Laccase-Catalyzed Oxidation of Allylbenzene Derivatives: Towards a Green Equivalent of Ozonolysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Enzymatic Reaction of Methyleugenol
3.1.1. Controls
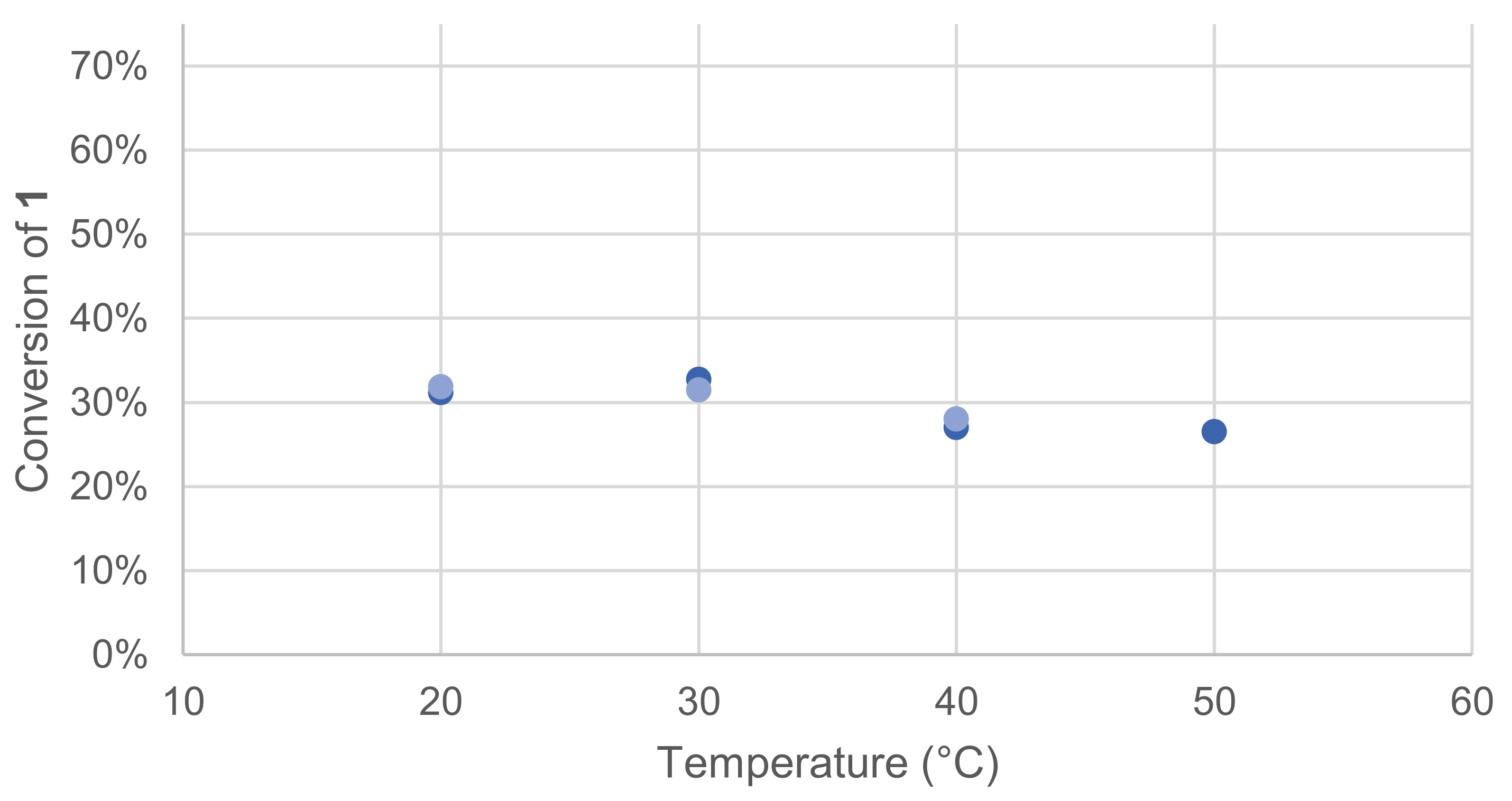
3.1.2. Effect of pH and Temperature
3.1.3. Effect of Co-Solvent
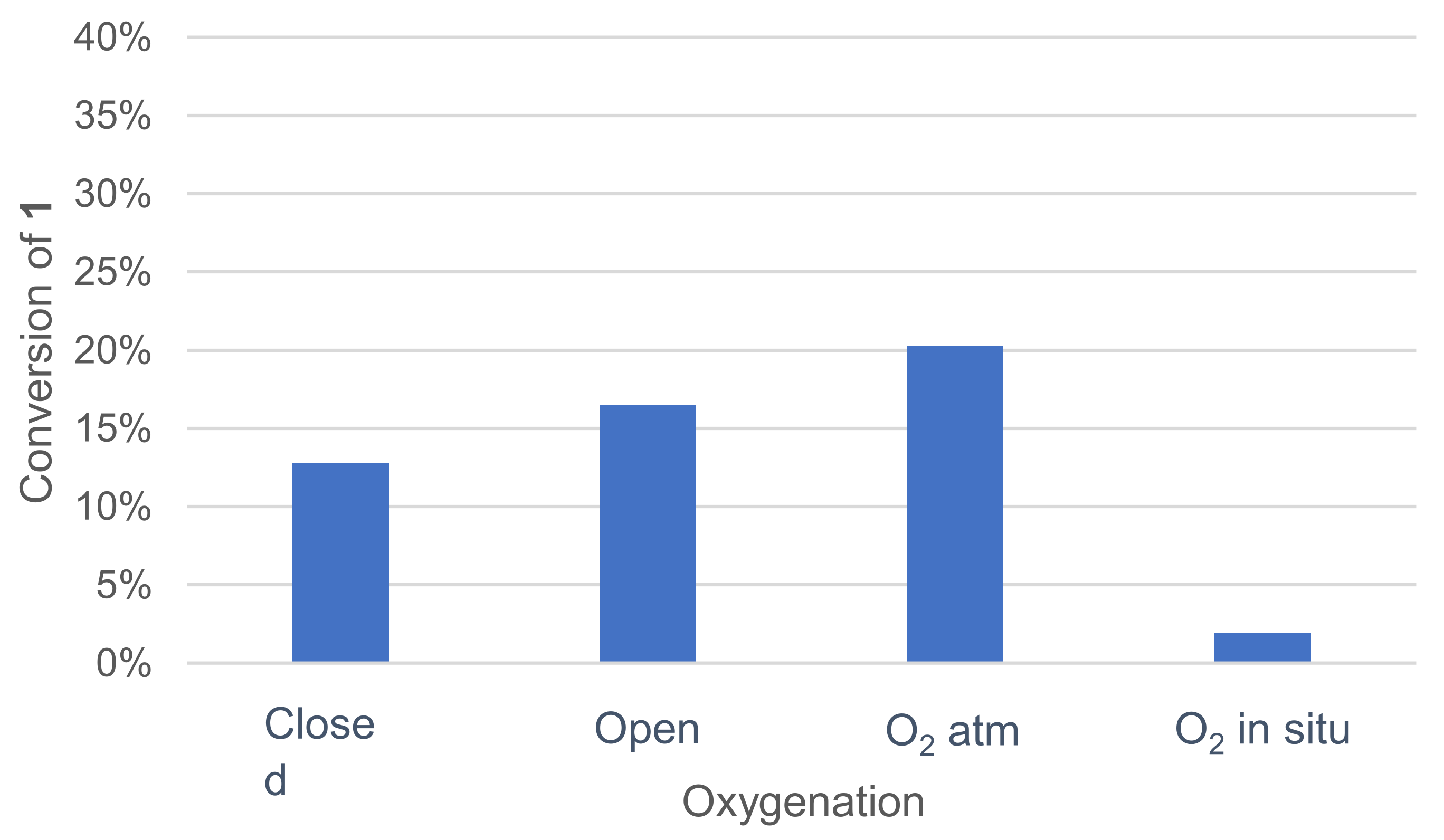
3.1.4. Effect of Oxygenation of the Medium
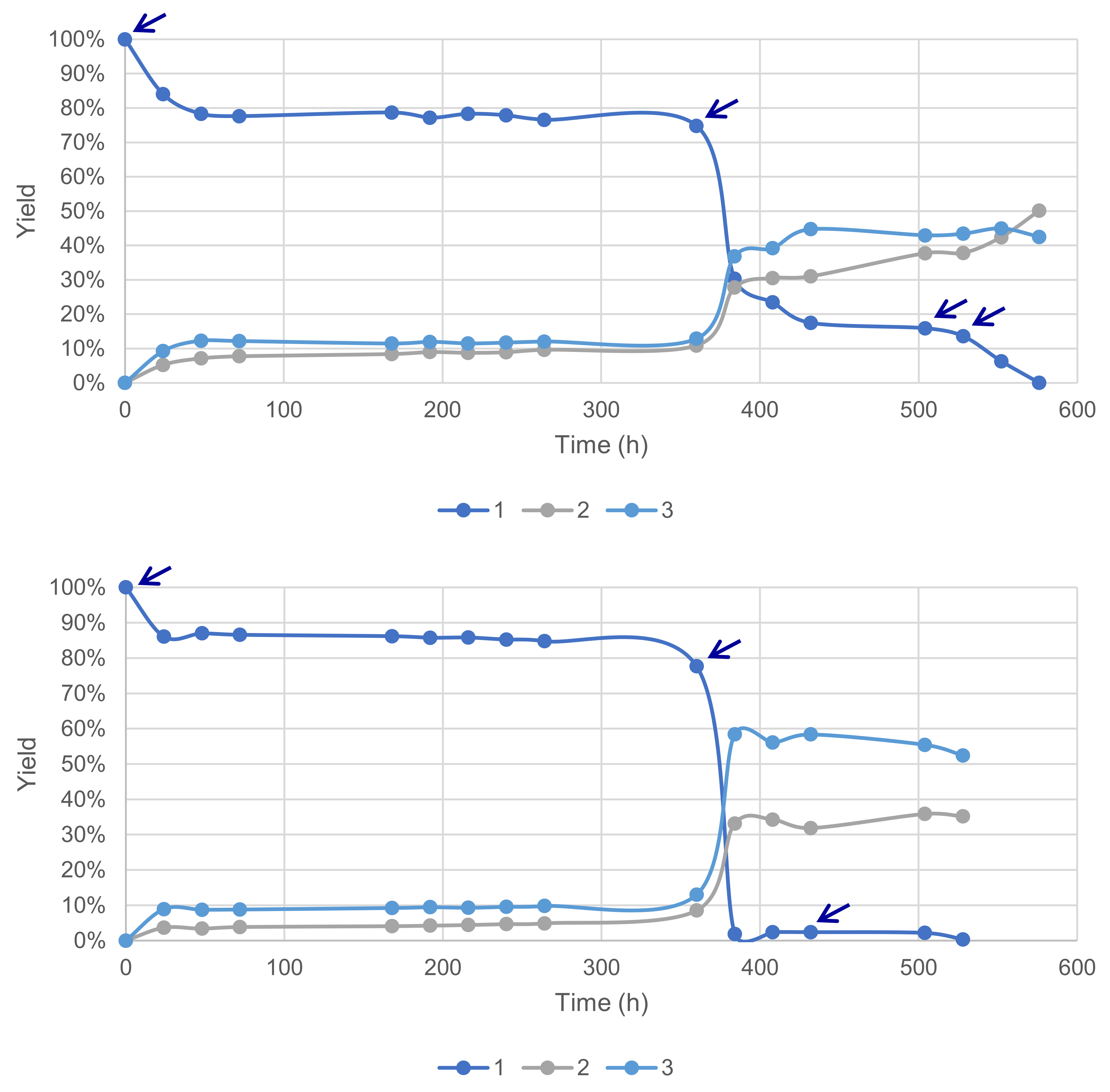
3.1.5. Effect of Reaction Time
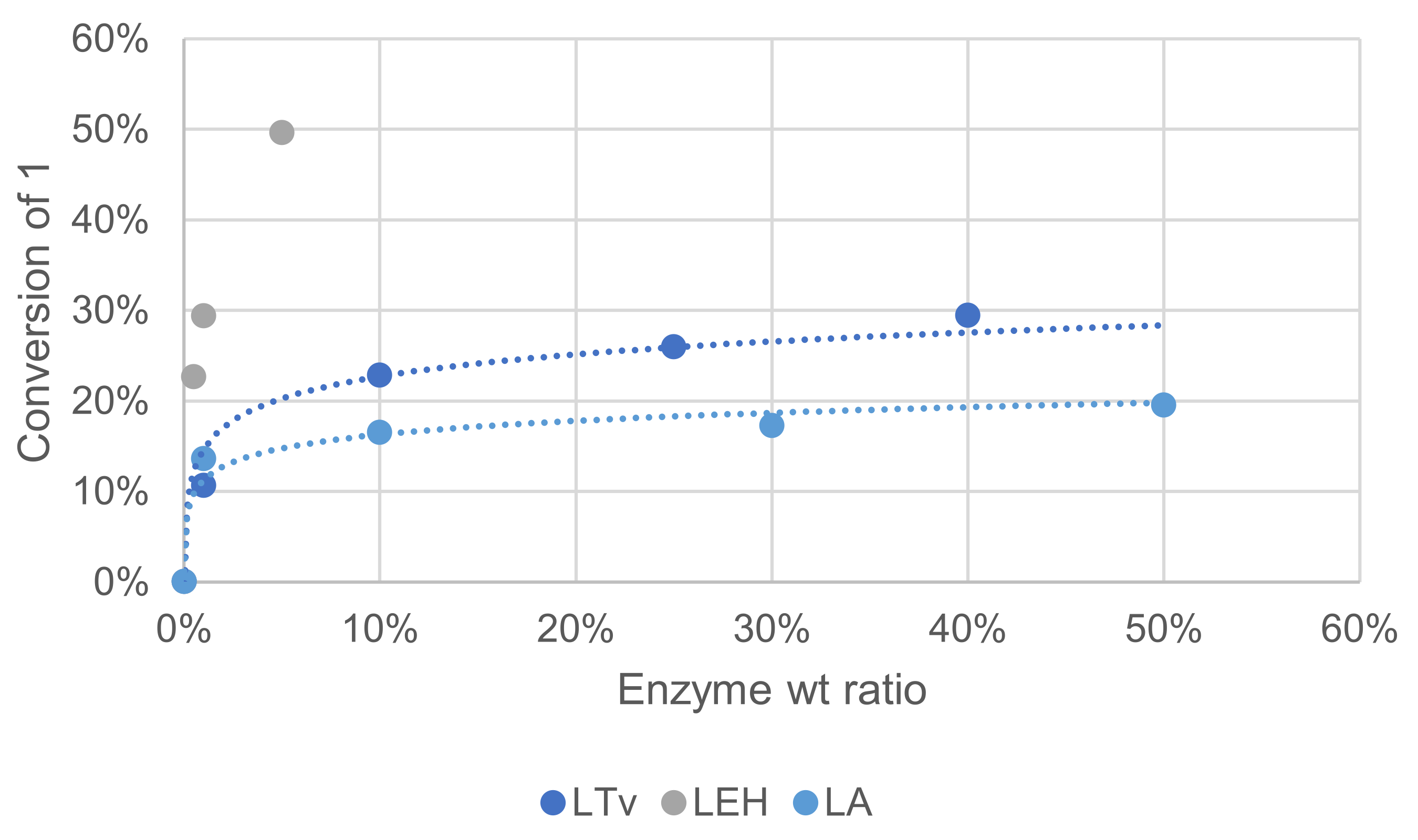
3.1.6. Effect of Enzyme Source and Concentration
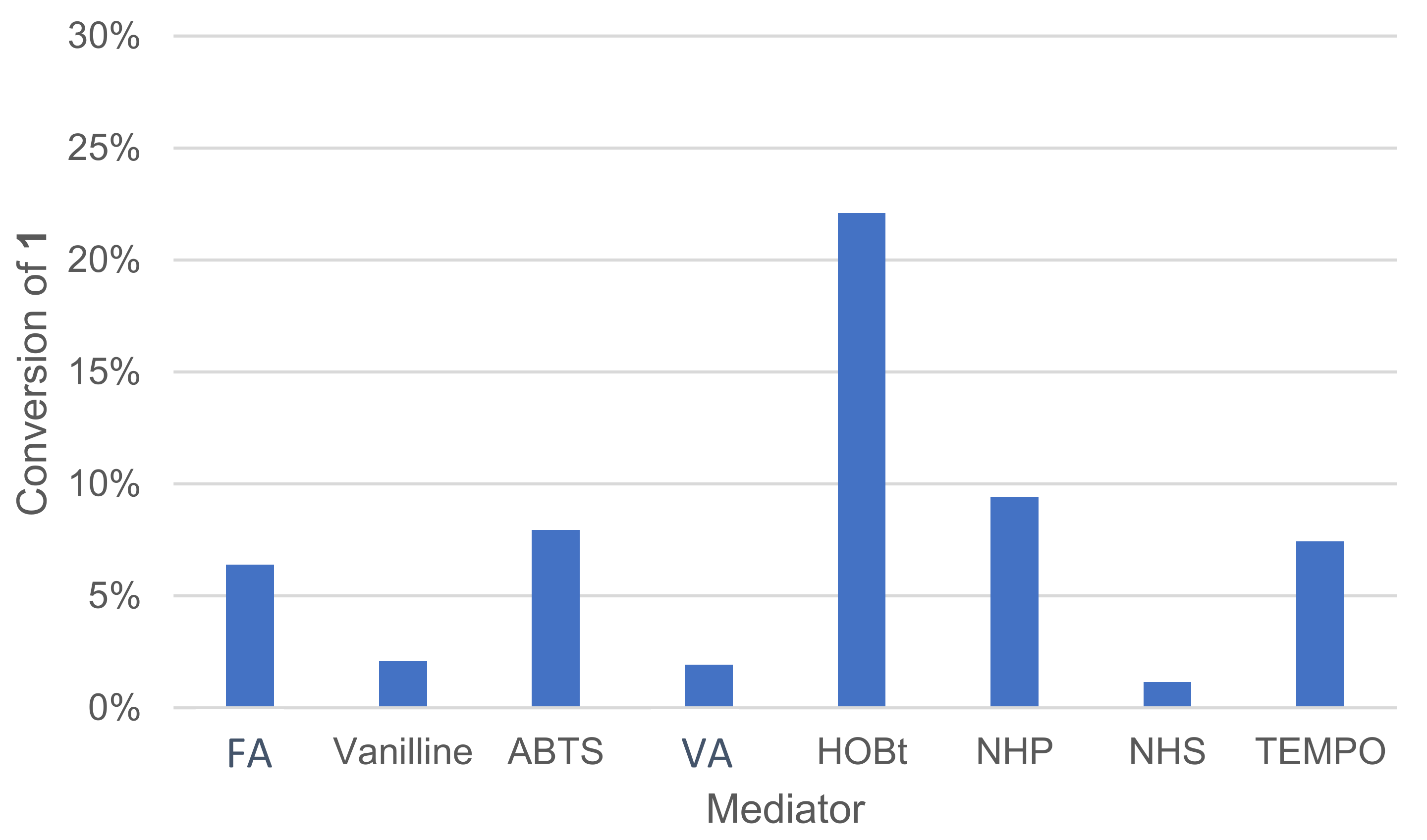
3.1.7. Effect of the Mediator Type
3.1.8. Effect of Mediator Concentration
3.1.9. Hypothesis of a Fast Deactivation and Sequential Supply of Fresh Enzyme and Mediator
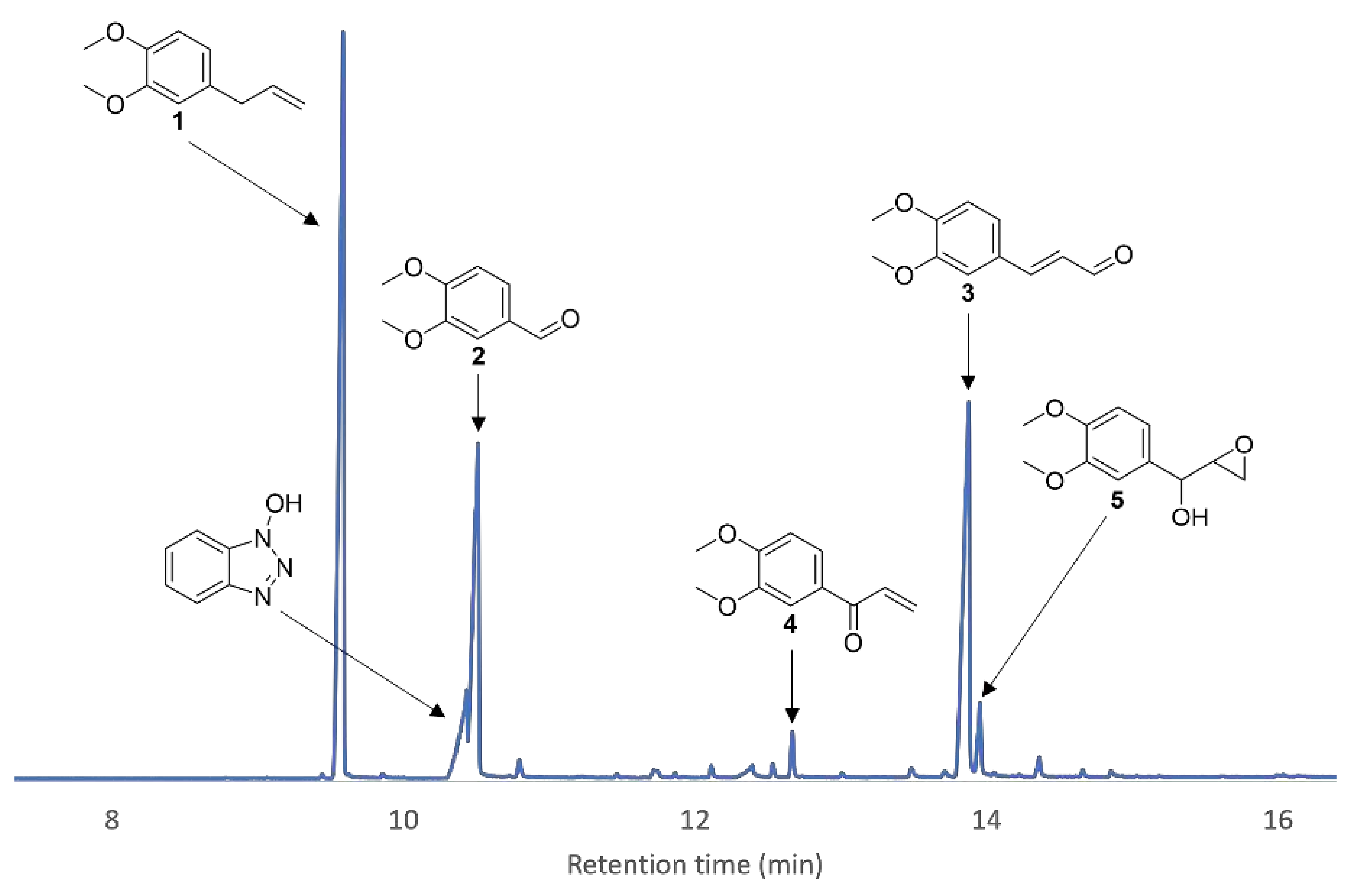
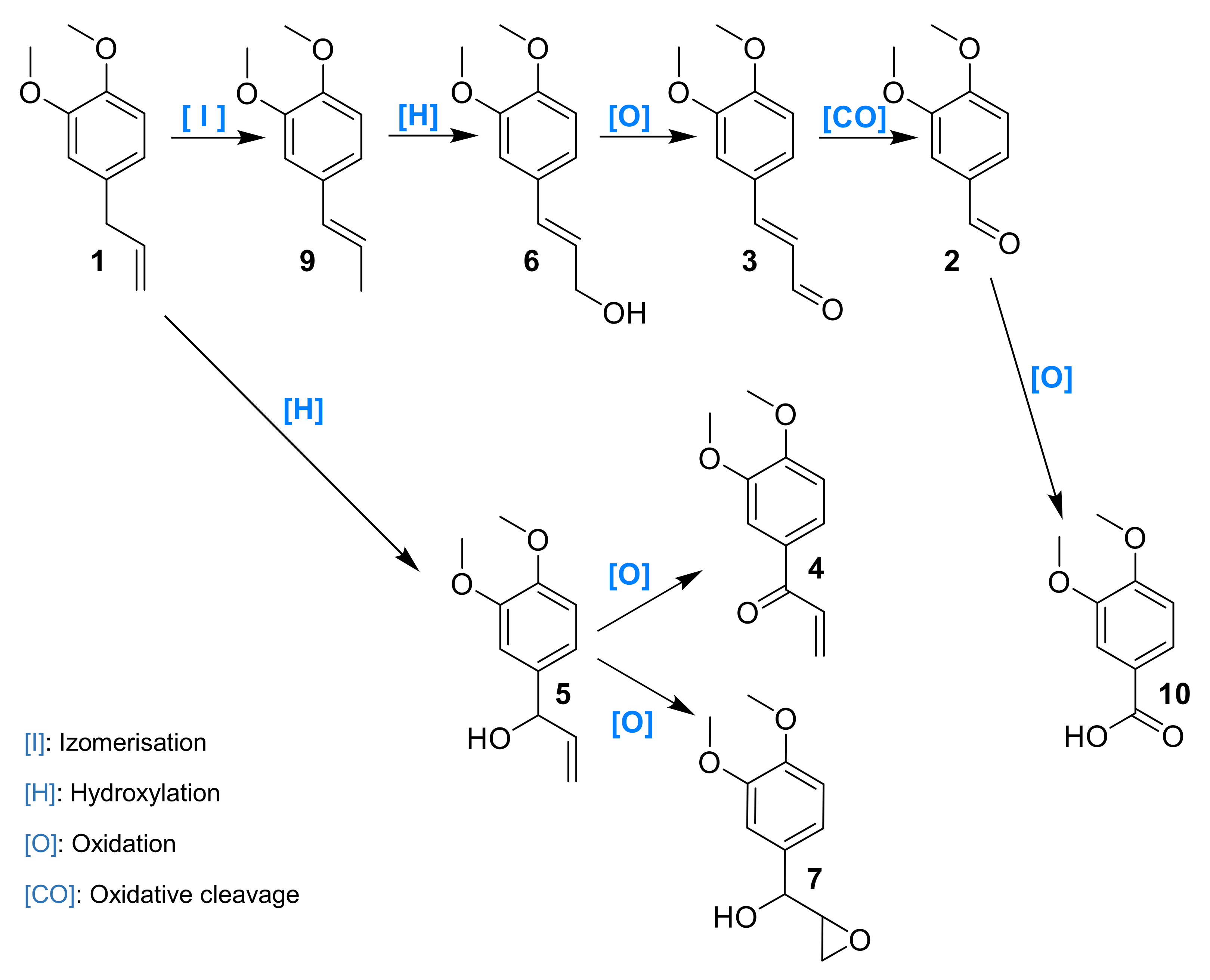
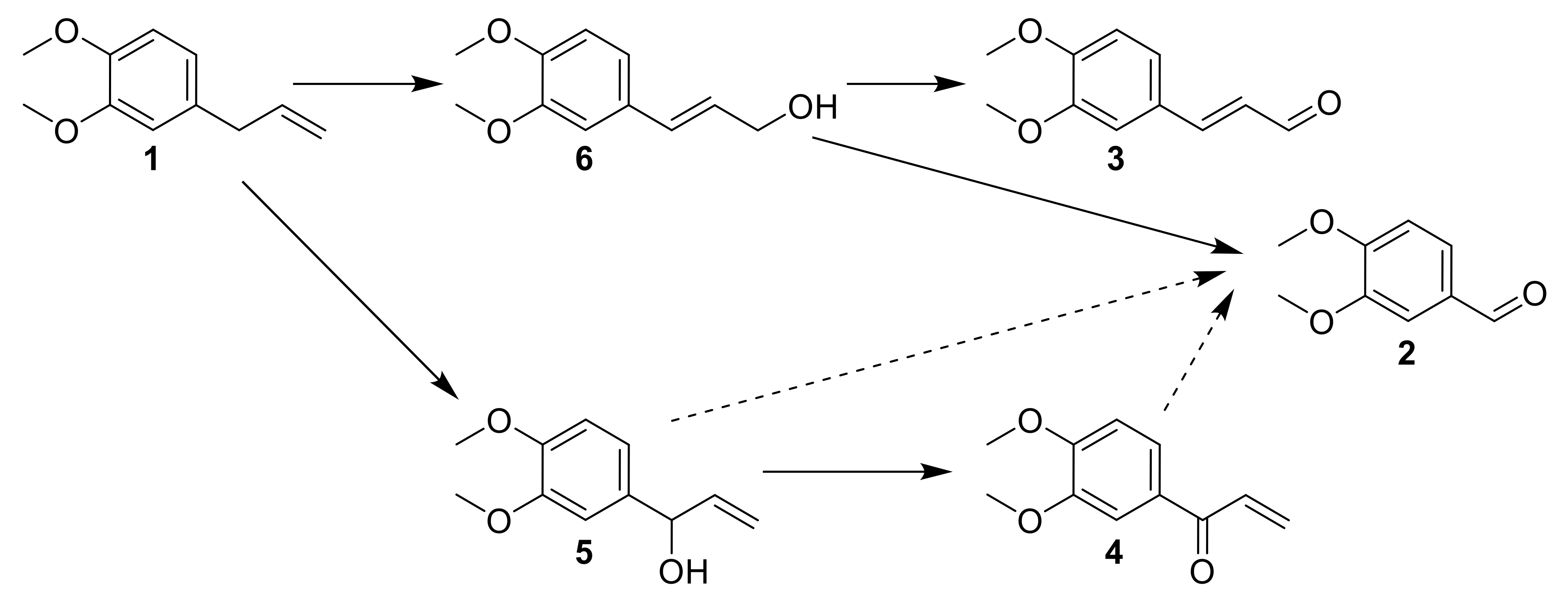
3.2. Mechanistic Investigations
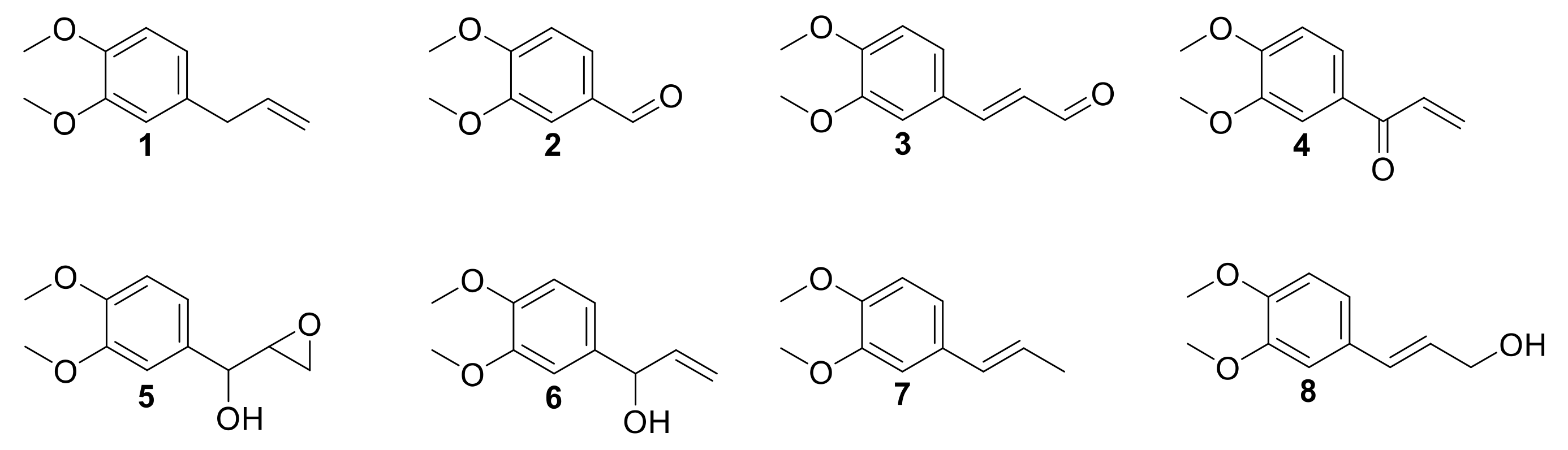
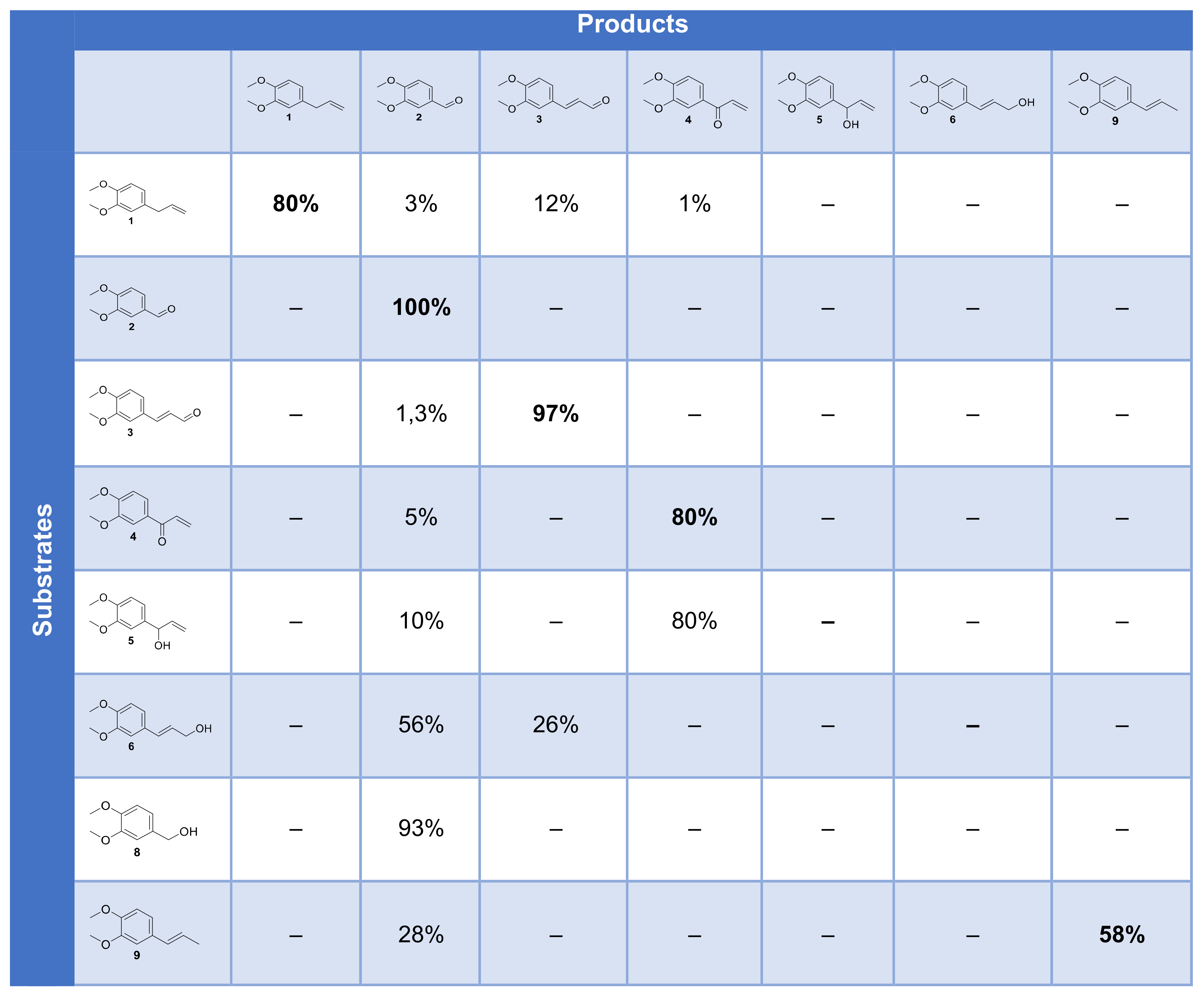
3.3. Scope and Limitations on Various Allylbenzene Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability: Samples of the compounds are not available from the authors. |
References
- Ricci, G.P.; de Faria, E.H.; Marcal, L.; Saltarelli, M.; Calefi, P.S.; Nassar, E.J.; Ciuffi, K.J. Heavy Metals, Synthetic Dyes, and Industrial Oxidation Reactions: Green Alternatives from Materials Chemistry; Luque, R., Ed.; Nova Publishers: New York, NY, USA, 2012; pp. 127–147. [Google Scholar]
- Poechlauer, P.; Skranc, W. Innovative oxidation methods in fine chemicals synthesis. Curr. Opin. Drug Discov. Dev. 2002, 5, 1000–1008. [Google Scholar] [CrossRef]
- Antoniotti, S.; Duñach, E. Studies on the catalytic oxidation of epoxides to α-diketones by Bi(0)/O2 in DMSO. J. Mol. Catal. A Chem. 2004, 208, 135–145. [Google Scholar] [CrossRef]
- Cousin, T.; Chatel, G.; Kardos, N.; Andrioletti, B.; Draye, M. Recent trends in the development of sustainable catalytic systems for the oxidative cleavage of cycloalkenes by hydrogen peroxide. Catal. Sci. Technol. 2019, 9, 5256–5278. [Google Scholar] [CrossRef]
- Albanese, D.C.M.; Foschi, F.; Penso, M. Sustainable Oxidations under Phase-Transfer Catalysis Conditions. Org. Process. Res. Dev. 2016, 20, 129–139. [Google Scholar] [CrossRef]
- Dong, J.; Fernández-Fueyo, E.; Hollmann, F.; Paul, C.E.; Pesic, M.; Schmidt, S.; Wang, Y.; Younes, S.; Zhang, W. Biocatalytic Oxidation Reactions: A Chemist’s Perspective. Angew. Chem. Int. Ed. 2018, 57, 9238–9261. [Google Scholar] [CrossRef]
- Monti, D.; Ottolina, G.; Carrea, G.; Riva, S. Redox Reactions Catalyzed by Isolated Enzymes. Chem. Rev. 2011, 111, 4111–4140. [Google Scholar] [CrossRef]
- Leonowicz, A.; Edgehill, R.U.; Bollag, J.-M. The effect of pH on the transformation of syringic and vanillic acids by the laccases of Rhizoctonia praticola and Trametes versicolor. Arch. Microbiol. 1984, 137, 89–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Nielsen, J.; Liu, Z. Engineering yeast metabolism for production of terpenoids for use as perfume ingredients, pharmaceuticals and biofuels. FEMS Yeast Res. 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Deguerry, F.; Pastore, L.; Wu, S.; Clark, A.; Chappell, J.; Schalk, M. The diverse sesquiterpene profile of patchouli, Pogostemon cablin, is correlated with a limited number of sesquiterpene synthases. Arch. Biochem. Biophys. 2006, 454, 123–136. [Google Scholar] [CrossRef]
- Krings, U.; Berger, R.G. Biotechnological production of flavours and fragrances. Appl. Microbiol. Biotechnol. 1998, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pogorevc, M.; Kroutil, W.; Wallner, S.R.; Faber, K. Enantioselective Stereoinversion in the Kinetic Resolution of rac-sec-Alkyl Sulfate Esters by Hydrolysis with an Alkylsulfatase from Rhodococcus ruber DSM 44541 Furnishes Homochiral Products. Angew. Chem. Int. Ed. 2002, 41, 4052–4054. [Google Scholar] [CrossRef]
- Lecourt, M.; Antoniotti, S. Chemistry, Sustainability and Naturality of Perfumery Biotech Ingredients. ChemSusChem 2020, 13, 5600–5610. [Google Scholar] [CrossRef]
- Cañas, A.; Camarero, S. Laccases and their natural mediators: Biotechnological tools for sustainable eco-friendly processes. Biotechnol. Adv. 2010, 28, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Witayakran, S.; Ragauskas, A.J. Synthetic Applications of Laccase in Green Chemistry. Adv. Synth. Catal. 2009, 351, 1187–1209. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Brady, D. Broadening the Scope of Biocatalysis in Sustainable Organic Synthesis. ChemSusChem 2019, 12, 2859–2881. [Google Scholar] [CrossRef]
- Morozova, O.V.; Shumakovich, G.P.; Shleev, S.; Yaropolov, Y.I. Laccase-mediator systems and their applications: A review. Appl. Biochem. Microbiol. 2007, 43, 523–535. [Google Scholar] [CrossRef]
- Fabbrini, M.; Galli, C.; Gentili, P. Comparing the catalytic efficiency of some mediators of laccase. J. Mol. Catal. B Enzym. 2002, 16, 231–240. [Google Scholar] [CrossRef]
- Fåhraeus, G.; Ljunggren, H. Substrate specificity of a purified fungal laccase. Biochim. Biophys. Acta 1961, 46, 22–32. [Google Scholar] [CrossRef]
- Morozova, O.V.; Shumakovich, G.P.; Gorbacheva, M.A.; Shleev, S.V.; Yaropolov, A.I. “Blue” laccases. Biochem. Mosc. 2007, 72, 1136–1150. [Google Scholar] [CrossRef]
- Niku-Paavola, M.-L.; Viikari, L. Enzymatic oxidation of alkenes. J. Mol. Catal. B Enzym. 2000, 10, 435–444. [Google Scholar] [CrossRef]
- Majcherczyk, A.; Johannes, C.; Hüttermann, A. Oxidation of Polycyclic Aromatic Hydrocarbons (PAH) by Laccase of Trametes versicolor. Enzym. Microb. Technol. 1998, 22, 335–341. [Google Scholar] [CrossRef]
- Ibrahim, V.; Mendoza, L.; Mamo, G.; Hatti-Kaul, R. Blue laccase from Galerina sp.: Properties and potential for Kraft lignin demethylation. Process. Biochem. 2011, 46, 379–384. [Google Scholar] [CrossRef]
- Aracri, E.; Colom, J.F.; Vidal, T. Application of laccase-natural mediator systems to sisal pulp: An effective approach to biobleaching or functionalizing pulp fibres? Bioresour. Technol. 2009, 100, 5911–5916. [Google Scholar] [CrossRef] [PubMed]
- Camarero, S.; Ibarra, D.; Martínez, Á.T.; Romero, J.; Gutiérrez, A.; del Río, J.C. Paper pulp delignification using laccase and natural mediators. Enzym. Microb. Technol. 2007, 40, 1264–1271. [Google Scholar] [CrossRef] [Green Version]
- Stoilova, I.; Dimitrova, G.; Angelova, G.; Krastanov, A. Biodegradation of phenol, catechol and 2, 4-dichlorophenol at higher initial inhibitory concentrations by Trametes versicolor 1 in a “fed-batch” process. Bulg. J. Agric. Sci. 2017, 23, 988–993. [Google Scholar]
- Bollag, J.M.; Chu, H.-L.; Rao, M.A.; Gianfreda, L. Enzymatic Oxidative Transformation of Chlorophenol Mixtures. J. Environ. Qual. 2003, 32, 63–69. [Google Scholar] [CrossRef]
- Riva, S. Laccases: Blue enzymes for green chemistry. Trends Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef]
- Yao, B.; Kolla, P.; Koodali, R.; Balaranjan, S.; Shrestha, S.; Smirnova, A. Laccase—Natural mediator systems for “green” synthesis of phenolic monomers from alkali lignin. Sustain. Energy Fuels 2017, 1, 1573–1579. [Google Scholar] [CrossRef]
- Smith, R.; Adams, T.; Doull, J.; Feron, V.; Goodman, J.; Marnett, L.; Portoghese, P.; Waddell, W.; Wagner, B.; Rogers, A.; et al. Safety assessment of allylalkoxybenzene derivatives used as flavouring substances—Methyl eugenol and estragole. Food Chem. Toxicol. 2002, 40, 851–870. [Google Scholar] [CrossRef]
- Johnson, J.D.; Abdo, K.M. Methyleugenol in the diet: Toxic and pathological aspects. Rev. Food Nutr. Toxic. 2005, 3, 1–60. [Google Scholar]
- Auerbach, S.S.; Shah, R.R.; Mav, D.; Smith, C.S.; Walker, N.; Vallant, M.K.; Boorman, G.A.; Irwin, R.D. Predicting the hepatocarcinogenic potential of alkenylbenzene flavoring agents using toxicogenomics and machine learning. Toxicol. Appl. Pharmacol. 2010, 243, 300–314. [Google Scholar] [CrossRef]
- FDA. Available online: https://www.fda.gov/food/cfsan-constituent-updates/fda-removes-7-synthetic-flavoring-substances-food-additives-list (accessed on 30 September 2021).
- European Commission. Opinion on Fragrance Allergens in Cosmetic Products; European Commission: Brussels, Belgium, 2012; p. 334. [Google Scholar]
- European Commission. Opinion of the Scientific Committee on Food on Methyleugenol (4-Allyl-1,2-dimethoxybenzene); European Commission: Brussels, Belgium, 2001. [Google Scholar]
- Baba, M.; Yamada, K.-I.; Ito, M. Cloning and Expression of a Perilla frutescens Cytochrome P450 Enzyme Catalyzing the Hydroxylation of Phenylpropenes. Plants 2020, 9, 577. [Google Scholar] [CrossRef]
- Jeurissen, S.M.F.; Punt, A.; Boersma, M.G.; Bogaards, J.J.P.; Fiamegos, Y.C.; Schilter, B.; van Bladeren, P.J.; Cnubben, N.H.P.; Rietjens, I.M.C.M. Human Cytochrome P450 Enzyme Specificity for the Bioactivation of Estragole and Related Alkenylbenzenes. Chem. Res. Toxicol. 2007, 20, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Voda, K.; Boh, B.; Vrtačnik, M.; Pohleven, F. Effect of the antifungal activity of oxygenated aromatic essential oil compounds on the white-rot Trametes versicolor and the brown-rot Coniophora puteana. Int. Biodeterior. Biodegrad. 2003, 51, 51–59. [Google Scholar] [CrossRef]
- Burkey, J.L.; Sauer, J.-M.; McQueen, C.A.; Sipes, I.G. Cytotoxicity and genotoxicity of methyleugenol and related congeners—A mechanism of activation for methyleugenol. Mutat. Res. Fund. Mol. M 2000, 453, 25–33. [Google Scholar] [CrossRef]
- Llevot, A.; Grau, E.; Carlotti, S.; Grelier, S.; Cramail, H. Selective laccase-catalyzed dimerization of phenolic compounds derived from lignin: Towards original symmetrical bio-based (bis) aromatic monomers. J. Mol. Catal. B Enzym. 2016, 125, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.-B.; Wang, X.-L.; Shi, T.; Liu, S.; Xu, Z.-H.; Li, X.; Shi, X.; Xu, P.; Zhao, Y.-L. Multicomponent kinetic analysis and theoretical studies on the phenolic intermediates in the oxidation of eugenol and isoeugenol catalyzed by laccase. Phys. Chem. Chem. Phys. 2015, 17, 29597–29607. [Google Scholar] [CrossRef]
- Adelakun, O.E.; Kudanga, T.; Green, I.R.; le Roes-Hill, M.; Burton, S.G. Enzymatic modification of 2,6-dimethoxyphenol for the synthesis of dimers with high antioxidant capacity. Process. Biochem. 2012, 47, 1926–1932. [Google Scholar] [CrossRef] [Green Version]
- Xing, W.; Yin, M.; Lv, Q.; Hu, Y.; Liu, C.; Zhang, J. Oxygen Solubility, Diffusion Coefficient, and Solution Viscosity. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Xing, W., Yin, G., Zhang, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–31. [Google Scholar]
- Ramilijaona, J.; Raynaud, E.; Bouhlel, C.; Sarrazin, E.; Fernandez, X.; Antoniotti, S. Enzymatic Modification of Palmarosa Essential Oil: Chemical Analysis and Olfactory Evaluation of Acylated Products. Chem. Biodivers. 2013, 10, 2291–2301. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecourt, M.; Chietera, G.; Blerot, B.; Antoniotti, S. Laccase-Catalyzed Oxidation of Allylbenzene Derivatives: Towards a Green Equivalent of Ozonolysis. Molecules 2021, 26, 6053. https://doi.org/10.3390/molecules26196053
Lecourt M, Chietera G, Blerot B, Antoniotti S. Laccase-Catalyzed Oxidation of Allylbenzene Derivatives: Towards a Green Equivalent of Ozonolysis. Molecules. 2021; 26(19):6053. https://doi.org/10.3390/molecules26196053
Chicago/Turabian StyleLecourt, Mathilde, Giorgiana Chietera, Bernard Blerot, and Sylvain Antoniotti. 2021. "Laccase-Catalyzed Oxidation of Allylbenzene Derivatives: Towards a Green Equivalent of Ozonolysis" Molecules 26, no. 19: 6053. https://doi.org/10.3390/molecules26196053
APA StyleLecourt, M., Chietera, G., Blerot, B., & Antoniotti, S. (2021). Laccase-Catalyzed Oxidation of Allylbenzene Derivatives: Towards a Green Equivalent of Ozonolysis. Molecules, 26(19), 6053. https://doi.org/10.3390/molecules26196053






