Cannabidiol Attenuates MK-801-Induced Cognitive Symptoms of Schizophrenia in the Passive Avoidance Test in Mice
Abstract
:1. Introduction
2. Results
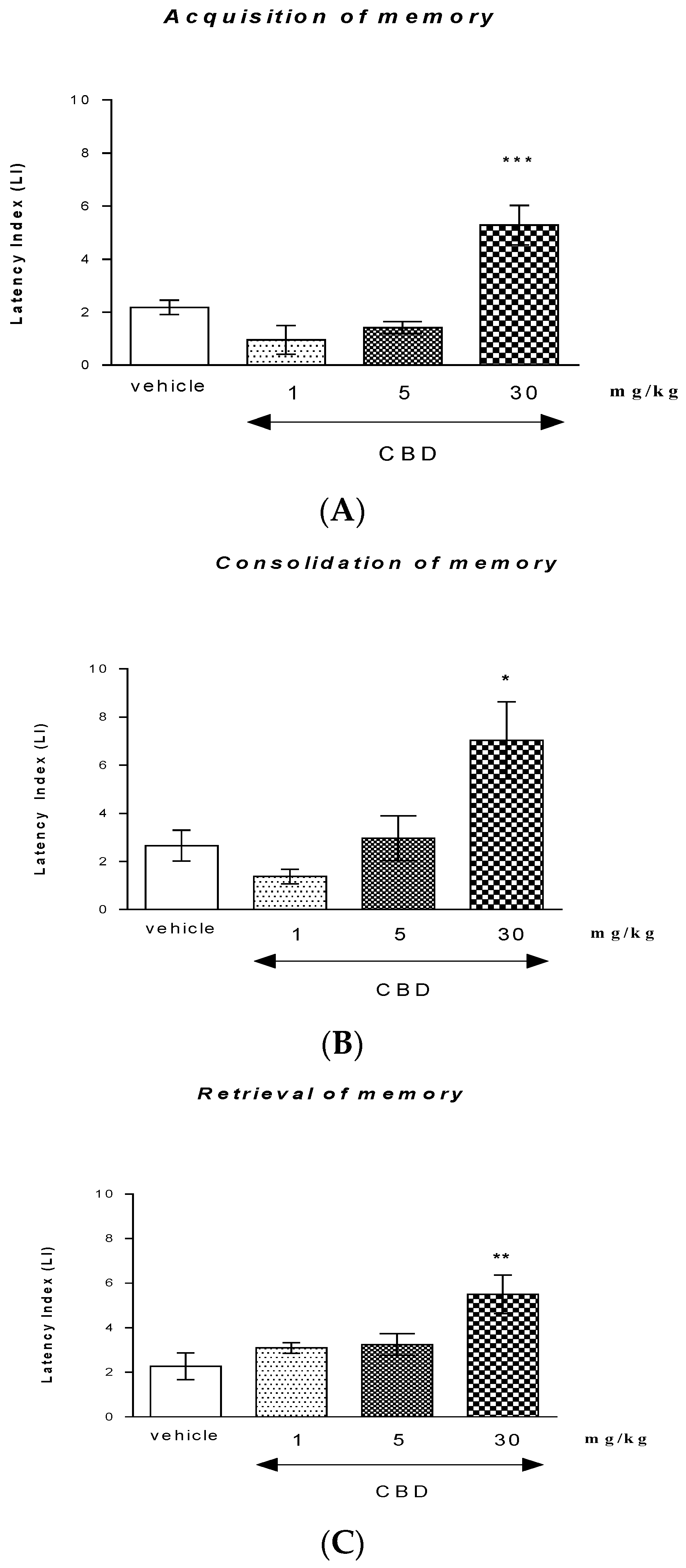
2.1. Memory-Related Responses
2.1.1. The Influence of an Acute Injection of CBD on the Long-Term Memory in Mice in the PA Test
Acquisition of Memory
Consolidation of Memory
Retrieval of Memory
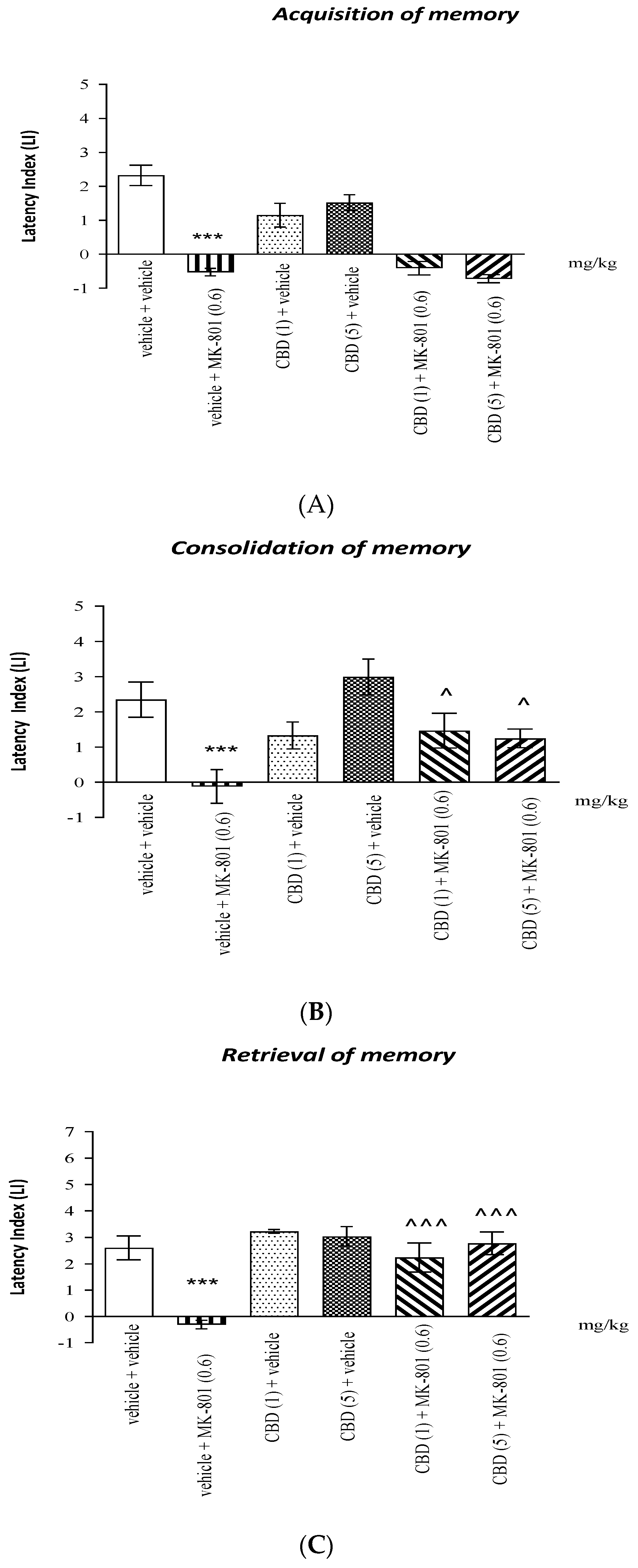
2.1.2. The Influence of the Administration of CBD on the Memory Impairment Provoked by an Acute Administration of MK-801 in the PA Test in Mice
Acquisition of Memory
Consolidation of Memory
Retrieval of Memory
2.2. Locomotor Activity
3. Discussion
3.1. CBD Impacts on Different Memory Stages
3.2. CBD’s Effects on MK-801-Induced Memory Impairment
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Experimental Procedure
4.3.1. Memory and Learning
PA Test Apparatus
PA Test Experimental Procedures
4.3.2. Locomotor Activity
4.4. Treatment
4.4.1. Memory-Related Responses
Acquisition of Memory Processes
Consolidation of Memory Processes
Retrieval of Memory Processes
Acquisition of Memory Processes
Consolidation of Memory Processes
Retrieval of Memory Processes
4.4.2. Locomotor Activity
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Dollfus, S.; Lynede, J. Negative symptoms: History of the concept and their position in diagnosis of schizophrenia. Schizophr. Res. 2017, 186, 3–7. [Google Scholar] [CrossRef]
- Lewis, D.A.; Lieberman, J.A. Catching up on schizophrenia: Natural history and neurobiology. Neuron 2000, 28, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Riedel, G.; Platt, B.; Micheau, J. Glutamate receptor function in learning and memory. Behav. Brain Res. 2003, 140, 1–47. [Google Scholar] [CrossRef]
- Shapiro, M. Plasticity, hippocampal place cells, and cognitive maps. Arch. Neurol. 2001, 58, 874–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadelha, A.; Noto, C.; Mari, J. Pharmacological treatment of schizophrenia. Int. Rev. Psychiatry 2012, 24, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Arseneault, L.; Cannon, M.; Witton, J.; Murray, R.M. Causal association between cannabis and psychosis: Examination of the evidence. Br. J. Psychiatry 2004, 184, 110–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grotenhermen, F. Pharmacology of cannabinoids. Neuro Endocrinol. Lett. 2004, 25, 14–23. [Google Scholar]
- Moore, C.; Rana, S.; Coulter, C. Simultaneous identification of 2-carboxy-tetrahydrocannabinol, tetrahydrocannabinol, cannabinol and cannabidiol in oral fluid. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 852, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Roser, P.; Haussleiter, I.S. Antipsychotic-like effects of cannabidiol and rimonabant: Systematic review of animal and human studies. Curr. Pharm. Des. 2012, 18, 5141–5155. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, R.; Almeida, V.; Peres, F.F.; Calzavara, M.B.; da Silva, N.D.; Akimi Suiama, M.; Tamie Niigaki, S.; Waldo Zuardi, A.; Eduardo Cecilio Hallak, J.; Alexandre Crippa, J.; et al. Antipsychotic profile of cannabidiol and rimonabant in an animal model of emotional context processing in schizophrenia. Curr. Pharm. Des. 2012, 18, 4960–4965. [Google Scholar] [CrossRef]
- Manseau, M.W.; Goff, D.C. Cannabinoids and Schizophrenia: Risks and Therapeutic Potential. Neurotherapeutics 2015, 12, 816–824. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Schubart, C.D.; Sommer, I.E.; van Gastel, W.A.; Goetgebuer, R.L.; Kahn, R.S.; Boks, M.P. Cannabis with high cannabidiol content is associated with fewer psychotic experiences. Schizophr. Res. 2011, 130, 216–221. [Google Scholar] [CrossRef] [Green Version]
- Kucerova, J.; Tabiova, K.; Drago, F.; Micale, V. Therapeutic potential of cannabinoids in schizophrenia. Recent Pat. CNS Drug Discov. 2014, 9, 13–25. [Google Scholar] [CrossRef]
- Bubeníková-Valešová, V.; Horáček, J.; Vrajová, M.; Höschl, C. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci. Biobehav. Rev. 2008, 32, 1014–1023. [Google Scholar] [CrossRef]
- Kruk-Slomka, M.; Biala, G. CB1 receptors in the formation of the different phases of memory-related processes in the inhibitory avoidance test in mice. Behav. Brain Res. 2016, 301, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Pamplona, F.A.; Takahashi, R.N. WIN 55212-2 impairs contextual fear conditioning through the activation of CB1 cannabinoid receptors. Neurosci. Lett. 2006, 397, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, A.H. SR 141716A enhances spatial memory as assessed in a radial-arm maze task in rats. Eur. J. Pharmacol. 2000, 404, 175–179. [Google Scholar] [CrossRef]
- Takahashi, R.N.; Pamplona, F.A.; Fernandes, M.S. The cannabinoid antagonist SR141716A facilitates memory acquisition and consolidation in the mouse elevated T-maze. Neurosci. Lett. 2005, 380, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta 9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [Green Version]
- Campos, A.; Fogac, M.; Sonego, A.; Guimaraes, F. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef]
- Osborne, A.L.; Solowij, N.; Weston-Green, K. A systematic review of the effect of cannabidiol on cognitive function: Relevance to schizophrenia. Neurosci. Biobehav. Rev. 2017, 72, 310–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resstel, L.B.; Joca, S.R.; Moreira, F.A.; Corrêa, F.M.; Guimarães, F.S. Effects of cannabidiol and diazepam on behavioral and cardiovascular responses induced by contextual conditioned fear in rats. Behav. Brain Res. 2006, 172, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.I.; Resstel, L.B.; Guimarães, F.S. Involvement of the prelimbic prefrontal cortex on cannabidiol-induced attenuation of contextual conditioned fear in rats. Behav. Brain Res. 2010, 207, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Jurkus, R.; Day, H.L.; Guimarães, F.S.; Lee, J.L.; Bertoglio, L.J.; Stevenson, C.W. Cannabidiol regulation of learned fear: Implications for treating anxiety-related disorders. Front. Pharmacol. 2016, 7, 454–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ElBatsh, M.M.; Assareh, N.; Marsden, C.A.; Kendall, D.A. Anxiogenic-like effects of chronic cannabidiol administration in rats. Psychopharmacology (Berl) 2012, 221, 239–247. [Google Scholar] [CrossRef]
- Cheng, D.; Low, J.K.; Logge, W.; Garner, B.; Karl, T. Chronic cannabidiol treatment improves social and object recognition in double transgenic APPswe/PS1∆E9 mice. Psychopharmacology (Berl) 2014, 231, 3009–3017. [Google Scholar] [CrossRef]
- Stern, C.A.; Gazarini, L.; Takahashi, R.N.; Guimarães, F.S.; Bertoglio, L.J. On disruption of fear memory by reconsolidation blockade: Evidence from cannabidiol treatment. Neuropsychopharmacology 2012, 37, 2132–2142. [Google Scholar] [CrossRef] [Green Version]
- Stern, C.A.; Gazarini, L.; Vanvossen, A.C.; Zuardi, A.W.; Galve-Roperh, I.; Guimaraes, F.S.; Takahashi, R.N.; Bertoglio, L.J. Δ9-Tetrahydrocannabinol alone and combined with cannabidiol mitigate fear memory through reconsolidation disruption. Eur. Neuropsychopharmacol. 2015, 25, 958–965. [Google Scholar] [CrossRef]
- Wright, M.J.; Vanderwater, S.A.; Taffr, M.A. Cannabidiol attenuates deficits of visuolspatial associative memory induced by Δ9tetrahydrocannabinol. Br. J. Pharmacol. 2013, 170, 1365–1373. [Google Scholar] [CrossRef] [Green Version]
- Osborne, A.; Solowij, N.; Babic, I.; Huang, X.; Weston-Green, K. Improved social interaction, recognition and working memory with cannabidiol treatment in a prenatal infection (poly I:C) rat model. Neuropsychopharmacology 2017, 42, 1447–1457. [Google Scholar] [CrossRef]
- Fagherazzi, E.V.; Garcia, V.A.; Maurmann, N.; Bervanger, T.; Halmenschlager, L.H.; Busato, S.B.; Hallak, J.E.; Zuardi, A.Z.; Crippa, J.A.; Schröder, N. Memory-rescuing effects of cannabidiol in an animal model of cognitive impairment relevant to neurodegenerative disorders. Psychopharmacology 2012, 219, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Gururajan, A.; Taylor, D.A.; Malone, D.T. Cannabidiol and clozapine reverse MK-801-induced deficits in social interaction and hyperactivity in Sprague-Dawley rats. J. Psychopharmacol. 2012, 26, 1317–1332. [Google Scholar] [CrossRef]
- Long, L.E.; Malone, D.T.; Taylor, D.A. Cannabidiol reverses MK-801-induced disruption of prepulse inhibition in mice. Neuropsychopharmacology 2006, 31, 795–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, F.V.; Llorente, R.; Del Bel, E.A.; Viveros, M.-P.; López-Gallardo, M.; Guimarães, F.S. Decreased glial reactivity could be involved in the antipsychotic-like effect of cannabidiol. Schizophr. Res. 2015, 164, 155–163. [Google Scholar] [CrossRef]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Kikuchi, T.; Stott, C.; Riedel, G. MK-801-induced deficits in social recognition in rats: Reversal by aripiprazole, but not olanzapine, risperidone, or cannabidiol. Behav. Pharmacol. 2015, 26, 748–765. [Google Scholar] [CrossRef]
- Fadda, P.; Robinson, L.; Fratta, W.; Pertwee, R.G.; Riedel, G. Differential effects of THC- and CBD-rich cannabis-extracts on working memory in rats. Neuropharmacology 2004, 47, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A. Human Cannabinoid Pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Blázquez, P.; Rodríguez-Muñoz, M.; Garzón, J. The cannabinoid receptor 1 associates with NMDA receptors to produce glutamatergic hypofunction: Implications in psychosis and schizophrenia. Front. Pharmacol. 2014, 4, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB 1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Schubart, C.D.; Sommer, I.E.C.; Fusar-Poli, P.; de Witte, L.; Kahn, R.S.; Boks, M.P.M. Cannabidiol as a potential treatment for psychosis. Eur. Neuropsychopharmacol. 2014, 24, 51–64. [Google Scholar] [CrossRef]
- Hampson, A.J.; Grimaldi, M. Cannabidiol and (−) Delta-9-tetrahydrocannabinol are neuroprotective. PNAS 1998, 95, 8268–8273. [Google Scholar] [CrossRef] [Green Version]
- Hajos, N.; Ledent, C.; Freund, T.F. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience 2001, 106, 1–4. [Google Scholar] [CrossRef]
- Pan, H.; Mukhopadhyay, P.; Rajesh, M.; Patel, V.; Mukhopadhyay, B.; Gao, B.; Haskó, G.; Pacher, P. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J. Pharmacol. Exp. Ther. 2008, 328, 708–714. [Google Scholar] [CrossRef] [Green Version]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Haskó, G.; Liaudet, L.; Drel, V.R.; Obrosova, I.G.; Pacher, P. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, 610–619. [Google Scholar] [CrossRef] [Green Version]
- Hamelink, C.; Hampson, A.; Wink, D.A.; Eiden, L.E.; Eskay, R.L. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J. Pharmacol. Exp. Ther. 2005, 314, 780–788. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Mukhopadhyay, P.; Cao, Z.; Wang, H.; Feng, D.; Haskó, G.; Mechoulam, R.; Gao, B.; Pacher, P. Cannabidiol attenuates alcohol-induced liver steatosis, metabolic dysregulation, inflammation and neutrophil-mediated injury. Sci. Rep. 2017, 7, 12064. [Google Scholar] [CrossRef] [Green Version]
- De Petrocellis, L.; Nabissi, M.; Santoni, G.; Ligresti, A. Actions and Regulation of Ionotropic Cannabinoid Receptors. Adv. Pharmacol. 2017, 80, 249–289. [Google Scholar]
- Ghovanloo, M.R.; Shuart, N.G.; Mezeyova, J.; Dean, R.A.; Ruben, P.C.; Goodchild, S.J. Inhibitory effects of cannabidiol on voltage-dependent sodium currents. J. Biol. Chem. 2018, 293, 16546–16558. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.L.C.; Bertoglio, L.J.; Guimaraes, F.S.; Stevenson, C.W. Cannabidiol regulation of emotion and emotional memory processing: Relevance for treating anxiety-related and substance abuse disorders. Br. J. Pharmacol. 2017, 174, 3242–3256. [Google Scholar] [CrossRef] [Green Version]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef]
- De Gregorio, D.; McLaughlin, R.J.; Posa, L.; Ochoa-Sanchez, R.; Enns, J.; Lopez-Canul, M.; Aboud, M.; Maione, S.; Comai, S.; Gobbi, G. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain 2019, 160, 136–150. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- Rock, E.M.; Bolognini, D.; Limebeer, L.M.; Cascio, M.G.; Anavi-Goffer, S.; Fletcher, P.J.; Mechoulam, R.; Pertwee, R.G.; Parker, L.A. Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT1A somatodendritic autoreceptors in the dorsal raphe nucleus. Br. J. Pharmacol. 2012, 165, 2620–2634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haj-Dahmane, S.; Roh-Yu Shen, R. Modulation of the Serotonin System by Endocannabinoid Signaling. Neuropharmacology 2011, 61, 414–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azouzi, S.; Santuz, H.; Morandat, S.; Pereira, C.; Côté, F.; Hermine, O.; El Kirat, K.; Colin, Y.; Le Van Kim, C.; Etchebest, C.; et al. Antioxidant and membrane binding properties of serotonin protect lipids from oxidation. Biophys. J. 2017, 112, 1863–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karl, T.; Garner, B.; Cheng, D. The therapeutic potential of the phytocannabinoid cannabidiol for Alzheimer’s disease. Behav. Pharmacol. 2017, 28, 142–160. [Google Scholar] [CrossRef] [Green Version]
- Kruk-Slomka, M.; Banaszkiewicz, I.; Slomka, T.; Biala, G. Effects of Fatty Acid Amide Hydrolase Inhibitors Acute Administration on the Positive and Cognitive Symptoms of Schizophrenia in Mice. Mol. Neurobiol. 2019, 56, 7251–7266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruk-Slomka, M.; Budzynska, B.; Slomka, T.; Banaszkiewicz, I.; Biala, G. The Influence of the CB1 Receptor Ligands on the Schizophrenia-Like Effects in Mice Induced byMK-801. Neurotox. Res. 2016, 30, 658–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, Y.; Hata, T.; Kawabata, A.; Itoh, E.; Kita, T. Impairment of passive avoidance performance in SART-stressed mice and the action of drugs. Jpn. J. Pharmacol. 1989, 49, 111–117. [Google Scholar] [CrossRef]
- Javadi-Paydar, M.; Zakeri, M.; Norouzi, A.; Rastegar, H.; Mirazi, N.; Dehpour, A. Involvement of nitric oxide in granisetron improving effect on scopolamine-induced memory impairment in mice. Brain Res. Rev. 2012, 1429, 61–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimakurthy, J.; Talasila, M. Effects of curcumin on pentylenetetrazole-induced anxiety-like behaviors and associated changes in cognition and monoamine levels. Psychol. Neurosci. 2010, 3, 239–244. [Google Scholar] [CrossRef] [Green Version]

| A | |||||
| Acquisition of memory | |||||
| PA test | drug administration | interval | TL1 | interval | TL2 |
| Long-term memory | CBD (1, 5, 30 mg/kg) | 30 min | + | 24 h | + |
| vehicle | 30 min | + | 24 h | + | |
| B | |||||
| Consolidation of memory | |||||
| PA test | TL1 | interval | drug administration | interval | TL2 |
| Long-term memory | + | 0 min | CBD (1, 5, 30 mg/kg) | 24 h | + |
| + | 0 min | vehicle | 24 h | + | |
| C | |||||
| Retrieval of memory | |||||
| PA test | TL1 | interval | drug administration | interval | TL2 |
| Long-term memory | + | 24 h | CBD (1, 5, 30 mg/kg) | 30 min | + |
| + | 24 h | vehicle | 30 min | + | |
| A | |||||||
| Acquisition of Memory | |||||||
| PA test | drug administration | interval | drug administration | interval | TL1 | interval | TL2 |
| Long-term memory | CBD (1 or 5 mg/kg) | 15 min | MK-801 (0.6 mg/kg) or vehicle | 15 min | + | 24 h | + |
| vehicle (control group) | 15 min | MK-801 (0.6 mg/kg) or vehicle | 15 min | + | 24 h | + | |
| B | |||||||
| Consolidation of Memory | |||||||
| PA test | TL1 | interval | drug administration | interval | drug administration | interval | TL2 |
| Long-term memory | + | 0 min | CBD (1, 5, 30 mg/kg) | 15 min | MK-801 (0.6 mg/kg) or vehicle | 24 h | + |
| + | 0 min | vehicle | 15 min | MK-801 (0.6 mg/kg) or vehicle | 24 h | + | |
| C | |||||||
| Retrieval of Memory | |||||||
| PA test | TL1 | interval | drug administration | interval | drug administration | interval | TL2 |
| Long-term memory | + | 24 h | CBD (1, 5, 30 mg/kg) | 15 min | MK-801 (0.6 mg/kg) or vehicle | 15 min | + |
| + | 24 h | vehicle | 15 min | MK-801 (0.6 mg/kg) or vehicle | 15 min | + | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruk-Slomka, M.; Biala, G. Cannabidiol Attenuates MK-801-Induced Cognitive Symptoms of Schizophrenia in the Passive Avoidance Test in Mice. Molecules 2021, 26, 5977. https://doi.org/10.3390/molecules26195977
Kruk-Slomka M, Biala G. Cannabidiol Attenuates MK-801-Induced Cognitive Symptoms of Schizophrenia in the Passive Avoidance Test in Mice. Molecules. 2021; 26(19):5977. https://doi.org/10.3390/molecules26195977
Chicago/Turabian StyleKruk-Slomka, Marta, and Grazyna Biala. 2021. "Cannabidiol Attenuates MK-801-Induced Cognitive Symptoms of Schizophrenia in the Passive Avoidance Test in Mice" Molecules 26, no. 19: 5977. https://doi.org/10.3390/molecules26195977






