Design, Synthesis, and Antibacterial Evaluation of Novel Ocotillol Derivatives and Their Synergistic Effects with Conventional Antibiotics
Abstract
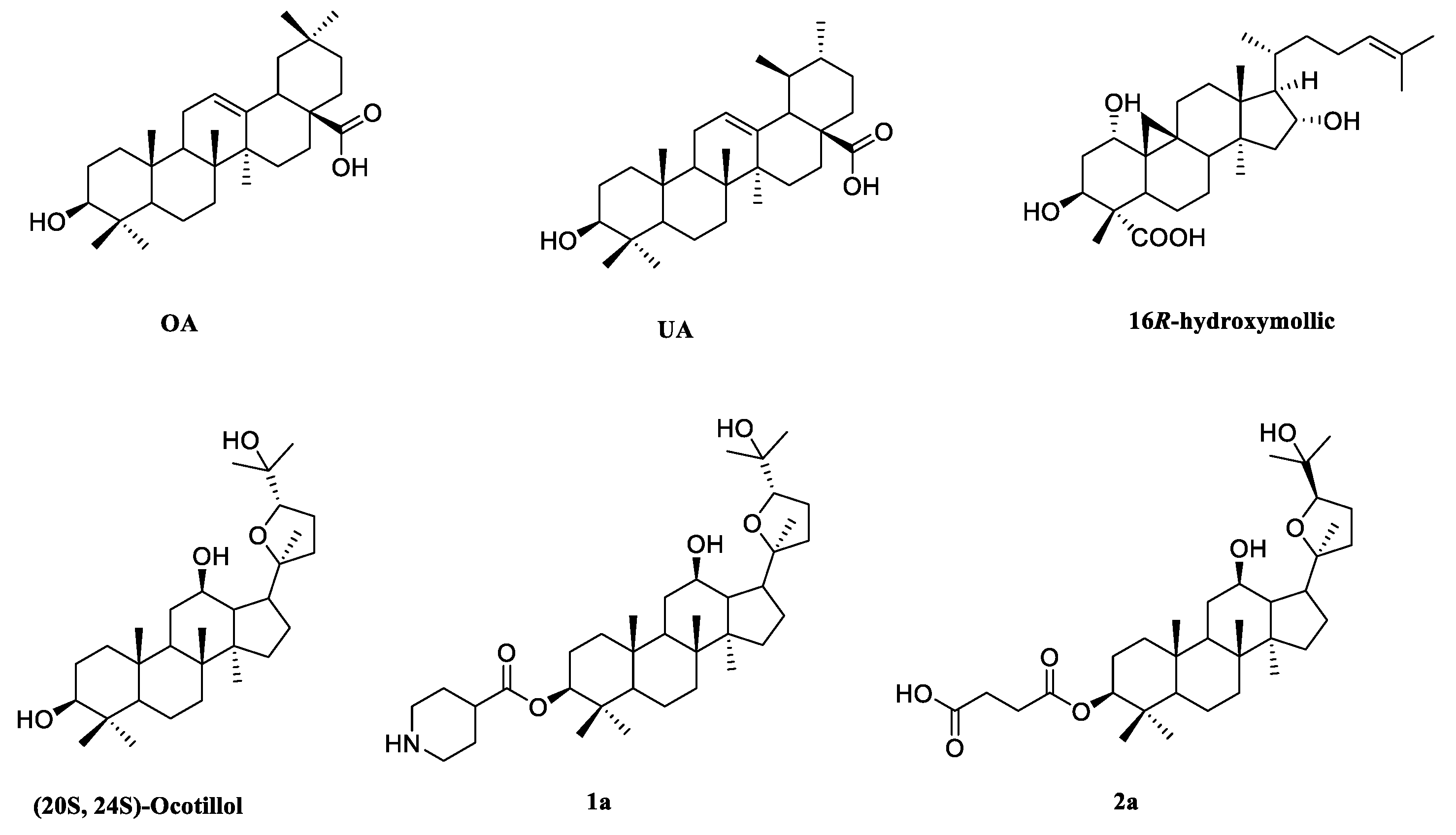
:1. Introduction
2. Results and Discussion
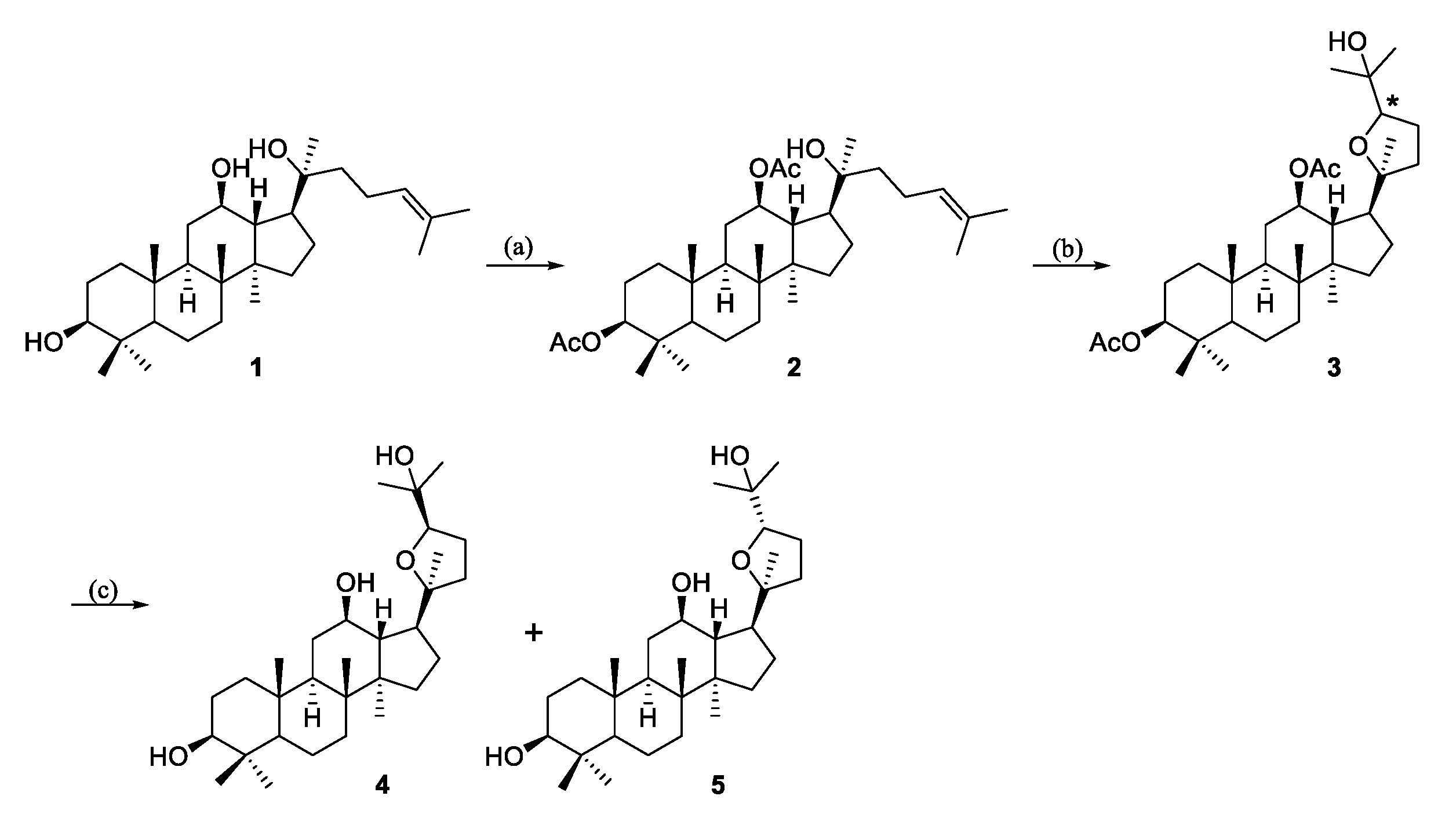
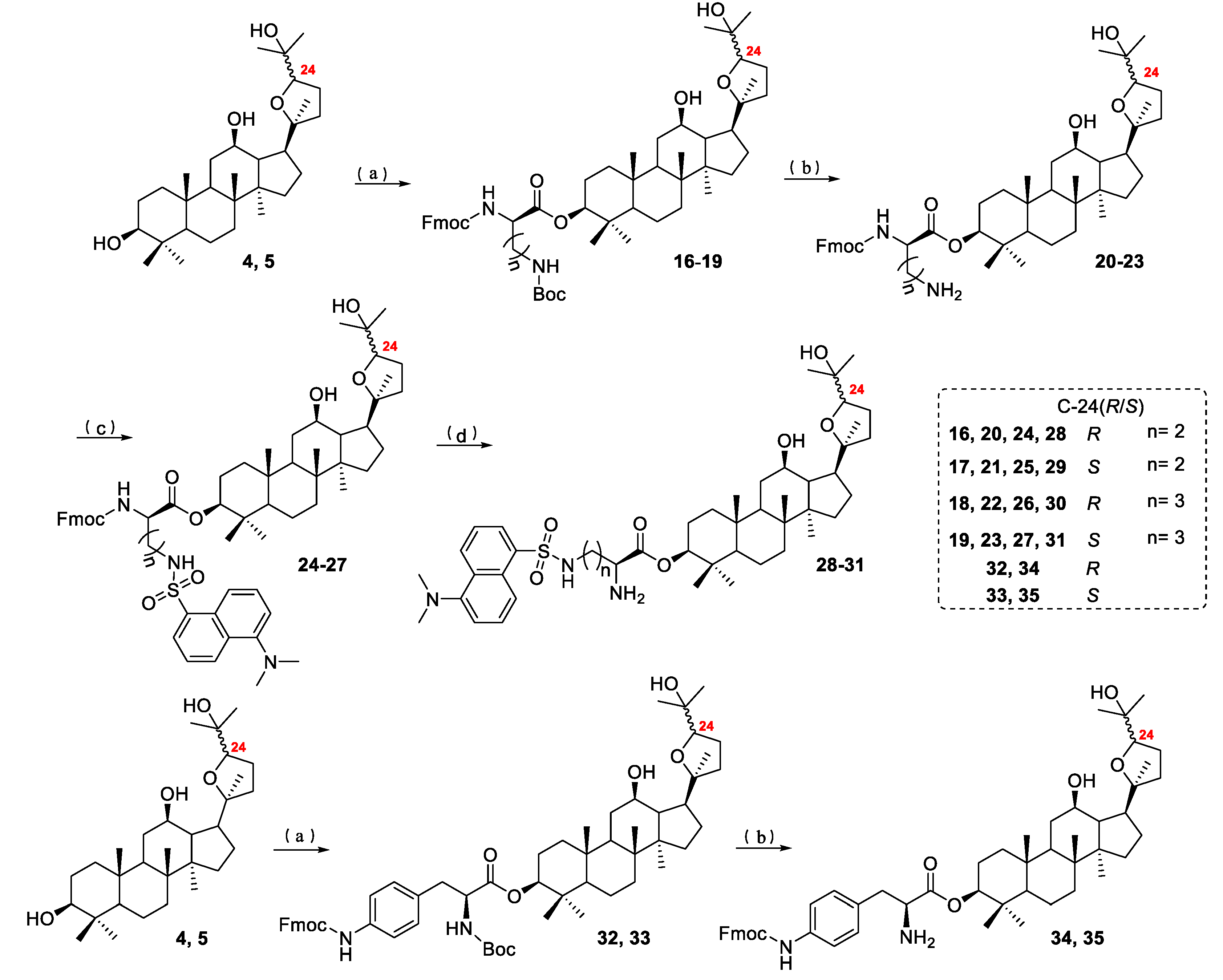
2.1. Chemistry
2.2. Antibacterial Activity
2.3. Synergistic Antibacterial Activity
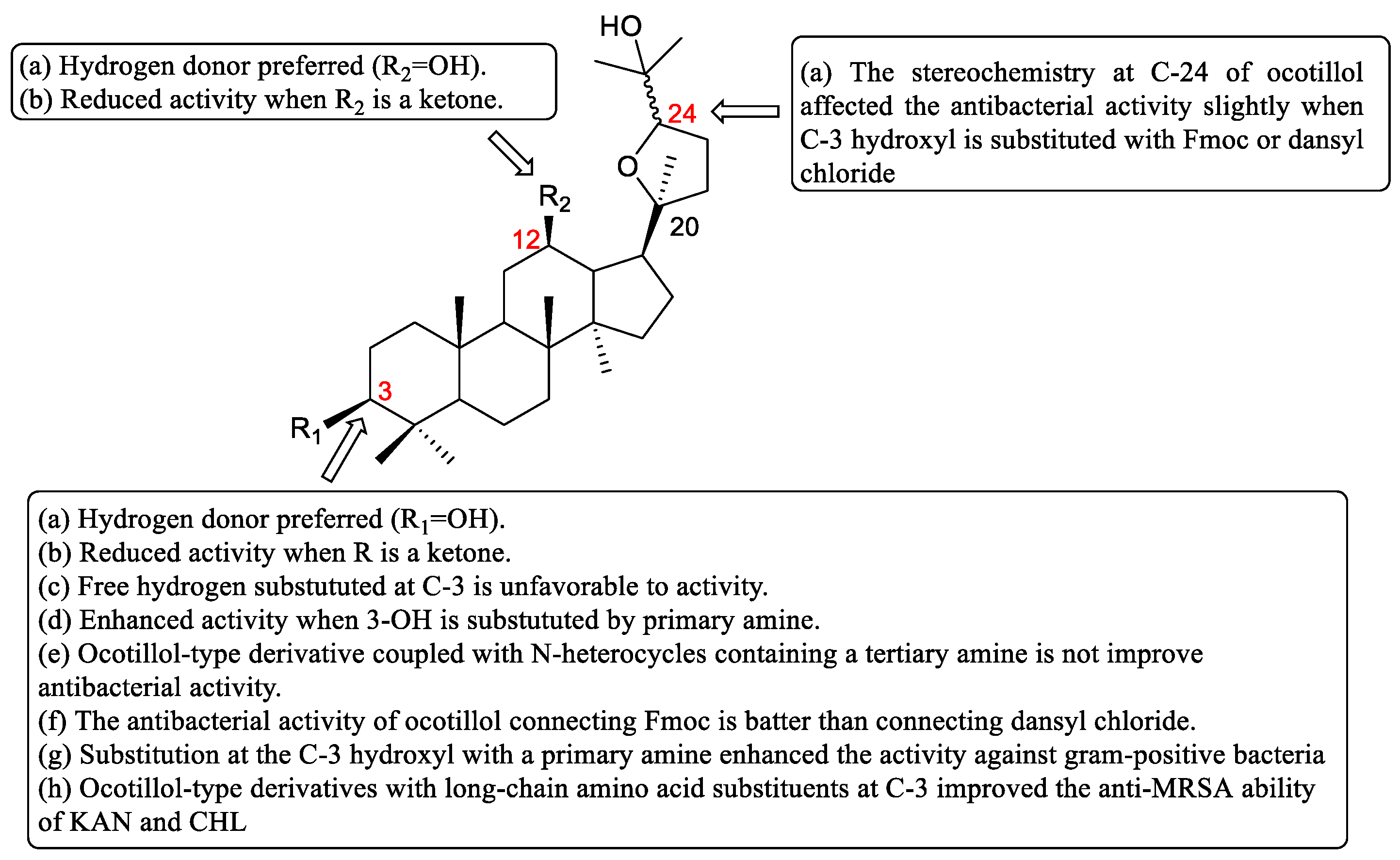
2.4. Structure–Activity Relationships (SARs)
3. Materials and Methods
3.1. Chemical Reagents and Instruments
3.2. The Synthesis of Compounds 8–15
3.3. The Synthesis of Compounds 20–23, 28–31, 34, and 35
3.3.1. (20S, 24R)-Epoxy-3β-O-[2-(N’-Fmoc)-5-amino butyryl]-dammarane-12β, 25-diol (20)
3.3.2. (20S, 24S)-Epoxy-3β-O-[2-(N’-Fmoc)-5-amino butyryl]-dammarane-12β, 25-diol (21)
3.3.3. (20S, 24R)-Epoxy-3β-O-[2-(N’-Fmoc)-6-amino ornithyl]-dammarane-12β, 25-diol (22)
3.3.4. (20S, 24S)-Epoxy-3β-O-[2-(N’-Fmoc)-6-amino ornithyl]-dammarane-12β, 25-diol (23)
3.3.5. (20S, 24R)-Epoxy-3β-O-[2-amino-(1-dansylamino)-5-dimethyl amino butyr-yl]-dammarane-12β, 25-diol (28)
3.3.6. (20S, 24S)-Epoxy-3β-O-[2-amino-(1-dansylamino)-5-dimethyl amino butyr-yl]-dammarane-12β, 25-diol (29)
3.3.7. (20S, 24R)-Epoxy-3β-O-[2-amino-(1-dansylamino)-5-dimethyl amino val-eryl]-dammarane-12β, 25-diol (30)
3.3.8. (20S, 24S)-Epoxy-3β-O-[2-amino-(1-dansylamino)-5-dimethyl amino val-eryl]-dammarane-12β, 25-diol (31)
3.3.9. (20S, 24R)-Epoxy-3β-O-[3-p-(N’-Fmoc) aromatic ring-2-alanine]-dammarane-12β, 25-diol (34)
3.3.10. (20S, 24S)-Epoxy-3β-O-[3-p-(N’-Fmoc) aromatic ring-2-alanine]-dammarane-12β, 25-diol (35)
3.4. Antibacterial Activity
3.5. Synergistic Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef] [Green Version]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef]
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [Green Version]
- Langeveld, W.T.; Veldhuizen, E.J.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Oloyede, H.O.B.; Ajiboye, H.O.; Salawu, M.O.; Ajiboye, T.O. Influence of oxidative stress on the antibacterial activity of betulin, betulinic acid and ursolic acid. Microb. Pathog. 2017, 111, 338–344. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [Green Version]
- Chung, P.Y.; Navaratnam, P.; Chung, L.Y. Synergistic antimicrobial activity between pentacyclic triterpenoids and antibiotics against Staphylococcus aureus strains. Ann. Clin. Microbiol. Antimicrob. 2011, 10. [Google Scholar] [CrossRef] [Green Version]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic interactions of phytochemicals with antimicrobial agents: Potential strategy to counteract drug resistance. Chem. Biol. Interact. 2019, 308, 294–303. [Google Scholar] [CrossRef]
- Cappiello, F.; Loffredo, M.R.; Del Plato, C.; Cammarone, S.; Casciaro, B.; Quaglio, D.; Mangoni, M.L.; Botta, B.; Ghirga, F. The Revaluation of Plant-Derived Terpenes to Fight Antibiotic-Resistant Infections. Antibiotics 2020, 9, 325. [Google Scholar] [CrossRef]
- Wolska, K.I.; Grudniak, A.M.; Fiecek, B.; Kraczkiewicz-Dowjat, A.; Kurek, A. Antibacterial activity of oleanolic and ursolic acids and their derivatives. Cent. Eur. J. Biol. 2010, 5, 543–553. [Google Scholar] [CrossRef]
- Kim, J.H. Pharmacological and medical applications of Panax ginseng and ginsenosides: A review for use in cardiovascular diseases. J. Ginseng Res. 2018, 42, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.H.; Kim, J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Xu, Y.; Yang, J.; Wang, W.; Zhang, J.; Zhang, R.; Meng, Q. Discovery, semisynthesis, biological activities, and metabolism of ocotillol-type saponins. J. Ginseng Res. 2017, 41, 373–378. [Google Scholar] [CrossRef]
- Bi, Y.; Ma, C.; Zhang, H.; Zhou, Z.; Yang, J.; Zhang, Z.; Meng, Q.; Lewis, P.J.; Xu, J. Novel 3-substituted ocotillol-type triterpenoid derivatives as antibacterial candidates. Chem. Biol. Drug Des. 2014, 84, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Yang, X.; Zhang, T.T.; Liu, Z.Y.; Zhang, X.C.; Lu, J.; Cheng, K.G.; Xu, J.Y.; Wang, H.B.; Lv, G.Y.; et al. Design, synthesis, nitric oxide release and antibacterial evaluation of novel nitrated ocotillol-type derivatives. Eur. J. Med. Chem. 2015, 101, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Ma, C.; Zhou, Z.; Zhang, T.; Zhang, H.; Zhang, X.; Lu, J.; Meng, Q.; Lewis, P.; Xu, J. Synthesis and Antibacterial Evaluation of Novel Hydrophilic Ocotillol-Type Triterpenoid Derivatives from 20(S)-Protopanaxadiol. Rec. Nat. Prod. 2015, 9, 356–368. [Google Scholar]
- Cao, Y.C.; Wang, K.Y.; Wang, J.L.; Cheng, H.R.; Ma, M.X.; Meng, Q.G.; Li, X.P.; Bi, Y. Design, synthesis and antibacterial evaluation of ocotillol derivatives with polycyclic nitrogen-containing groups. Future Med. Chem. 2021, 13. [Google Scholar] [CrossRef]
- Bi, Y.; Liu, X.X.; Zhang, H.Y.; Yang, X.; Liu, Z.Y.; Lu, J.; Lewis, P.J.; Wang, C.Z.; Xu, J.Y.; Meng, Q.G.; et al. Synthesis and Antibacterial Evaluation of Novel 3-Substituted Ocotillol-Type Derivatives as Leads. Molecules 2017, 22, 590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.C.; Wang, K.Y.; Xu, S.; Kong, L.T.; Bi, Y.; Li, X.P. Recent Advances in the Semisynthesis, Modifications and Biological Activities of Ocotillol-Type Triterpenoids. Molecules 2020, 25, 5562. [Google Scholar] [CrossRef]
- Ge, X.; Xu, Z. 1,2,4-Triazole hybrids with potential antibacterial activity against methicillin-resistant Staphylococcus aureus. Arch. Pharm. 2021, 354, e2000223. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.R.S.; Andrade, J.T.; Sousa, C.D.F.; Fernandes, J.S.; Carmo, L.F.; Araujo, M.G.F.; Ferreira, J.M.S.; Villar, J. Synthesis and Evaluation of the in vitro Antimicrobial Activity of Triazoles, Morpholines and Thiosemicarbazones. Med. Chem. 2019, 15, 38–50. [Google Scholar] [CrossRef]
- Yin, J.; Kwon, Y.; Kim, D.; Lee, D.; Kim, G.; Hu, Y.; Ryu, J.H.; Yoon, J. Preparation of a cyanine-based fluorescent probe for highly selective detection of glutathione and its use in living cells and tissues of mice. Nat. Protoc. 2015, 10, 1742–1754. [Google Scholar] [CrossRef]
- Lin, G.J.; Luo, S.P.; Zheng, X.; Ye, J.L.; Huang, P.Q. Enantiodivergent synthesis of trans-3,4-disubstituted succinimides by SmI(2)-mediated Reformatsky-type reaction. Tetrahedron Lett. 2008, 49, 4007–4010. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, N.-H.; Kim, E.-J.; Kim, J.H.; Lee, M.-Y.; Park, Y.-H.; Lee, J.R.; Park, S.-C.; Jang, M.-K. Antibacterial effects of amino acids-grafted water-soluble chitosan against drug-resistant bacteria. Biotechnol. Bioprocess Eng. 2016, 21, 183–189. [Google Scholar] [CrossRef]
- Gahane, A.Y.; Ranjan, P.; Singh, V.; Sharma, R.K.; Sinha, N.; Sharma, M.; Chaudhry, R.; Thakur, A.K. Fmoc-phenylalanine displays antibacterial activity against Gram-positive bacteria in gel and solution phases. Soft Matter 2018, 14, 2234–2244. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Chen, L.; Wang, J.; Wang, W.; Chen, D.; Li, L.; Li, B.; Deng, Y.; Xu, Z. Current methodologies on genotyping for nosocomial pathogen methicillin-resistant Staphylococcus aureus (MRSA). Microb. Pathog. 2017, 107, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Linden, P.K.; Friedman, B. Incidence, prevalence, and management of MRSA bacteremia across patient populations-a review of recent developments in MRSA management and treatment. Crit. Care 2017, 21, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefani, S.; Chung, D.R.; Lindsay, J.A.; Friedrich, A.W.; Kearns, A.M.; Westh, H.; Mackenzie, F.M. Meticillin-resistant Staphylococcus aureus (MRSA): Global epidemiology and harmonisation of typing methods. Int. J. Antimicrob. Agents 2012, 39, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ma, C.; Zhang, H.; Bi, Y.; Chen, X.; Tian, H.; Xie, X.; Meng, Q.; Lewis, P.J.; Xu, J. Synthesis and biological evaluation of novel ocotillol-type triterpenoid derivatives as antibacterial agents. Eur. J. Med. Chem. 2013, 68, 444–453. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [Green Version]



| Cpd. | Antimicrobial Screening MIC (μg/mL) | ||
|---|---|---|---|
| MRSA 18–19 | MRSA 18–20 | S. aureus ATCC 29213 | |
| 8 | >128 | >128 | >128 |
| 9 | >128 | >128 | >128 |
| 10 | >128 | >128 | >128 |
| 11 | >128 | >128 | >128 |
| 12 | >128 | >128 | >128 |
| 13 | >128 | >128 | >128 |
| 14 | >128 | >128 | >128 |
| 15 | >128 | >128 | >128 |
| 20 | 4 | 4 | 16 |
| 21 | 1 | 1 | 1 |
| 22 | 8 | 2 | 4 |
| 23 | 4 | 2 | 8 |
| 28 | 64 | 32 | 32 |
| 29 | 32 | 64 | 32 |
| 30 | 4 | 8 | 32 |
| 31 | 4 | 8 | 8 |
| 34 | >128 | >128 | >128 |
| 35 | >128 | >128 | >128 |
| 4 | >128 | >128 | >128 |
| 5 | 64 | 64 | 64 |
| Levofloxacin | 8 | 0.125 | 0.25 |
| MIC (μg/mL) | MBC (μg/mL) | FICI | |
|---|---|---|---|
| MRSA 18–15 | MRSA 18–15 | MRSA 18–15 | |
| CHL | 8 | >32 | - |
| 20 | 4 | >64 | - |
| 21 | 2 | >128 | - |
| 20 + CHL | 2 | NE | 0.5 |
| 21 + CHL | 1 | NE | 0.375 |
| MIC (μg/mL) | MBC (μg/mL) | FICI | |
|---|---|---|---|
| MRSA 18–15 | MRSA 18–15 | MRSA 18–15 | |
| KAN | 2 | >8 | - |
| 20 | 4 | >64 | - |
| 21 | 1 | >128 | - |
| 20 + KAN | 0.5 | NE | 0.3125 |
| 21 + KAN | 0.25 | NE | 0.375 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Cao, Y.; Wang, K.; Shi, Z.; Wang, R.; Meng, Q.; Bi, Y. Design, Synthesis, and Antibacterial Evaluation of Novel Ocotillol Derivatives and Their Synergistic Effects with Conventional Antibiotics. Molecules 2021, 26, 5969. https://doi.org/10.3390/molecules26195969
Zhang D, Cao Y, Wang K, Shi Z, Wang R, Meng Q, Bi Y. Design, Synthesis, and Antibacterial Evaluation of Novel Ocotillol Derivatives and Their Synergistic Effects with Conventional Antibiotics. Molecules. 2021; 26(19):5969. https://doi.org/10.3390/molecules26195969
Chicago/Turabian StyleZhang, Doudou, Yucheng Cao, Kaiyi Wang, Zhuoyue Shi, Ruodong Wang, Qingguo Meng, and Yi Bi. 2021. "Design, Synthesis, and Antibacterial Evaluation of Novel Ocotillol Derivatives and Their Synergistic Effects with Conventional Antibiotics" Molecules 26, no. 19: 5969. https://doi.org/10.3390/molecules26195969






