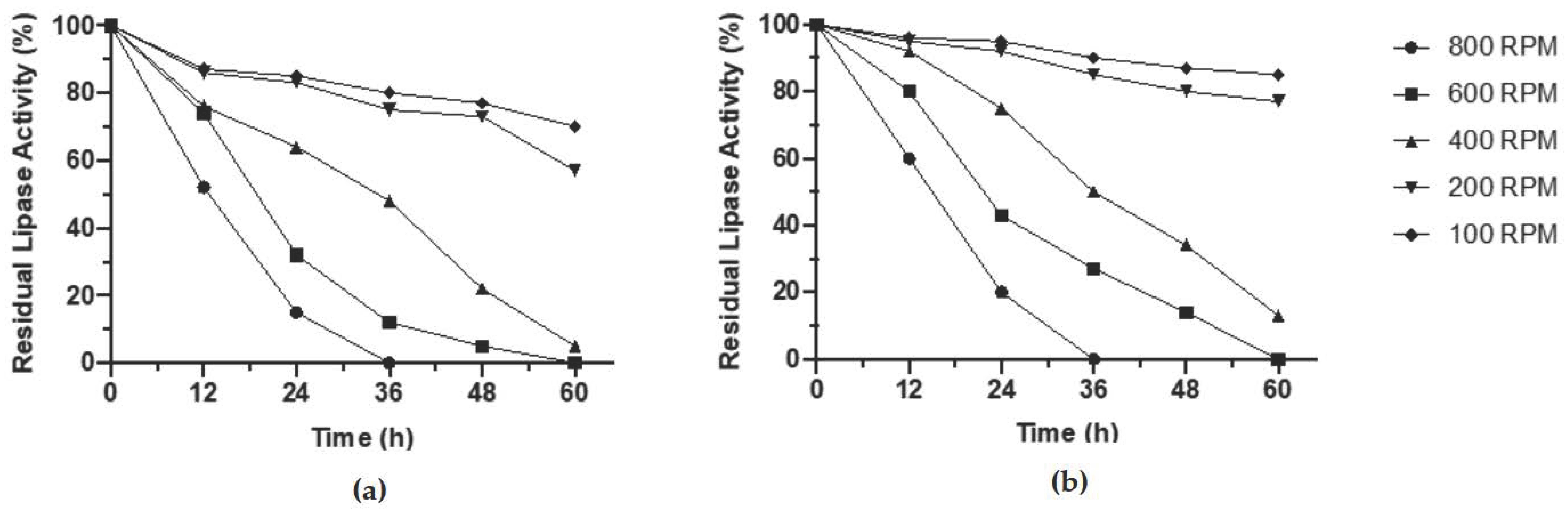
2.3. Effect of Co-Solvent Concentration on Enzyme Stability
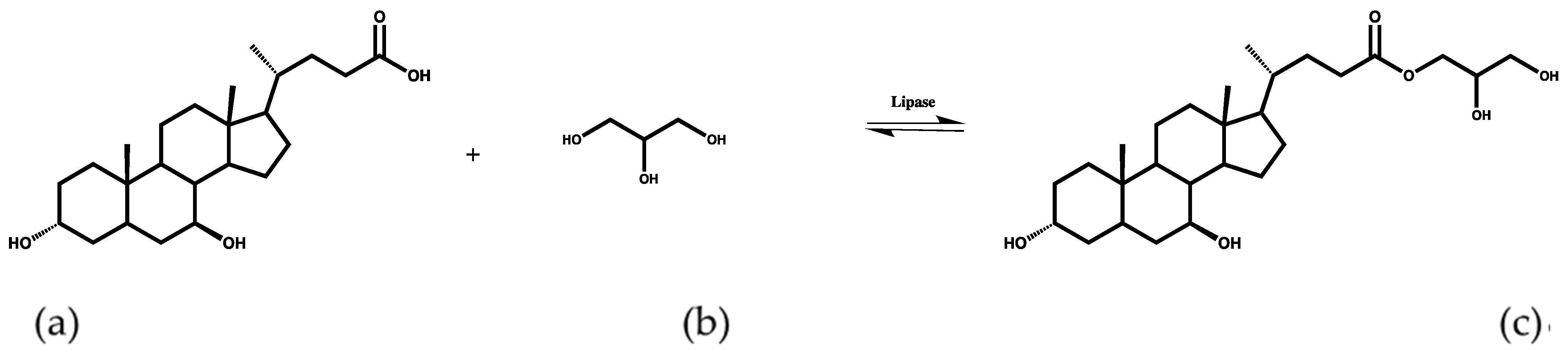
2.4. Effect of Esterification Parameters
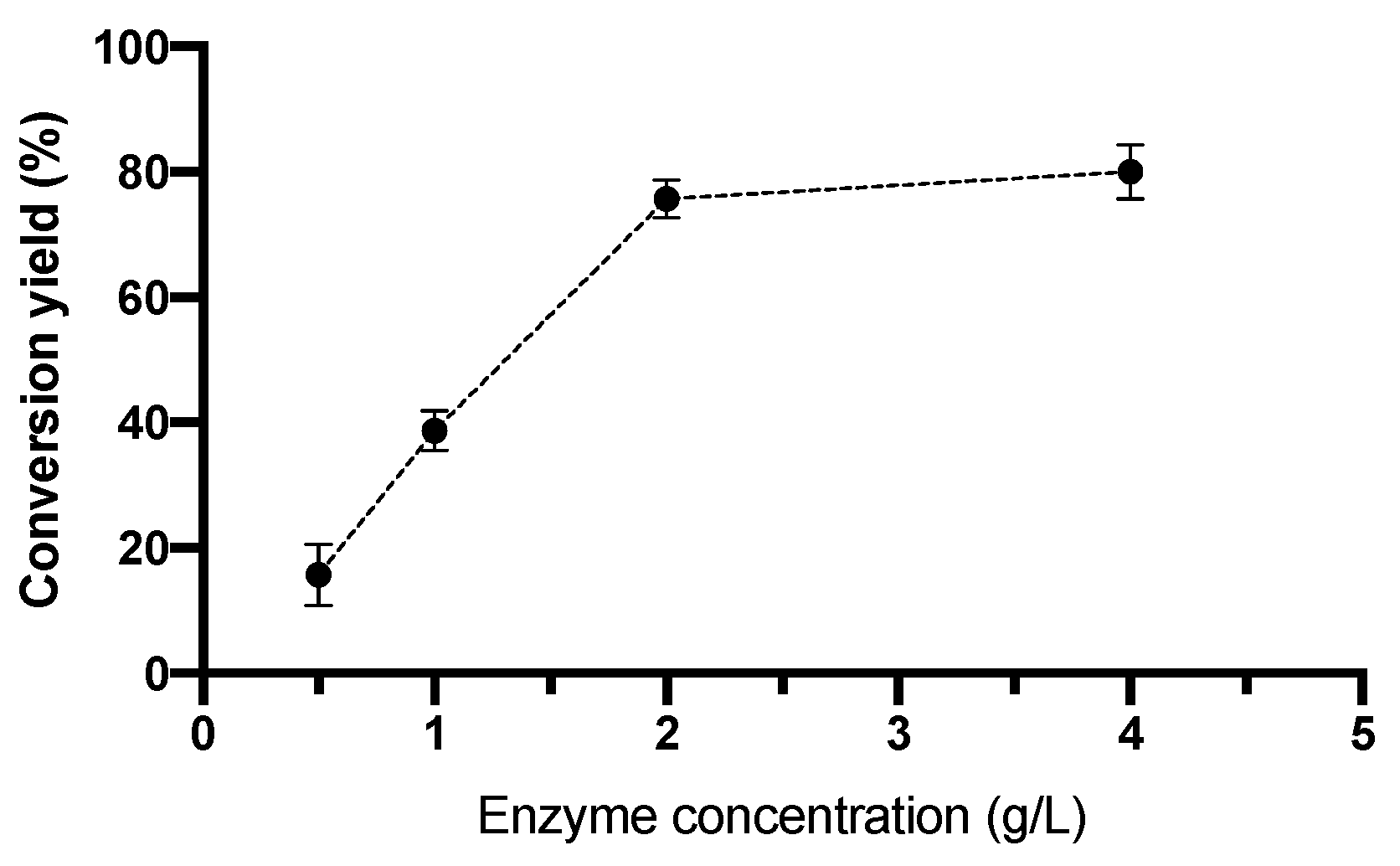
2.4.1. Effect of Enzyme Concentration on Conversion Yield
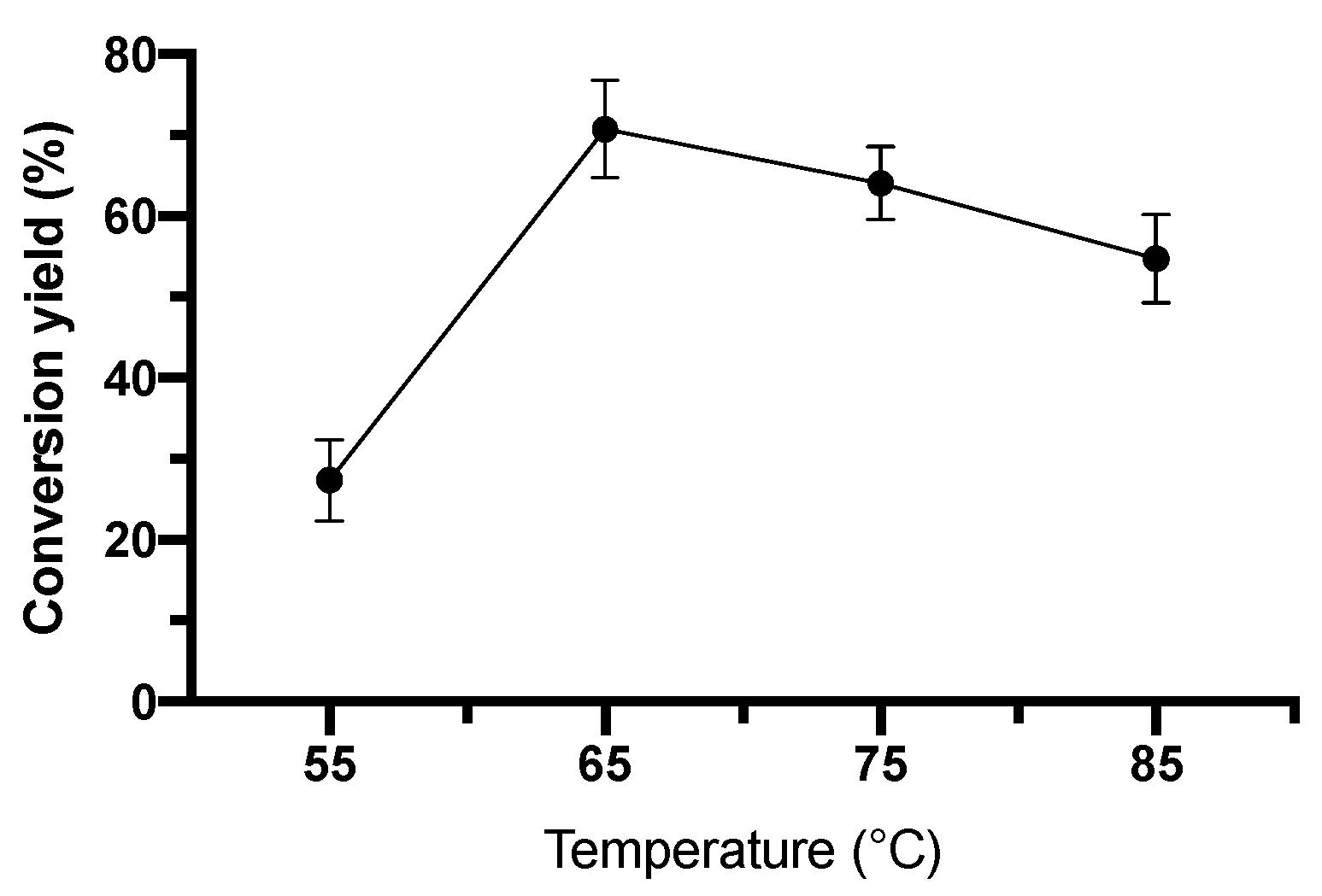
2.4.2. Effect of Temperature on Conversion Yield
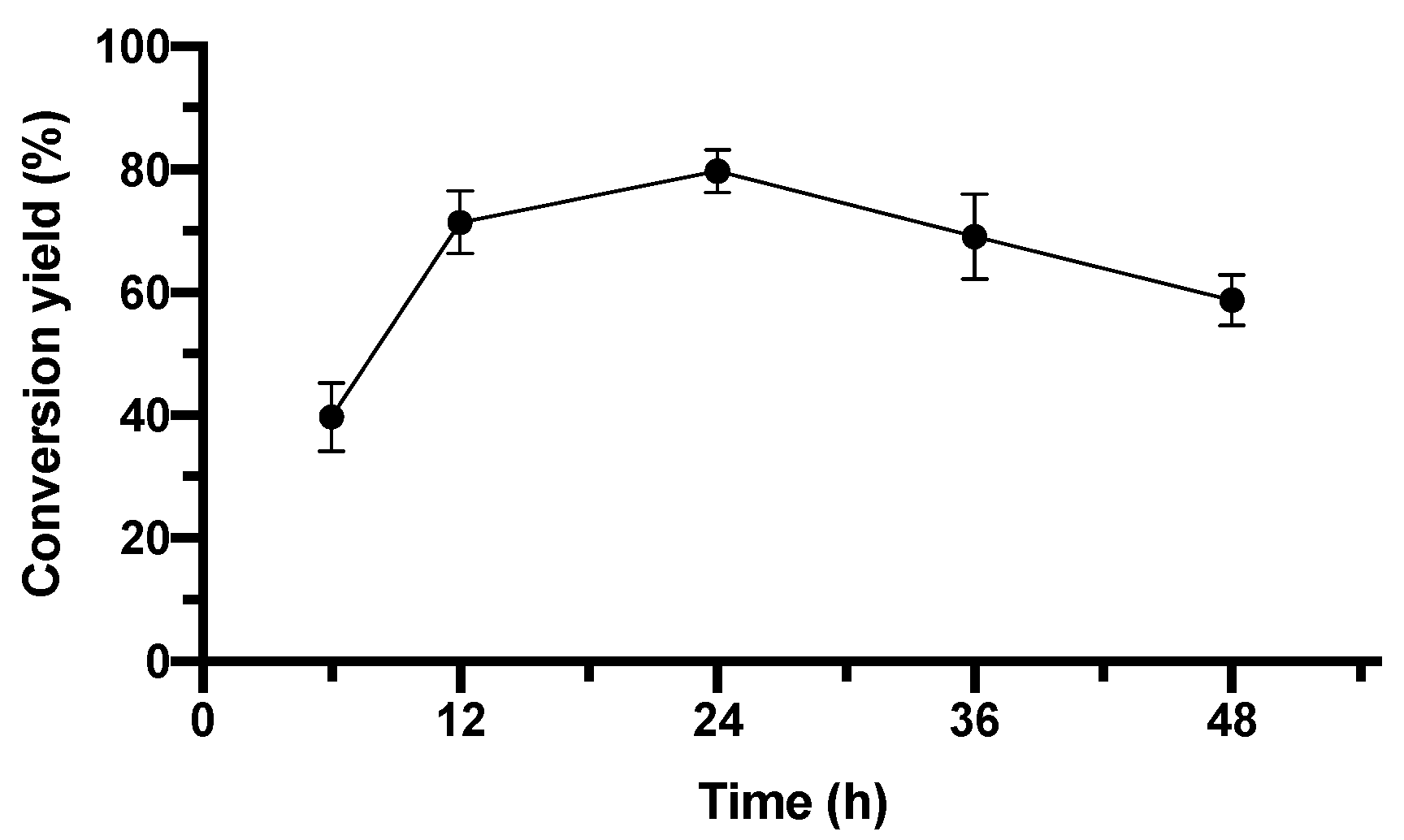
2.4.3. Effect of Reaction Time on Conversion Yield
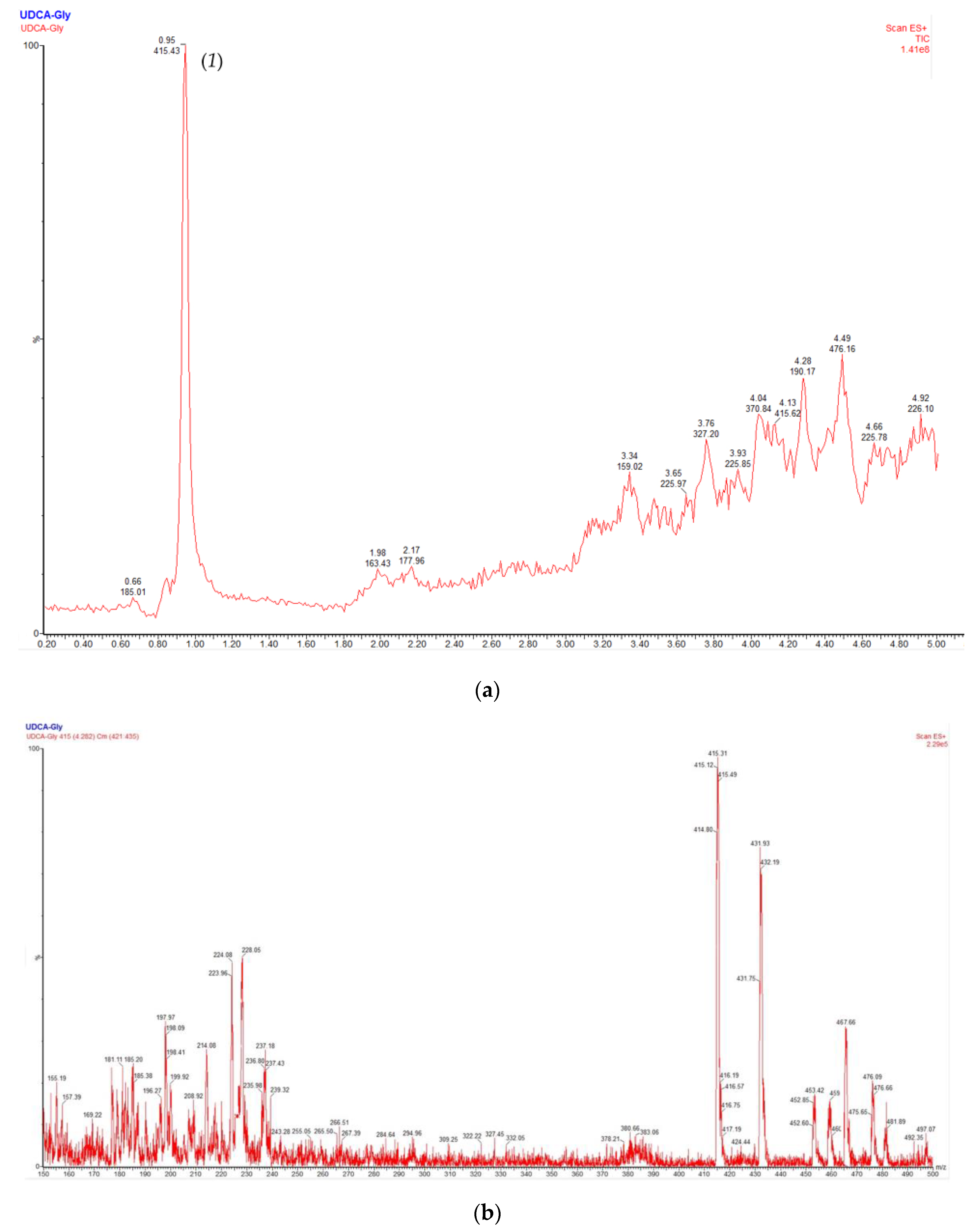
2.5. NMR and uHPLC–MS Characterization of UDCA-Monoglyceride
3. Materials and Methods
3.1. Enzyme, Chemicals, and Materials
3.2. Lipase Activity
3.3. Effect of the Esterification Parameters
3.4. Thin-Layer Chromatography (TLC)
3.5. Purification and Spectroscopic Characterization of Glycerol Ester of UDCA
3.6. Analytical HPLC Methods
3.7. Analytical uHPLC–MS Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alvaro, D.; Cantafora, A.; Attili, A.F.; Ginanni Corradini, S.; De Luca, C.; Minervini, G.; Di Blase, A.; Angelico, M. Relationships between Bile Salts Hydrophilicity and Phospholipid Composition in Bile of Various Animal Species. Comp. Biochem. Physiol. Part B Biochem. 1986, 83, 551–554. [Google Scholar] [CrossRef]
- Woollett, L.A.; Buckley, D.D.; Yao, L.; Jones, P.J.H.; Granholm, N.A.; Tolley, E.A.; Heubi, J.E. Effect of Ursodeoxycholic Acid on Cholesterol Absorption and Metabolism in Humans. J. Lipid Res. 2003, 44, 935–942. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Simental-Mendía, M.; Sánchez-García, A.; Banach, M.; Serban, M.C.; Cicero, A.F.G.; Sahebkar, A. Impact of Ursodeoxycholic Acid on Circulating Lipid Concentrations: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. Lipids Health Dis. 2019, 18, 88. [Google Scholar] [CrossRef]
- Crosignani, A.; Battezzati, P.M.; Setchell, K.D.R.; Camisasca, M.; Bertolini, E.; Roda, A.; Zuin, M.; Podda, M. Effects of Ursodeoxycholic Acid on Serum Liver Enzymes and Bile Acid Metabolism in Chronic Active Hepatitis: A Dose-response Study. Hepatology 1991, 13, 339–344. [Google Scholar] [CrossRef]
- Colombo, C.; Crosignani, A.; Assaisso, M.; Battezzati, P.M.; Podda, M.; Giunta, A.; Zimmer-Nechemias, L.; Setchell, K.D.R. Ursodeoxycholic Acid Therapy in Cystic Fibrosis—Associated Liver Disease: A Dose-response Study. Hepatology 1992, 16, 924–930. [Google Scholar] [CrossRef]
- Palma, J.; Reyes, H.; Ribalta, J.; Hernández, I.; Sandoval, L.; Almuna, R.; Liepins, J.; Lira, F.; Sedano, M.; Silva, O.; et al. Ursodeoxycholic Acid in the Treatment of Cholestasis of Pregnancy: A Randomized, Double-Blind Study Controlled with Placebo. J. Hepatol. 1997, 27, 1022–1028. [Google Scholar] [CrossRef]
- Yue, P.F.; Zhang, W.J.; Yuan, H.L.; Yang, M.; Zhu, W.F.; Cai, P.L.; Xiao, X.H. Process Optimization, Characterization and Pharmacokinetic Evaluation in Rats of Ursodeoxycholic Acid-Phospholipid Complex. AAPS PharmSciTech 2008, 9, 322–329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Friman, S.; Svanvik, J. A Possible Role of Ursodeoxycholic Acid in Liver Transplantation. Scand. J. Gastroenterol. 1994, 29, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Beuers, U. Drug Insight: Mechanisms and Sites of Action of Ursodeoxycholic Acid in Cholestasis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F.; Hagey, L.R. Bile Acids: Chemistry, Pathochemistry, Biology, Pathobiology, and Therapeutics. Cell. Mol. Life Sci. 2008, 65, 2461–2483. [Google Scholar] [CrossRef] [PubMed]
- Navacchia, M.L.; Marchesi, E.; Perrone, D. Bile Acid Conjugates with Anticancer Activity: Most Recent Research. Molecules 2020, 26, 25. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.A.; Tirendi, S.; Puglisi, G.; Bousquet, E.; Panza, L. Improvement of Water Solubility and Dissolution Rate of Ursodeoxycholic Acid and Chenodeoxycholic Acid by Complexation with Natural and Modified β-Cyclodextrins. Int. J. Pharm. 1997, 149, 1–13. [Google Scholar] [CrossRef]
- Maharjan, P.; Kim, D.; Jin, M.; Ko, H.J.; Song, Y.H.; Lee, Y.; Ahn, B.N.; Kim, S.K.; Lee, Y.; Shin, M.C.; et al. Preclinical Evaluation of UDCA-Containing Oral Formulation in Mice for the Treatment of Wet Age-Related Macular Degeneration. Pharmaceutics 2019, 11, 561. [Google Scholar] [CrossRef]
- Hu, J.; Johnston, K.P.; Williams, R.O. Nanoparticle Engineering Processes for Enhancing the Dissolution Rates of Poorly Water Soluble Drugs. Drug Dev. Ind. Pharm. 2004, 30, 233–245. [Google Scholar] [CrossRef]
- Kondo, M.; Niwa, T.; Okamoto, H.; Danjo, K. Particle Characterization of Poorly Water-Soluble Drugs Using a Spray Freeze Drying Technique. Chem. Pharm. Bull. 2009, 57, 657–662. [Google Scholar] [CrossRef][Green Version]
- Shakeel, F.; Faisal, M.S. Nanoemulsion: A Promising Tool for Solubility and Dissolution Enhancement of Celecoxib. Pharm. Dev. Technol. 2010, 15, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Carli, S.; Fioravanti, G.; Armirotti, A.; Ciarpella, F.; Prato, M.; Ottonello, G.; Salerno, M.; Scarpellini, A.; Perrone, D.; Marchesi, E.; et al. A New Drug Delivery System Based on Tauroursodeoxycholic Acid and PEDOT. Chem.—A Eur. J. 2019, 25, 2322–2329. [Google Scholar] [CrossRef]
- Chung, H.Y.; Yonemochi, E.; Saitoh, T.; Terada, K.; Tozuka, Y.; Oguchi, T.; Yamamoto, K.; Chung, H.Y.; Choi, W.S. Factors Affecting the Apparent Solubility of Ursodeoxycholic Acid in the Grinding Process. Int. J. Pharm. 2003, 255, 49–56. [Google Scholar] [CrossRef]
- Dosa, P.I.; Ward, T.; Castro, R.E.; Rodrigues, C.M.P.; Steer, C.J. Synthesis and Evaluation of Water-Soluble Prodrugs of Ursodeoxycholic Acid (UDCA), an Anti-Apoptotic Bile Acid. ChemMedChem 2013, 8, 1002–1011. [Google Scholar] [CrossRef]
- Sinokrot, H.; Smerat, T.; Najjar, A.; Karaman, R. Advanced Prodrug Strategies in Nucleoside and Non-Nucleoside Antiviral Agents: A Review of the Recent Five Years. Molecules 2017, 22, 1736. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.E.; Deng, Y.N.; Hsu, J.L.; Leu, W.J.; Marchesi, E.; Capobianco, M.L.; Marchetti, P.; Navacchia, M.L.; Guh, J.H.; Perrone, D.; et al. Evaluation of the Anticancer Activity of a Bile Acid-Dihydroartemisinin Hybrid Ursodeoxycholic-Dihydroartemisinin in Hepatocellular Carcinoma Cells. Front. Pharmacol. 2020, 11, 1776. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Park, H.; Park, S.; Kim, D.; Baek, I.; Jeong, L.; Kim, B.J.; Park, K.; Lee, D.; Lee, S.H. Bile Acid-Based Dual-Functional Prodrug Nanoparticles for Bone Regeneration through Hydrogen Peroxide Scavenging and Osteogenic Differentiation of Mesenchymal Stem Cells. J. Control. Release 2020, 328, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Di Guida, F.; Pirozzi, C.; Magliocca, S.; Santoro, A.; Lama, A.; Russo, R.; Nieddu, M.; Burrai, L.; Boatto, G.; Mollica, M.P.; et al. A Galactosylated Pro-Drug of Ursodeoxycholic Acid: Design, Synthesis, Characterization, and Pharmacological Effects in a Rat Model of Estrogen-Induced Cholestasis. Mol. Pharm. 2018, 15, 21–30. [Google Scholar] [CrossRef]
- de Oliveira Junior, E.R.; Truzzi, E.; Ferraro, L.; Fogagnolo, M.; Pavan, B.; Beggiato, S.; Rustichelli, C.; Maretti, E.; Lima, E.M.; Leo, E.; et al. Nasal Administration of Nanoencapsulated Geraniol/Ursodeoxycholic Acid Conjugate: Towards a New Approach for the Management of Parkinson’s Disease. J. Control. Release 2020, 321, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, A.; Møller, A.H.; Danielsen, M.; Madsen, B.; Joernsgaard, B.; Vaerbak, S.; Adlercreutz, P.; Dalsgaard, T.K. Hydrophilization of Bixin by Lipase-Catalyzed Transesterification with Sorbitol. Food Chem. 2018, 268, 203–209. [Google Scholar] [CrossRef]
- Ravelo, M.; Fuente, E.; Blanco, Á.; Ladero, M.; García-Ochoa, F. Esterification of Glycerol and Ibuprofen in Solventless Media Catalyzed by Free CALB: Kinetic Modelling. Biochem. Eng. J. 2015, 101, 228–236. [Google Scholar] [CrossRef]
- Tamayo, J.J.; Ladero, M.; Santos, V.E.; García-Ochoa, F. Esterification of Benzoic Acid and Glycerol to α-Monobenzoate Glycerol in Solventless Media Using an Industrial Free Candida antarctica Lipase B. Process Biochem. 2012, 47, 243–250. [Google Scholar] [CrossRef]
- Zappaterra, F.; Elena, M.; Rodriguez, M.; Summa, D.; Semeraro, B.; Costa, S.; Tamburini, E. Biocatalytic Approach for Direct Esterification of Ibuprofen with Sorbitol in Biphasic Media. Int. J. Mol. Sci. 2021, 22, 3066. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Della Pina, C. From Glycerol to Value-Added Products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef]
- Bradbury, S.L.; Jakoby, W.B. Glycerol as an Enzyme-Stabilizing Agent: Effects on Aldehyde Dehydrogenase. Proc. Natl. Acad. Sci. USA 1972, 69, 2373–2376. [Google Scholar] [CrossRef]
- Ohtake, S.; Kita, Y.; Arakawa, T. Interactions of Formulation Excipients with Proteins in Solution and in the Dried State. Adv. Drug Deliv. Rev. 2011, 63, 1053–1073. [Google Scholar] [CrossRef]
- Vagenende, V.; Yap, M.G.S.; Trout, B.L. Mechanisms of Protein Stabilization and Prevention of Protein Aggregation by Glycerol. Biochemistry 2009, 48, 11084–11096. [Google Scholar] [CrossRef]
- Kumar, V.; Chari, R.; Sharma, V.K.; Kalonia, D.S. Modulation of the Thermodynamic Stability of Proteins by Polyols: Significance of Polyol Hydrophobicity and Impact on the Chemical Potential of Water. Int. J. Pharm. 2011, 413, 19–28. [Google Scholar] [CrossRef]
- Wolfson, A.; Dlugy, C.; Shotland, Y. Glycerol as a Green Solvent for High Product Yields and Selectivities. Environ. Chem. Lett. 2007, 5, 67–71. [Google Scholar] [CrossRef]
- Wolfson, A.; Atyya, A.; Dlugy, C.; Tavor, D. Glycerol Triacetate as Solvent and Acyl Donor in the Production of Isoamyl Acetate with Candida antarctica Lipase B. Bioprocess Biosyst. Eng. 2010, 33, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Ravelo, M.; Esteban, J.; Ladero, M.; García-Ochoa, F. Enzymatic Synthesis of Ibuprofen Monoglycerides Catalyzed by Free: Candida antarctica Lipase B in a Toluene-Glycerol Biphasic Medium. RSC Adv. 2016, 6, 69658–69669. [Google Scholar] [CrossRef]
- Zappaterra, F.; Summa, D.; Semeraro, B.; Buzzi, R.; Trapella, C.; Ladero, M.; Costa, S.; Tamburini, E. Enzymatic Esterification as Potential Strategy to Enhance the Sorbic Acid Behavior as Food and Beverage Preservative. Fermentation 2020, 6, 96. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; Dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “Perfect” Lipase Immobilized Biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Foresti, M.L.; Galle, M.; Ferreira, M.L.; Briand, L.E. Enantioselective Esterification of Ibuprofen with Ethanol as Reactant and Solvent Catalyzed by Immobilized Lipase: Experimental Andmolecular Modeling Aspects. J. Chem. Technol. Biotechnol. 2009, 84, 1461–1473. [Google Scholar] [CrossRef]
- Trodler, P.; Pleiss, J. Modeling Structure and Flexibility of Candida antarctica Lipase B in Organic Solvents. BMC Struct. Biol. 2008, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.L.; Kamaruddin, A.H.; Bhatia, S.; Long, W.S.; Lim, S.T.; Kumari, R. Performance of Free Candida antarctica Lipase B in the Enantioselective Esterification of (R)-Ketoprofen. Enzyme Microb. Technol. 2006, 39, 924–929. [Google Scholar] [CrossRef]
- Nordblad, M.; Adlercreutz, P. Immobilisation Procedure and Reaction Conditions for Optimal Performance of Candida antarctica Lipase B in Transesterification and Hydrolysis. Biocatal. Biotransformation 2013, 31, 237–245. [Google Scholar] [CrossRef]
- Manoel, E.A.; dos Santos, J.C.S.; Freire, D.M.G.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of Lipases on Hydrophobic Supports Involves the Open Form of the Enzyme. Enzyme Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef]
- Edy Susanto, M. Water-Insoluble Drug Formulation; CSC Press: Boca Raton, FL, USA, 2019; Volume 53. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Yang, Y.X.; Cai, J.L.; Lv, W.H.; Xie, W.C.; Wang, Y.Z.; Gross, R.A. Poly(Oleic Diacid-Co-Glycerol): Comparison of Polymer Structure Resulting from Chemical and Lipase Catalysis. ACS Symp. Ser. 2012, 1105, 111–129. [Google Scholar] [CrossRef]
- Riva, S.; Chopineau, J.; Kieboom, A.P.G.; Klibanov, A.M. Protease-Catalyzed Regioselective Esterification of Sugars and Related Compounds in Anhydrous Dimethylformamide. J. Am. Chem. Soc. 1988, 110, 584–589. [Google Scholar] [CrossRef]
- Li, G.; Yao, D.; Zong, M. Lipase-Catalyzed Synthesis of Biodegradable Copolymer Containing Malic Acid Units in Solvent-Free System. Eur. Polym. J. 2008, 44, 1123–1129. [Google Scholar] [CrossRef]
| Solvent | Boiling Point (°C) | LogP | Solubility in Water | Miscibility with Glycerol |
|---|---|---|---|---|
| Toluene | 110.6 | 2.43 | 0.52 mg mL−1 | Immiscible |
| 2-Methylbutan-2-ol | 102.4 | 0.89 | 120 mg mL−1 | Miscible |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zappaterra, F.; Costa, S.; Summa, D.; Semeraro, B.; Cristofori, V.; Trapella, C.; Tamburini, E. Glyceric Prodrug of Ursodeoxycholic Acid (UDCA): Novozym 435-Catalyzed Synthesis of UDCA-Monoglyceride. Molecules 2021, 26, 5966. https://doi.org/10.3390/molecules26195966
Zappaterra F, Costa S, Summa D, Semeraro B, Cristofori V, Trapella C, Tamburini E. Glyceric Prodrug of Ursodeoxycholic Acid (UDCA): Novozym 435-Catalyzed Synthesis of UDCA-Monoglyceride. Molecules. 2021; 26(19):5966. https://doi.org/10.3390/molecules26195966
Chicago/Turabian StyleZappaterra, Federico, Stefania Costa, Daniela Summa, Bruno Semeraro, Virginia Cristofori, Claudio Trapella, and Elena Tamburini. 2021. "Glyceric Prodrug of Ursodeoxycholic Acid (UDCA): Novozym 435-Catalyzed Synthesis of UDCA-Monoglyceride" Molecules 26, no. 19: 5966. https://doi.org/10.3390/molecules26195966
APA StyleZappaterra, F., Costa, S., Summa, D., Semeraro, B., Cristofori, V., Trapella, C., & Tamburini, E. (2021). Glyceric Prodrug of Ursodeoxycholic Acid (UDCA): Novozym 435-Catalyzed Synthesis of UDCA-Monoglyceride. Molecules, 26(19), 5966. https://doi.org/10.3390/molecules26195966









