Synthesis and Photocatalytic Activity of TiO2/CdS Nanocomposites with Co-Exposed Anatase Highly Reactive Facets
Abstract
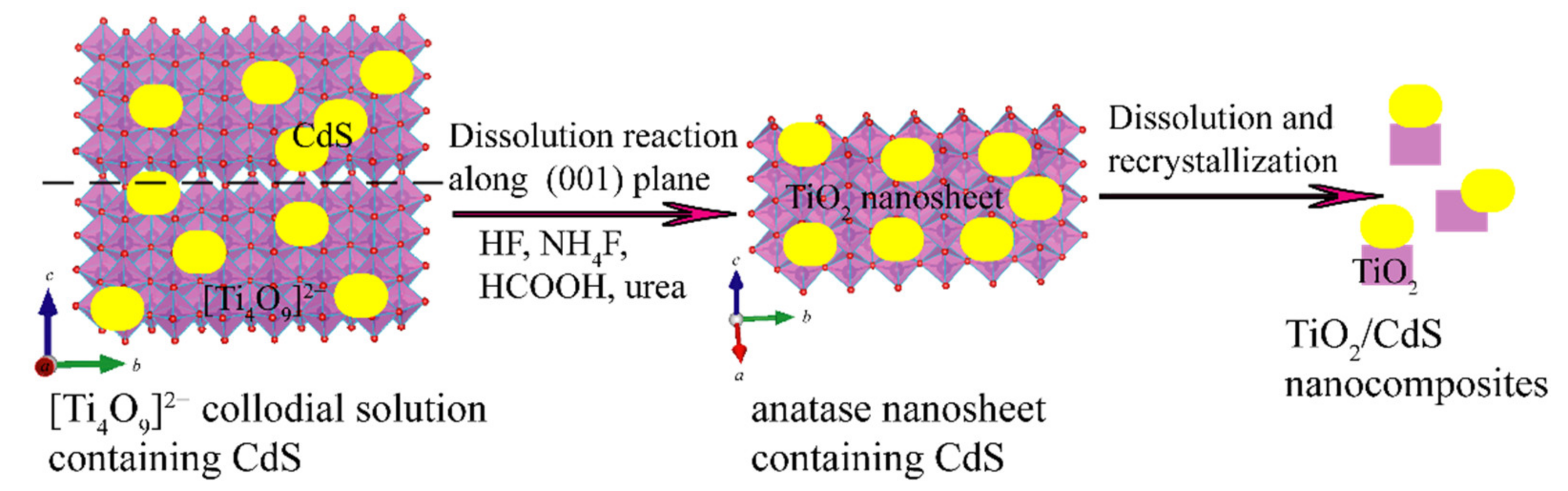
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CdS Nanoparticles
2.2. Preparation of [Ti4O9]2− Colloidal Solution from H2Ti4O9
2.3. Characterization
2.4. Measurement of Photocatalytic Activity
3. Results and Discussion
3.1. XRD Analysis
3.2. FESEM and FESEM-EDS Analysis
3.3. TEM and HRTEM Analysis
3.4. Nitrogen Adsorption-Desorption Isotherms Analysis
3.5. Photoluminescence Analysis
3.6. Electrochemical Impedance Spectroscopy Analysis
3.7. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Gangwar, J.; Srivastava, A.K. Multiphase TiO2 nanostructures: A review of efficient synthesis, growth mechanism, probing capabilities, and applications in bio-safety and health. RSC Adv. 2017, 7, 44199–44224. [Google Scholar] [CrossRef] [Green Version]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Shi, H.; Jin, B.; Qin, X.; Wang, G.; Li, K.; Zhang, T.; Zhang, H. In-site synthesis of an inorganic-framework molecular imprinted TiO2/CdS heterostructure for the photoelectrochemical sensing of bisphenol A. Anal. Methods 2021, 13, 2857–2864. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Feng, S.Q.; Tang, N.; Zhang, S.B.; Zhang, X.Y.; Liu, B. Fabrication of TiO2/Fe2O3/CdS systems: Effects of Fe2O3 and CdS content on superior photocatalytic activity. RSC Adv. 2021, 11, 10300–10308. [Google Scholar] [CrossRef]
- Xu, K.; Liu, Z.; Qi, S.; Yin, Z.; Deng, S.; Zhang, M.; Sun, Z. Construction of Ag-modified TiO2/ZnO heterojunction nanotree arrays with superior photo-catalytic and photoelectrochemical properties. RSC Adv. 2020, 10, 34702–34711. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Abdi, V.; Sourinejad, I. Green fabrication of Ag/AgCl@TiO2 superior plasmonic nanocomposite: Biosynthesis, characterization and photocatalytic activity under sunlight. J. Alloys Compd. 2020, 841, 155593. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhao, S.; Han, Z.; Zhang, Y.; Zhao, X.; Kang, L. A novel preparation of Ag@TiO2 tubes and their potent photocatalytic degradation efficiency. CrystEngComm 2016, 18, 8756–8761. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Liu, L.; Hu, J.; Cui, W. Highly ordered TiO2 nanotube arrays wrapped with g-C3N4 nanoparticles for efficient charge separation and increased photoelectrocatalytic degradation of phenol. J. Hazard. Mater. 2018, 344, 369–380. [Google Scholar] [CrossRef]
- Liu, J.; Meeprasert, J.; Namuangruk, S.; Zha, K.; Li, H.; Huang, L.; Shi, L.-Y.; Zhang, D. Facet-activity relationship of TiO2 in Fe2O3/TiO2 nanocatalysts for selective cat-alytic reduction of NO with NH3: In situ DRIFTs and DFT studies. J. Phys. Chem. C 2017, 121, 4970–4979. [Google Scholar] [CrossRef]
- David, S.; Mahadik, M.A.; Chung, H.S.; Ryu, J.H.; Jang, J.S. Facile hydrothermally synthesized a novel CdS nanoflower/rutile-TiO2 nanorod heterojunction photoanode used for photoelectrocatalytic hydrogen generation. ACS Sustain. Chem. Eng. 2017, 5, 7537–7548. [Google Scholar] [CrossRef]
- Li, G.; Huang, J.; Chen, J.; Deng, Z.; Huang, Q.; Liu, Z.; Guo, W.; Cao, R. Highly active photocatalyst of Cu2O/TiO2 octahedron for hydrogen generation. ACS Omega 2019, 4, 3392–3397. [Google Scholar] [CrossRef]
- Du, Y.E.; Li, W.; Bai, Y.; Huangfu, Z.; Wang, W.; Chai, R.; Feng, Q. Facile synthesis of TiO2/Ag3PO4 composites with co-exposed high-energy facets for efficient photodegradation of rhodamine B solution under visible light irradiation. RSC Adv. 2020, 10, 24555–24569. [Google Scholar] [CrossRef]
- Luo, X.; Deng, F.; Min, L.; Luo, S.; Guo, B.; Zeng, G.; Au, C. Facile one-step synthesis of inorganic-framework molecularly imprinted TiO2/WO3 nano-composite and its molecular recognitive photocatalytic degradation of target contaminant. Environ. Sci. Technol. 2013, 47, 7404–7412. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, X.; Zhang, L.; Lu, Z.; Zhang, E.; Wang, H.; Ji, Z. Constructing a novel n–p–n dual heterojunction between anatase TiO2 nanosheets with co-exposed {101}, {001} facets and porous ZnS for enhancing photocatalytic activity. J. Phys. Chem. C 2017, 121, 6133–6140. [Google Scholar] [CrossRef]
- Wan, Y.; Han, M.; Yu, L.; Yi, G.; Jia, J. 3D Bi2S3salix leaf-like nanosheet/TiO2nanorod branched heterostructure arrays for improving photoelectrochemical properties. CrystEngComm 2016, 18, 1577–1584. [Google Scholar] [CrossRef]
- Devaraji, P.; Gao, R.; Xiong, L.; Jia, X.; Huang, L.; Chen, W.; Liu, S.; Mao, L. Usage of natural leaf as a bio-template to inorganic leaf: Leaf structure black TiO2/CdS heterostructure for efficient photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2021, 46, 14369–14383. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Robert, D.; Weber, J.V. Bi2S3/TiO2 and CdS/TiO2 heterojunctions as an available configuration for photo-catalytic degradation of organic pollutant. J. Photoch. Photobio. A 2004, 163, 569–580. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, C.-F. Recent advances in the TiO2/CdS nanocomposite used for photocatalytic hydrogen production and quantum-dot-sensitized solar cells. Renew. Sustain. Energy Rev. 2016, 54, 1048–1059. [Google Scholar] [CrossRef]
- Gao, B.; Yuan, X.; Lu, P.; Lin, B.; Chen, Y. Enhanced visible-light-driven photocatalytic H2-production activity of CdS-loaded TiO2 microspheres with exposed (001) facets. J. Phys. Chem. Solids 2015, 87, 171–176. [Google Scholar] [CrossRef]
- Wang, X.; Liu, G.; Wang, L.; Pan, J.; Lu, G.Q.M.; Cheng, H.M. TiO2 films with oriented anatase {001} facets and their photoelectrochemical behavior as CdS nanoparticle sensitized photoanodes. J. Mater. Chem. 2011, 21, 869–873. [Google Scholar] [CrossRef]
- Dai, K.; Lv, J.; Zhang, J.; Zhu, G.; Geng, L.; Liang, C. Efficient visible-light-driven splitting of water into hydrogen over surface-fluorinated anatase TiO2 nanosheets with exposed {001} facets/layered CdS-diethylenetriamine nanobelts. ACS Sustain. Chem. Eng. 2018, 6, 12817–12826. [Google Scholar] [CrossRef]
- Ma, Z.; Shi, L.; Qu, W.; Hu, Q.; Chen, R.; Wang, Y.; Chen, Z. Microwave-assisted synthesis of an RGO/CdS/TiO2 step-scheme with exposed TiO2 {001} facets and enhanced visible photocatalytic activity. RSC Adv. 2020, 10, 43447–43458. [Google Scholar] [CrossRef]
- Liu, M.; Piao, L.; Zhao, L.; Ju, S.; Yan, Z.; He, T.; Wang, W. Anatase TiO2 single crystals with exposed {001} and {110} facets: Facile synthesis and en-hanced photocatalysis. Chem. Commun. 2010, 46, 1664–1666. [Google Scholar] [CrossRef]
- Xu, H.; Reunchan, P.; Ouyang, S.; Tong, H.; Umezawa, N.; Kako, T.; Ye, J. Anatase TiO2 single crystals exposed with high-reactive {111} facets toward efficient H2 evolution. Chem. Mater. 2013, 25, 405–411. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Chai, S.P.; Yong, S.T.; Mohamed, A.R. Highly reactive {001} facets of TiO2-based composites: Synthesis, formation mechanism and characterization. Nanoscale 2014, 6, 1946–2008. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [Green Version]
- Sadovnikov, A.; Baranchikov, A.; Zubavichus, Y.; Ivanova, O.; Murzin, V.; Kozik, V.; Ivanov, V. Photocatalytically active fluorinated nano-titania synthesized by microwave-assisted hydrothermal treatment. J. Photochem. Photobiol. A 2015, 303, 36–43. [Google Scholar] [CrossRef]
- Han, X.; Wang, X.; Xie, S.; Kuang, Q.; Ouyang, J.; Xie, Z.; Zheng, L. Carbonate ions-assisted syntheses of anatase TiO2 nanoparticles exposed with high energy (001) facets. RSC Adv. 2012, 2, 3251–3253. [Google Scholar] [CrossRef]
- Yang, W.; Li, J.; Wang, Y.; Zhu, F.; Shi, W.; Wan, F.; Xu, D.A. facile synthesis of anatase TiO2 nanosheets-based hierarchical spheres with over 90% {001} facets for dye-sensitized solar cells. Chem. Commun. 2011, 47, 1809–1811. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, W.; Chen, C.; Ji, H.; Zhao, J. Anatase TiO2 mesocrystals enclosed by (001) and (101) Facets: Synergistic Effects between Ti3+and Facets for Their Photocatalytic Performance. Chem.-A Eur. J. 2012, 18, 12584–12589. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Du, Y.-E.; Niu, X.; Li, W.; Li, J.; Yang, X.; Feng, Q. Synthesis, transformation mechanism and photocatalytic properties of various morphologies anatase TiO2 nanocrystals derived from tetratitanate nanobelts. Chemistry 2018, 3, 9953–9959. [Google Scholar] [CrossRef]
- Du, Y.E.; Niu, X.; Bai, Y.; Qi, H.; Guo, Y.; Chen, Y.; Feng, Q. Synthesis of anatase TiO2 Nanocrystals with defined morphologies from exfoliated nanorib-bons: Photocatalytic performance and application in dye-sensitized solar cell. ChemistrySelect 2019, 4, 4443–4457. [Google Scholar] [CrossRef]
- Du, Y.E.; Bai, Y.; Liu, Y.; Guo, Y.; Cai, X.; Feng, Q. One-pot synthesis of [111]-/{010} facets coexisting anatase nanocrystals with enhanced dye-sensitized solar cell performance. ChemistrySelect 2016, 1, 6632–6640. [Google Scholar] [CrossRef]
- Dong, S.; Ding, X.; Guo, T.; Yue, X.; Han, X.; Sun, J. Self-assembled hollow sphere shaped Bi 2 WO 6 /RGO composites for efficient sunlight-driven photocatalytic degradation of organic pollutants. Chem. Eng. J. 2017, 316, 778–789. [Google Scholar] [CrossRef]
- Yu, T.; Hu, W.L.; Jia, L.; Tan, X.; Huang, J.; Huang, X. Enhanced photoelectrochemical performance of coaxial-nanocoupled strontium-rich SrTiO3/TiO2 {001} nanotube arrays. Ind. Eng. Chem. Res. 2015, 54, 8193–8200. [Google Scholar] [CrossRef]
- Du, Y.E.; Feng, Q.; Chen, C.; Tanaka, Y.; Yang, X. Photocatalytic and dye-sensitized solar cell performances of {010}-faceted and [111]-faceted anatase TiO2 nanocrystals synthesized from tetratitanate nanoribbons. ACS Appl. Mater. Interf. 2014, 6, 16007–16019. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, S.; Wang, L.; Liu, R.; Zeng, Y.; Xia, X.; Liu, Y.; Luo, S. Facile synthesis of bird’s nest-like TiO 2 microstructure with exposed (001) facets for photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2017, 391, 228–235. [Google Scholar] [CrossRef]
- Cao, Y.; Zong, L.; Li, Q.; Li, C.; Li, J.; Yang, J. Solvothermal synthesis of TiO2 nanocrystals with {001} facets using titanic acid nanobelts for superior photocatalytic activity. Appl. Surf. Sci. 2017, 391, 311–317. [Google Scholar] [CrossRef]
- Liu, G.M.; Jia, W.Y.; Jiang, Q.S.; Cheng, Z.Q. Controllable growth of three-dimensional CdS nanoparticles on TiO2 nanotubes to en-hance photocatalytic activity. RSC Adv. 2020, 10, 16776–16782. [Google Scholar] [CrossRef]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Shang, Q.; Huang, X.; Tan, X.; Yu, T. High activity Ti3+-modified brookite TiO2/graphene nanocomposites with specific facets exposed for water splitting. Ind. Eng. Chem. Res. 2017, 56, 9098–9106. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Yu, W.; Jiang, F.; Zhang, E.; Wang, H.; Ji, Z. Novel dual heterojunction between MoS2 and anatase TiO2 with coexposed {101} and {001} facets. J. Am. Ceram. Soc. 2017, 100, 5274–5285. [Google Scholar] [CrossRef]
- Yu, J.C.C.; Nguyen, V.H.; Lasek, J.; Wu, J.C. Titania nanosheet photocatalysts with dominantly exposed (001) reactive facets for photocatalytic NOx abatement. Appl. Catal. B-Environ. 2017, 219, 391–400. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, J.; Wang, Y.; Wu, M.; Meng, M.; Tian, Y.; Gong, J. Insights into the effects of surface/bulk defects on photocatalytic hydrogen evolution over TiO2 with exposed {001} facets. Appl. Catal. B-Environ. 2018, 220, 126–136. [Google Scholar] [CrossRef]
- Liu, X.; Du, G.; Li, M. True photoreactivity origin of Ti3+-doped anatase TiO2 crystals with respectively dominated exposed {001}, {101}, and {100} facets. ACS Omega 2019, 4, 14902–14912. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Tang, Y.; Tay, Q.; Zhang, Y.; Malyi, O.I.; Wang, D.; Leng, J.; Lai, Y.; Zhou, H.; Chen, Z.; et al. Understanding the role of nanostructures for efficient hydrogen generation on immo-bilized photocatalysts. Adv. Energy Mater. 2013, 3, 1368–1380. [Google Scholar] [CrossRef]
- Kong, M.; Li, Y.; Chen, X.; Tian, T.; Fang, P.; Zheng, F.; Zhao, X. Tuning the relative concentration ratio of bulk defects to surface defects in TiO2 nanocrystals leads to high photocatalytic efficiency. J. Am. Chem. Soc. 2011, 133, 16414–16417. [Google Scholar] [CrossRef]
- Wei, X.-X.; Cui, B.; Wang, X.; Cao, Y.; Gao, L.; Guo, S.; Chen, C.-M. Tuning the physico-chemical properties of BiOBr via solvent adjustment: Towards an efficient photocatalyst for water treatment. CrystEngComm 2019, 21, 1750–1757. [Google Scholar] [CrossRef]
- Zheng, Y.; Lv, K.; Wang, Z.; Deng, K.; Li, M. Microwave-assisted rapid synthesis of anatase TiO2 nanocrystals with exposed {001} facets. J. Mol. Catal. A-Chem. 2012, 356, 137–143. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, W.; Tanaka, Y.; Kusunose, N.; Peng, Y.; Feng, Q. Mesocrystalline nanocomposites of TiO2 polymorphs: Topochemical mesocrystal conversion, characterization, and photocatalytic response. Cryst. Growth Des. 2015, 15, 1214–1225. [Google Scholar] [CrossRef]
- Pan, J.; Liu, G.; Lu, G.Q.; Cheng, H.M. On the true photoreactivity order of {001}, {010}, and {101} facets of anatase TiO2 crystals. Angew. Chem. Int. Ed. 2011, 50, 2133–2137. [Google Scholar] [CrossRef]









| Sample | K2Ti4O9 | H2Ti4O9 | CdS | |||
|---|---|---|---|---|---|---|
| Element | Atom% | Mass% | Atom% | Mass% | Atom% | Mass% |
| C | 4.869 | 2.019 | 0.000 | 0.000 | 0.889 | 0.136 |
| O | 49.968 | 27.593 | 47.102 | 23.269 | 0.051 | 0.010 |
| K | 11.328 | 15.286 | 2.027 | 2.448 | 0.000 | 0.000 |
| Ti | 33.835 | 35.103 | 50.005 | 73.908 | 0.000 | 0.000 |
| N | 0.000 | 0.000 | 0.866 | 0.375 | 1.010 | 0.180 |
| S | 0.000 | 0.000 | 0.000 | 0.000 | 39.849 | 16.286 |
| Cd | 0.000 | 0.000 | 0.000 | 0.000 | 58.202 | 83.387 |
| Sample | HF-TiO2/CdS | NH4F-TiO2/CdS | FMA-TiO2/CdS | Urea-TiO2/CdS | ||||
|---|---|---|---|---|---|---|---|---|
| Element | Atom% | Mass% | Atom% | Mass% | Atom% | Mass% | Atom% | Mass% |
| C | 0.844 | 0.201 | 3.319 | 1.107 | 4.586 | 1.608 | 2.927 | 0.988 |
| N | 4.887 | 1.358 | 1.985 | 0.772 | 2.218 | 0.907 | 2.092 | 0.823 |
| O | 30.291 | 9.614 | 38.045 | 16.909 | 45.319 | 21.175 | 42.897 | 19.288 |
| F | 0.087 | 0.033 | 2.391 | 1.262 | 0.000 | 0.000 | 0.000 | 0.000 |
| S | 19.117 | 12.161 | 4.897 | 4.362 | 3.851 | 3.607 | 4.257 | 3.837 |
| Ti | 18.127 | 17.212 | 43.815 | 58.260 | 38.106 | 53.268 | 41.916 | 56.388 |
| Cd | 26.648 | 59.422 | 5.549 | 17.328 | 5.920 | 19.435 | 5.911 | 18.675 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.-e.; Niu, X.; He, X.; Hou, K.; Liu, H.; Zhang, C. Synthesis and Photocatalytic Activity of TiO2/CdS Nanocomposites with Co-Exposed Anatase Highly Reactive Facets. Molecules 2021, 26, 6031. https://doi.org/10.3390/molecules26196031
Du Y-e, Niu X, He X, Hou K, Liu H, Zhang C. Synthesis and Photocatalytic Activity of TiO2/CdS Nanocomposites with Co-Exposed Anatase Highly Reactive Facets. Molecules. 2021; 26(19):6031. https://doi.org/10.3390/molecules26196031
Chicago/Turabian StyleDu, Yi-en, Xianjun Niu, Xinru He, Kai Hou, Huiling Liu, and Caifeng Zhang. 2021. "Synthesis and Photocatalytic Activity of TiO2/CdS Nanocomposites with Co-Exposed Anatase Highly Reactive Facets" Molecules 26, no. 19: 6031. https://doi.org/10.3390/molecules26196031





