Premix Membrane Emulsification: Preparation and Stability of Medium-Chain Triglyceride Emulsions with Droplet Sizes below 100 nm
Abstract
:1. Introduction
2. Results and Discussion
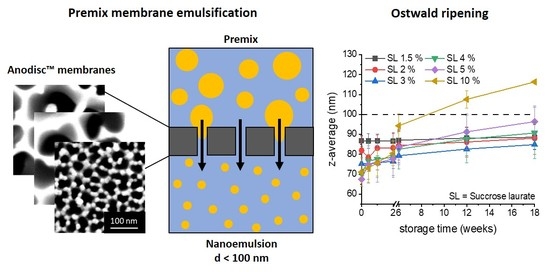
2.1. Membrane Selection and Characterisation
2.2. Determination of Minimum Required Emulsifier Concentration
2.3. Influence of Emulsifier Concentration on Emulsion Stability
2.4. Osmotic Stabilisation
3. Materials and Methods
3.1. Nanoemulsion Preparation
3.2. Osmotic Stabilisation of Nanoemulsions
3.3. Particle Size Measurements
3.4. Determination of Ostwald Ripening Rates
3.5. Scanning Electron Microscopy
3.6. Determination of Critical Micelle Concentration
3.7. Determination of the Concentration of Free SL (Vivaspin Method)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ganta, S.; Talekar, M.; Singh, A.; Coleman, T.P.; Amiji, M.M. Nanoemulsions in translational research—Opportunities and challenges in targeted cancer therapy. AAPS Pharm. Sci. Tech. 2014, 15, 694–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hörmann, K.; Zimmer, A. Drug delivery and drug targeting with parenteral lipid nanoemulsions—A review. J. Control Release 2016, 223, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, D.F. Lipid injectable emulsions: Pharmacopeial and safety issues. Pharm. Res. 2006, 23, 1959–1969. [Google Scholar] [CrossRef]
- Alliod, O.; Valour, J.-P.; Urbaniak, S.; Fessi, H.; Dupin, D.; Charcosset, C. Preparation of oil-in-water nanoemulsions at large-scale using premix membrane emulsification and Shirasu Porous Glass (SPG) membranes. Colloids Surf. A Physicochem. Eng. Asp. 2018, 557, 76–84. [Google Scholar] [CrossRef]
- Vladisavljević, G.T.; Lambrich, U.; Nakajima, M.; Schubert, H. Production of O/W emulsions using SPG membranes, ceramic α-aluminium oxide membranes, microfluidizer and a silicon microchannel plate—a comparative study. Colloids Surf. A Physicochem. Eng. Asp. 2004, 232, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, T.; Shimizu, M.; Kukizaki, M. Membrane emulsification by microporous glass. Key Eng. Mater. 1992, 61, 513–516. [Google Scholar] [CrossRef]
- Nazir, A.; Schroën, K.; Boom, R. Premix emulsification: A review. J. Membr. Sci. 2010, 362, 1–11. [Google Scholar] [CrossRef]
- Joseph, S.; Bunjes, H. Preparation of nanoemulsions and solid lipid nanoparticles by premix membrane emulsification. J. Pharm. Sci. 2012, 101, 2479–2489. [Google Scholar] [CrossRef]
- Suzuki, K.; Shuto, I.; Hagura, Y. Characteristics of the membrane emulsification method combined with preliminary emulsi-fication for preparing corn oil-in-water emulsions. Food Sci. Technol. Int. 1996, 2, 43–47. [Google Scholar]
- Vladisavljević, G.T.; Shimizu, M.; Nakashima, T. Preparation of monodisperse multiple emulsions at high production rates by multi-stage premix membrane emulsification. J. Membr. Sci. 2004, 244, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Kukizaki, M. Preparation of solid lipid microcapsules via solid-in-oil-in-water dispersions by premix membrane emulsification. Chem. Eng. J. 2009, 151, 387–396. [Google Scholar] [CrossRef]
- Vladisavljević, G.T.; Shimizu, M.; Nakashima, T. Production of multiple emulsions for drug delivery systems by repeated SPG membrane homogenization: Influence of mean pore size, interfacial tension and continuous phase viscosity. J. Membr. Sci. 2006, 284, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Nazir, A.; Schroën, K.; Boom, R. High-throughput premix membrane emulsification using nickel sieves having straight-through pores. J. Membr. Sci. 2011, 383, 116–123. [Google Scholar] [CrossRef]
- Trentin, A.; Güell, C.; López, F.; Ferrando, M. Microfiltration membranes to produce BSA-stabilized O/W emulsions by premix membrane emulsification. J. Membr. Sci. 2010, 356, 22–32. [Google Scholar] [CrossRef]
- Surh, J.; Jeong, Y.G.; Vladisavljević, G.T. On the preparation of lecithin-stabilized oil-in-water emulsions by multi-stage premix membrane emulsification. J. Food Eng. 2008, 89, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, H.S.; Rico, L.G.; Badolato, G.G.; Schubert, H. Production of O/W emulsions containing astaxanthin by repeated premix membrane emulsification. J. Food Sci. 2005, 70, E117–E123. [Google Scholar] [CrossRef]
- Gehrmann, S.; Bunjes, H. Instrumented small scale extruder to investigate the influence of process parameters during premix membrane emulsification. Chem. Eng. J. 2016, 284, 716–723. [Google Scholar] [CrossRef]
- Gehrmann, S.; Bunjes, H. Preparation of nanoemulsions by premix membrane emulsification: Which parameters have a sig-nificant influence on the resulting particle size? J. Pharm. Sci. 2017, 106, 2068–2076. [Google Scholar] [CrossRef]
- Joseph, S.; Bunjes, H. Influence of membrane structure on the preparation of colloidal lipid dispersions by premix membrane emulsification. Int. J. Pharm. 2013, 446, 59–62. [Google Scholar]
- Joseph, S.; Bunjes, H. Evaluation of Shirasu Porous Glass (SPG) membrane emulsification for the preparation of colloidal lipid drug carrier dispersions. Eur J Pharm Biopharm. 2014, 87, 178–186. [Google Scholar] [CrossRef]
- Zhou, Q.-Z.; Ma, G.-H.; Su, Z.-G. Effect of membrane parameters on the size and uniformity in preparing agarose beads by premix membrane emulsification. J. Membr. Sci. 2009, 326, 694–700. [Google Scholar] [CrossRef]
- Gehrmann, S.; Bunjes, H. Influence of membrane material on the production of colloidal emulsions by premix membrane emulsification. Eur. J. Pharm. Biopharm. 2018, 126, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.H.; Filippin-Monteiro, F.B.; Mattei, B.; Zanetti-Ramos, B.G.; Creczynski-Pasa, T.B. In vitro biocompatibility of solid lipid nanoparticles. Sci. Total Environ. 2012, 432, 382–388. [Google Scholar] [CrossRef]
- Whatman Anodisc Circle with Support Ring. Available online: https://www.cytivalifesciences.com/en/fr/shop/whatman-laboratory-filtration/membranes-circles-sheets-and-reels/anopore-membranes/anodisc-circle-with-support-ring-p-05830 (accessed on 12 July 2021).
- Silva, K.A.; Rocha-Leão, M.H.; Coelho, M.A.Z. Evaluation of aging mechanisms of olive oil–lemon juice emulsion through digital image analysis. J. Food Eng. 2010, 97, 335–340. [Google Scholar] [CrossRef]
- Schmitt, V.; Cattelet, C.; Leal-Calderon, F. Coarsening of alkane-in-water emulsions stabilized by nonionic poly(oxyethylene) surfactants: The role of molecular permeation and coalescence. Langmuir 2004, 20, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Urbina-Villalba, G.; Forgiarini, A.; Rahn, K.; Lozsán, A. Influence of flocculation and coalescence on the evolution of the average radius of an O/W emulsion. Is a linear slope of vs. t an unmistakable signature of Ostwald ripening? Phys. Chem. Chem. Phys. 2009, 11, 11184–11195. [Google Scholar] [CrossRef] [PubMed]
- Bunjes, H. Lipid nanoparticles for the delivery of poorly water-soluble drugs. J. Pharm. Pharmacol. 2010, 62, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Jäschke, C. Herstellung pharmazeutischer Nanoemulsionen mittels Premix-Membranemulgierung. Master thesis, Technische Universität Braunschweig, Braunschweig, Germany, 2017. [Google Scholar]
- Zhao, Y.; Liu, A.; Du, Y.; Cao, Y.; Zhang, E.; Zhou, Q.; Hai, H.; Zhen, Y.; Zhang, S. Effects of sucrose ester structures on lipo-some-mediated gene delivery. Acta Biomater. 2018, 72, 278–286. [Google Scholar] [CrossRef]
- Gehrmann, S.; Bunjes, H. Preparation of lipid nanoemulsions by premix membrane emulsification with disposable materials. Int. J. Pharm. 2016, 511, 741–744. [Google Scholar] [CrossRef]
- Anodisc Circle with Support Ring, 47 mm, 0.02 µm Pore Size: Product Support. Available online: https://www.cytivalifesciences.com/en/fr/support/products/anodisc-circle-with-support-ring-47-mm-0-02-mm-pore-size-28420411 (accessed on 12 July 2021).
- Hunter, D.G.; Frisken, B.J. Effect of extrusion pressure and lipid properties on the size and polydispersity of lipid vesicles. Biophys. J. 1998, 74, 2996–3002. [Google Scholar] [CrossRef] [Green Version]
- Binks, B.P.; Clint, J.H.; Fletcher, P.D.I.; Rippon, S.; Lubetkin, S.D.; Mulqueen, P.J. Kinetics of swelling of oil-in-water emulsions stabilized by different surfactants. Langmuir 1999, 15, 4495–4501. [Google Scholar] [CrossRef]
- Nguyen Hoang, T.K.; La, V.B.; Deriemaeker, L.; Finsy, R. Ostwald ripening and solubilization in alkane in water emulsions stabilized by different surfactants. Phys. Chem. Chem. Phys. 2004, 6, 1413–1422. [Google Scholar] [CrossRef]
- Ariyaprakai, S.; Dungan, S.R. Influence of surfactant structure on the contribution of micelles to Ostwald ripening in oil-in-water emulsions. J. Colloid Interface Sci. 2010, 343, 102–108. [Google Scholar] [CrossRef]
- Han, S.W.; Song, H.Y.; Moon, T.W.; Choi, S.J. Influence of emulsion interfacial membrane characteristics on Ostwald ripening in a model emulsion. Food Chem. 2018, 242, 91–97. [Google Scholar] [CrossRef]
- Weiss, J.; Canceliere, C.; McClements, D.J. Mass transport phenomena in oil-in-water emulsions containing surfactant micelles: Ostwald ripening. Langmuir 2000, 16, 6833–6838. [Google Scholar] [CrossRef]
- De Smet, Y.; Deriemaeker, L.; Parloo, E.; Finsy, R. On the determination of Ostwald ripening rates from dynamic light scattering measurements. Langmuir 1999, 15, 2327–2332. [Google Scholar] [CrossRef]
- Park, E.-J.; Lee, E.-S.; Hong, S.-T. A study on the formation and Ostwald ripening stability of nanoemulsion with various emulsifiers. J. Korean Oil Chem. Soc. 2015, 32, 536–545. [Google Scholar] [CrossRef]
- Walstra, P. Physical Chemistry of Foods; Marcel Dekker, Inc.: New York, NY, USA, 2003; p. 477. [Google Scholar]
- Wagner, C. Theorie der Alterung von Niederschlägen durch Umlösen (Ostwald-Reifung). Z. Elektrochem. 1961, 65, 581–591. [Google Scholar]
- Lifshitz, I.M.; Slyozov, V.V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Kabalnov, A.S. Can micelles mediate a mass transfer between oil droplets? Langmuir 1994, 10, 680–684. [Google Scholar] [CrossRef]
- Taylor, P. Otwald ripening in emulsions. Adv. Colloid Interface Sci. 1998, 75, 107–163. [Google Scholar] [CrossRef]
- Tadros, T.F. Formulation of Disperse Systems: Science and Technology; Wiley-VCH: Weinheim, Germany, 2014; p. 287. [Google Scholar]
- Landfester, K.; Bechthold, N.; Tiarks, F.; Antonietti, M. Formulation and stability mechanisms of polymerizable miniemulsions. Macromolecules 1999, 32, 5222–5228. [Google Scholar] [CrossRef]
- Wooster, T.J.; Golding, M.; Sanguansri, P. Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir 2008, 24, 12758–12765. [Google Scholar] [CrossRef]
- Jang, Y.; Park, J.; Song, H.Y.; Choi, S.J. Ostwald ripening rate of orange oil emulsions: Effects of molecular structure of emul-sifiers and their oil composition. J. Food Sci. 2019, 84, 440–447. [Google Scholar] [CrossRef]
- McClements, D.J.; Henson, L.; Popplewell, L.M.; Decker, E.A.; Choi, S.J. Inhibition of Ostwald ripening in model beverage emulsions by addition of poorly water soluble triglyceride oils. J. Food Sci. 2012, 77, C33–C38. [Google Scholar] [CrossRef]
- Webster, A.J.; Cates, M.E. Stabilization of emulsions by trapped species. Langmuir 1998, 14, 2068–2079. [Google Scholar] [CrossRef]
- Landfester, K. Miniemulsions for nanoparticle synthesis. Top. Curr. Chem. 2003, 227, 75–123. [Google Scholar]
- Alskär, L.C.; Porter, C.J.H.; Bergström, C.A.S. Tools for early prediction of drug loading in lipid-based formulations. Mol. Pharm. 2016, 13, 251–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, L.C.; Porter, C.J.H.; Charman, W.N.; Bergström, C.A.S. Computational prediction of drug solubility in lipid based formulation excipients. Pharm. Res. 2013, 30, 3225–3237. [Google Scholar] [CrossRef]







| Anodisc™ Membrane Type | Feretmax ± SD [nm] | Resulting Particle Size d50 ± SD [nm] 1 | d50/dp Feretmax Pore Size | d50/dp Nominal Pore Size |
|---|---|---|---|---|
| 200 nm 100 nm 20 nm | 156 ± 32 n = 1960 144 ± 33 n = 2024 86 ± 40 n = 283 | 166 ± 8 | 1.06 | 0.83 |
| 158 ± 12 | 1.10 | 1.58 | ||
| 81 ± 1 | 0.95 | 4.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Hawari, L.; Bunjes, H. Premix Membrane Emulsification: Preparation and Stability of Medium-Chain Triglyceride Emulsions with Droplet Sizes below 100 nm. Molecules 2021, 26, 6029. https://doi.org/10.3390/molecules26196029
El-Hawari L, Bunjes H. Premix Membrane Emulsification: Preparation and Stability of Medium-Chain Triglyceride Emulsions with Droplet Sizes below 100 nm. Molecules. 2021; 26(19):6029. https://doi.org/10.3390/molecules26196029
Chicago/Turabian StyleEl-Hawari, Lara, and Heike Bunjes. 2021. "Premix Membrane Emulsification: Preparation and Stability of Medium-Chain Triglyceride Emulsions with Droplet Sizes below 100 nm" Molecules 26, no. 19: 6029. https://doi.org/10.3390/molecules26196029