Unambiguous Stereochemical Assignment of Cyclo(Phe-Pro), Cyclo(Leu-Pro), and Cyclo(Val-Pro) by Electronic Circular Dichroic Spectroscopy
Abstract
:1. Introduction
2. Results
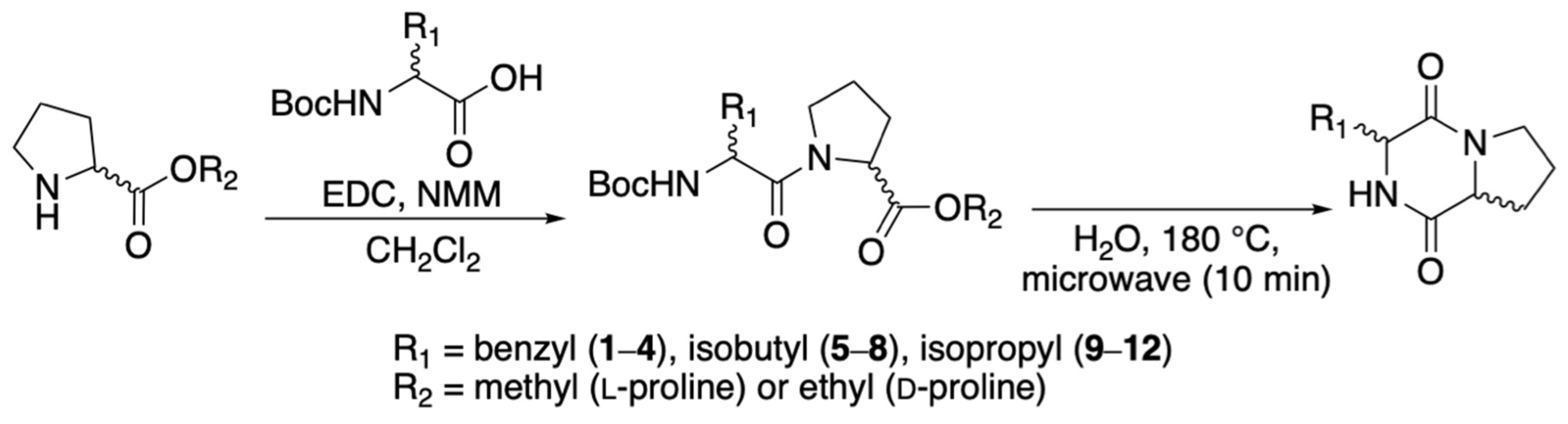
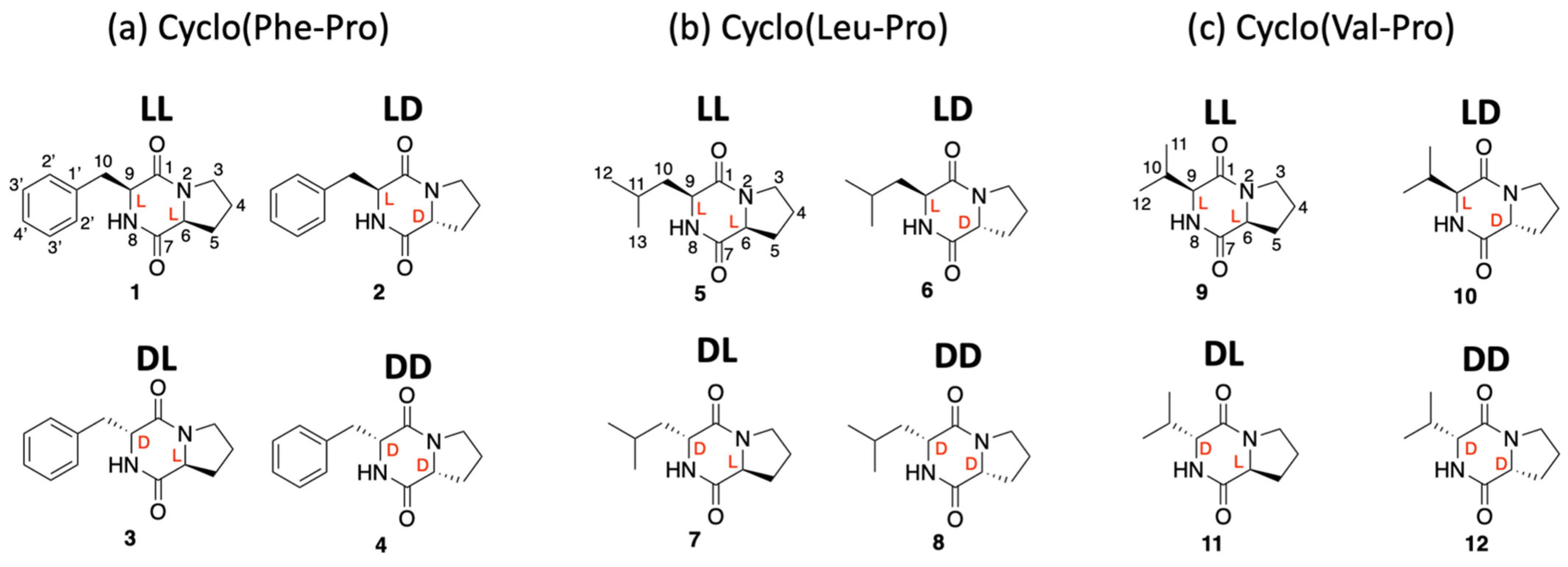
2.1. Synthesis of all Stereoisomers of Cyclo(Phe-Pro), Cyclo(Leu-Pro), and Cyclo(Val-Pro)
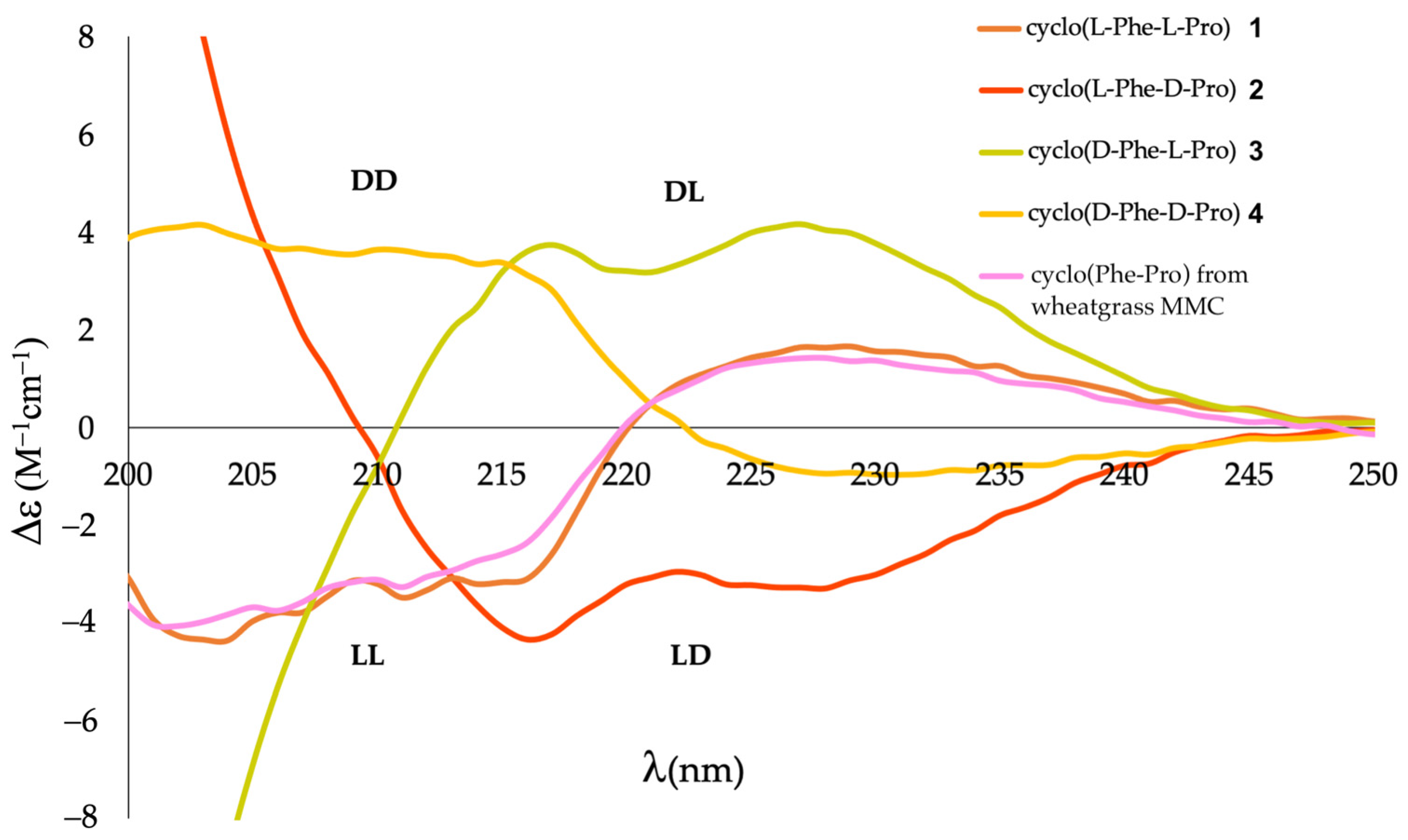
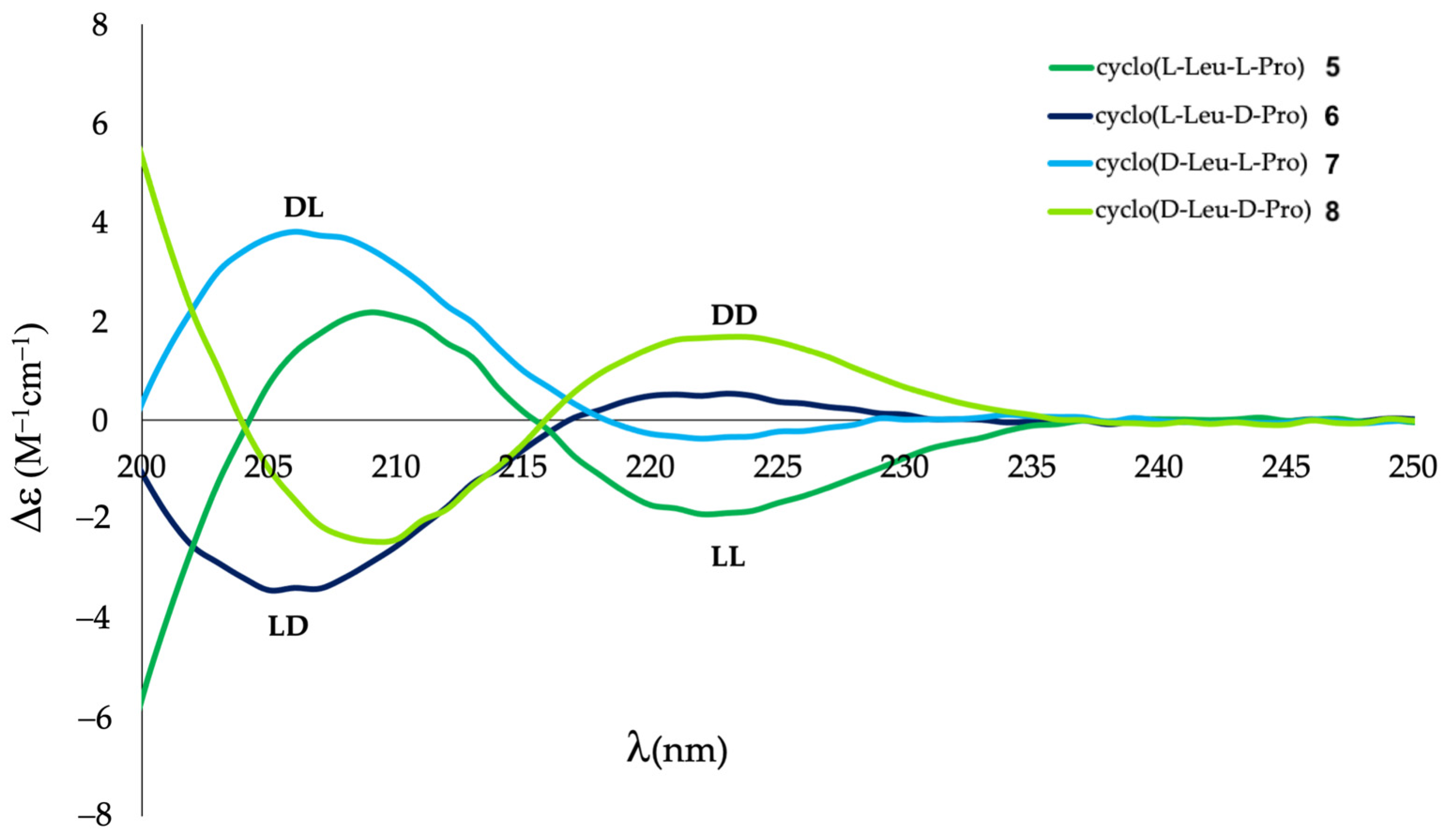
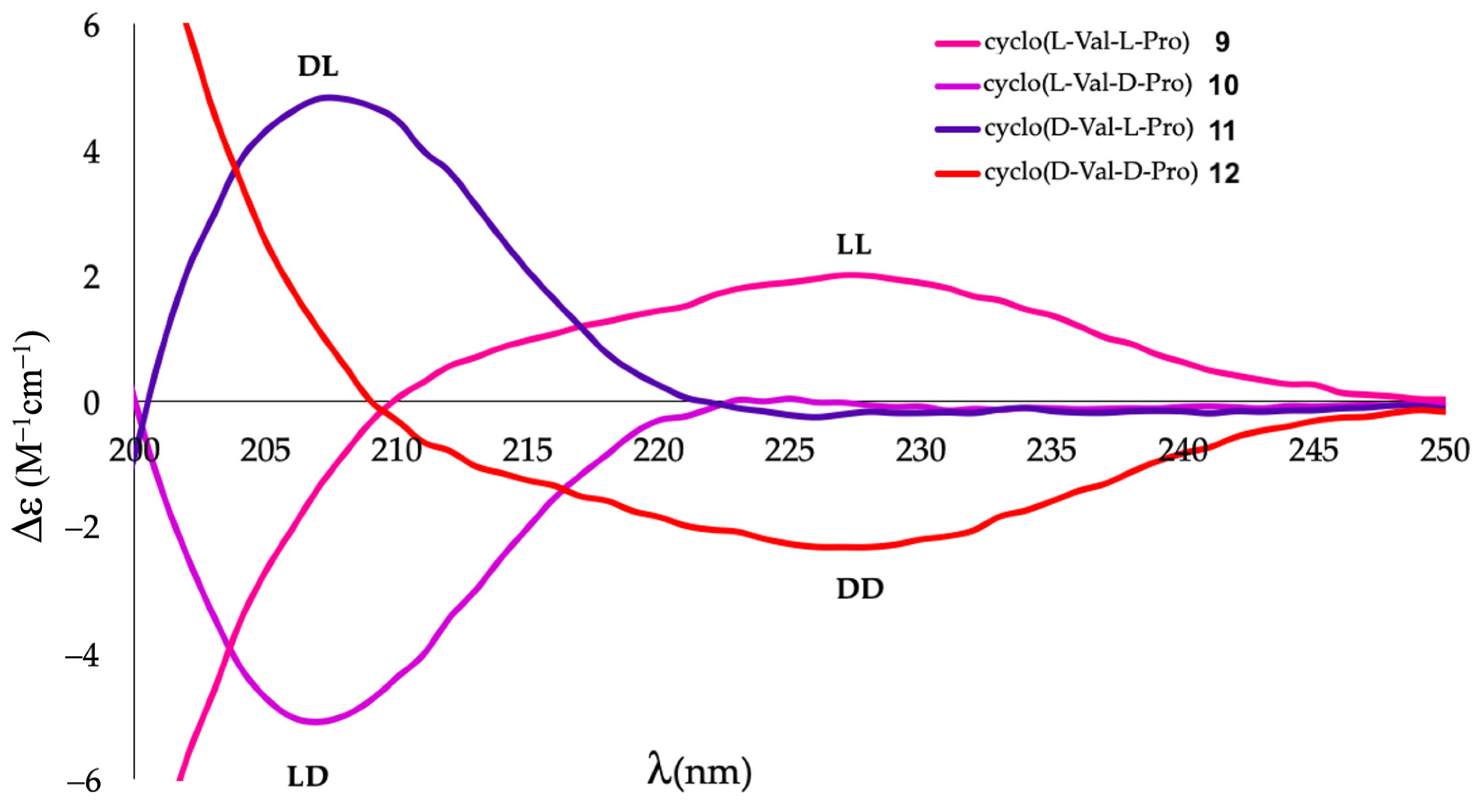
2.2. Differentiation of DKP Stereoisomers by ECD
2.3. Stereochemical Assignment of Cyclo(Phe-Pro) from Wheatgrass MMC
2.4. Stereochemistry–Activity Relationship of DKPs
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Purification and Isolation of Natural 2,5-diketopiperazine
4.3. Construction of Synthetic DKP Library
4.4. Purification of Synthetic DKPs
4.5. E. coli Growth and Biofilm Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.B.; Carvalho, I. Diketopiperazines: Biological activity and synthesis. Tetrahedron 2007, 63, 9923–9932. [Google Scholar] [CrossRef]
- Fdhila, F.; Vázquez, V.; Sánchez, J.L.; Riguera, R. dd-Diketopiperazines: Antibiotics Active againstVibrioanguillarumIsolated from Marine Bacteria Associated with Cultures ofPectenmaximus. J. Nat. Prod. 2003, 66, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, M.P. Antimicrobial and Biofilm Inhibiting Diketopiperazines. Curr. Med. Chem. 2012, 19, 3564–3577. [Google Scholar] [CrossRef] [PubMed]
- Cornacchia, C.; Cacciatore, I.; Baldassarre, L.; Mollica, A.; Feliciani, F.; Pinnen, F. 2,5-diketopiperazines as neuroprotective agents. Mini Rev. Med. Chem. 2012, 12, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, P.L. Optical rotation: Recent advances in determining the absolute configuration. Chirality 2002, 14, 768–781. [Google Scholar] [CrossRef]
- Pandiscia, L. Optical Rotation Measurement for Small Sample Amounts. Available online: https://jascoinc.com/applications/optical-rotation-small-volumes/ (accessed on 26 September 2021).
- Marfey, P. Determination ofD-amino acids. II. Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene. Carlsberg Res. Commun. 1984, 49, 591–596. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, S.; Bada, J.L. Diketopiperazine Formation During Investigations of Amino Acid Racemization in Dipeptides. Science 1981, 213, 544–545. [Google Scholar] [CrossRef]
- Adamczeski, M.; Reed, A.R.; Crews, P. New and Known Diketopiperazines from the Caribbean Sponge, Calyx cf. podatypa. J. Nat. Prod. 1995, 58, 201–208. [Google Scholar] [CrossRef]
- Jayatilake, G.S.; Thornton, M.P.; Leonard, A.C.; Grimwade, J.E.; Baker, B.J. Metabolites from an Antarctic Sponge-Associated Bacterium, Pseudomonas aeruginosa. J. Nat. Prod. 1996, 59, 293–296. [Google Scholar] [CrossRef]
- Bull, S.D.; Davies, S.G.; Parkin, R.M.; Sánchez-Sancho, F. The biosynthetic origin of diketopiperazines derived from D-proline. J. Chem. Soc. Perkin Trans. 1 1998, 1, 2313–2320. [Google Scholar] [CrossRef]
- Domzalski, A.; Perez, S.D.; Yoo, B.; Velasquez, A.; Vigo, V.; Pasolli, H.A.; Oldham, A.L.; Henderson, D.P.; Kawamura, A. Uncovering potential interspecies signaling factors in plant-derived mixed microbial culture. Bioorganic Med. Chem. 2021, 42, 116254. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madison, V.; Young, P.E.; Blout, E.R. Cyclic peptides. 14. Conformational energy and circular dichroism of proline-containing cyclic dipeptides. J. Am. Chem. Soc. 1976, 98, 5358–5364. [Google Scholar] [CrossRef]
- Tullberg, M.; Grøtli, M.; Luthman, K. Synthesis of Functionalized, Unsymmetrical 1,3,4,6-Tetrasubstituted 2,5-Diketopiperazines. J. Org. Chem. 2007, 72, 195–199. [Google Scholar] [CrossRef]
- Bofinger, M.R.; de Sousa, L.S.; Fontes, J.E.N.; Marsaioli, A.J. Diketopiperazines as Cross-Communication Quorum-Sensing Signals between Cronobacter sakazakii and Bacillus cereus. ACS Omega 2017, 2, 1003–1008. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.; Lin, Q.; Geske, G.D.; Blackwell, H.E. New and Unexpected Insights into the Modulation of LuxR-Type Quorum Sensing by Cyclic Dipeptides. ACS Chem. Biol. 2009, 4, 1051–1059. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, A.; Feng, L.; Gao, H. Quorum sensing signals affect spoilage of refrigerated large yellow croaker (Pseudosciaena crocea) by Shewanella baltica. Int. J. Food Microbiol. 2016, 217, 146–155. [Google Scholar] [CrossRef]
- Zhu, H.-J. Organic Stereochemistry: Experimental and Computational Methods; Wiley-VCHVerlag GmbH & Co.: Weinheim, Germany, 2015; ISBN 978-3-527-33822-1. [Google Scholar]
- Sun, S.; Ma, K.; Tao, Q.; Han, J.; Bao, L.; Liu, L.; Liu, H. Diketopiperazines and 2H-pyran-2-ones with antioxidant activity from the rice fermented with Aspergillus luchuensis. Fitoterapia 2018, 125, 266–272. [Google Scholar] [CrossRef]
- Ye, G.; Huang, C.; Li, J.; Chen, T.; Tang, J.; Liu, W.; Long, Y. Isolation, Structural Characterization and Antidiabetic Activity of New Diketopiperazine Alkaloids from Mangrove Endophytic Fungus Aspergillus sp. 16-5c. Mar. Drugs 2021, 19, 402. [Google Scholar] [CrossRef] [PubMed]
- Keiderling, T.A. Structure of Condensed Phase Peptides: Insights from Vibrational Circular Dichroism and Raman Optical Activity Techniques. Chem. Rev. 2020, 120, 3381–3419. [Google Scholar] [CrossRef] [PubMed]
- Declerck, V.; Mellor, A.F.P.; Guillot, R.; Aitken, D.J.; Mons, M.; Zehnacker, A. Vibrational circular dichroism as a probe of solid-state organisation of derivatives of cyclic β-amino acids: Cis - and trans -2-aminocyclobutane-1-carboxylic acid. Chirality 2019, 31, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mellor, A.; Zehnacker, A. Vibrational circular dichroism of a 2,5-diketopiperazine (DKP) peptide: Evidence for dimer formation in cyclo LL or LD diphenylalanine in the solid state. Chirality 2017, 29, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hopmann, K.H.; Hudecová, J.; Stensen, W.; Novotná, J.; Urbanová, M.; Svendsen, J.-S.; Bouř, P.; Ruud, K. Absolute Configuration of a Cyclic Dipeptide Reflected in Vibrational Optical Activity: Ab Initio and Experimental Investigation. J. Phys. Chem. A 2012, 116, 2554–2563. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.D.; Vitorino, I.; De La Cruz, M.; Díaz, C.; Cautain, B.; Annang, F.; Pérez-Moreno, G.; Gonzalez, I.; Tormo, J.R.; Martin, J.; et al. Diketopiperazines and other bioactive compounds from bacterial symbionts of marine sponges. Antonie van Leeuwenhoek 2020, 113, 875–887. [Google Scholar] [CrossRef]
- Kim, K.; Kim, N.-J.; Kim, S.Y.; Kim, I.H.; Kim, K.-S.; Lee, G.R. Cyclo(Phe-Pro) Produced by the Human Pathogen Vibrio vulnificus Inhibits Host Innate Immune Responses through the NF-κB Pathway. Infect. Immun. 2015, 83, 1150–1161. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, F.; Bao, X.; Feng, J.; Fu, L. Inhibition of Biogenic Amines in Shewanella baltica by Anthocyanins Involving a Quorum Sensing System. J. Food Prot. 2019, 82, 589–596. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation inPseudomonas fluorescensWCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef] [PubMed]

| Compounds | ε-value (220 nm) |
|---|---|
| 1, 4 | 3700 |
| 2, 3 | 4800 |
| 5, 8 | 1800 |
| 6, 7 | 1800 |
| 9, 12 | 1600 |
| 10, 11 | 1700 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domzalski, A.; Margent, L.; Vigo, V.; Dewan, F.; Pilarsetty, N.V.K.; Xu, Y.; Kawamura, A. Unambiguous Stereochemical Assignment of Cyclo(Phe-Pro), Cyclo(Leu-Pro), and Cyclo(Val-Pro) by Electronic Circular Dichroic Spectroscopy. Molecules 2021, 26, 5981. https://doi.org/10.3390/molecules26195981
Domzalski A, Margent L, Vigo V, Dewan F, Pilarsetty NVK, Xu Y, Kawamura A. Unambiguous Stereochemical Assignment of Cyclo(Phe-Pro), Cyclo(Leu-Pro), and Cyclo(Val-Pro) by Electronic Circular Dichroic Spectroscopy. Molecules. 2021; 26(19):5981. https://doi.org/10.3390/molecules26195981
Chicago/Turabian StyleDomzalski, Alison, Liliana Margent, Valeria Vigo, Faizunnahar Dewan, Naga Vara Kishore Pilarsetty, Yujia Xu, and Akira Kawamura. 2021. "Unambiguous Stereochemical Assignment of Cyclo(Phe-Pro), Cyclo(Leu-Pro), and Cyclo(Val-Pro) by Electronic Circular Dichroic Spectroscopy" Molecules 26, no. 19: 5981. https://doi.org/10.3390/molecules26195981
APA StyleDomzalski, A., Margent, L., Vigo, V., Dewan, F., Pilarsetty, N. V. K., Xu, Y., & Kawamura, A. (2021). Unambiguous Stereochemical Assignment of Cyclo(Phe-Pro), Cyclo(Leu-Pro), and Cyclo(Val-Pro) by Electronic Circular Dichroic Spectroscopy. Molecules, 26(19), 5981. https://doi.org/10.3390/molecules26195981







