Control of the Induced Handedness of Helical Nanofilaments Employing Cholesteric Liquid Crystal Fields
Abstract
:1. Introduction
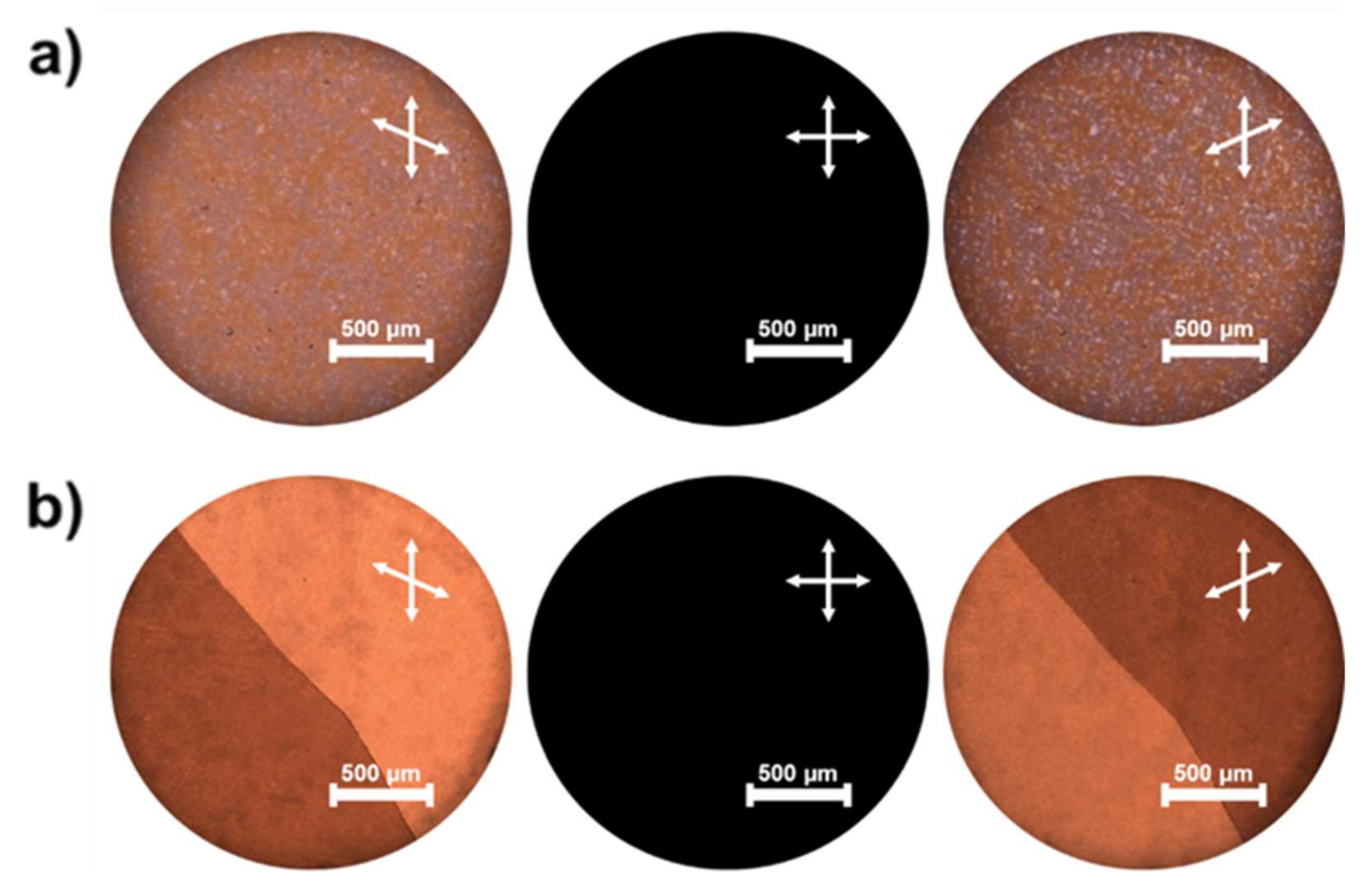
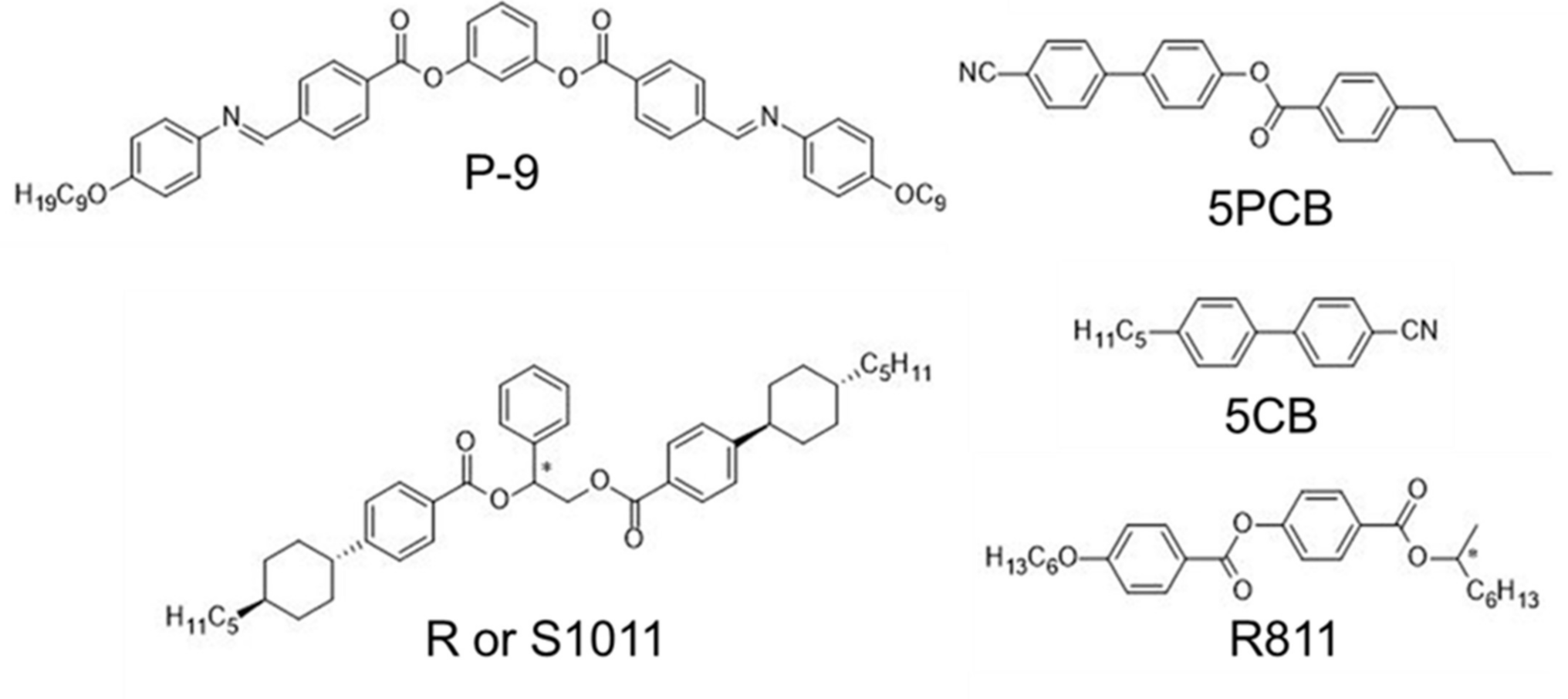
2. Results and Discussion
3. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pasteur, L. Sur les relations qui peuvent exister entre la forme crystalline, la composition chimique et le sens de la polarization rotatoire. Ann. Chim. Phys. 1848, 24, 442–459. [Google Scholar]
- Hur, S.-T.; Lee, B.R.; Gim, M.-J.; Park, K.-W.; Song, M.H.; Choi, S.-W. Liquid-Crystalline Blue Phase Laser with Widely Tunable Wavelength. Adv. Mater. 2013, 25, 3002–3006. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-W.; Kim, D.-Y.; Araoka, F.; Jeong, K.-W.; Choi, S.-W. Nanosegregated Chiral Materials with Self-Assembled Hierarchical Mesophases: Effect of Thermotropic and Photoinduced Polymorphism in Rodlike Molecules. Chem. Eur. J. 2017, 23, 17794–17799. [Google Scholar] [CrossRef]
- Kim, K.; Kim, H.; Jo, S.-Y.; Araoka, F.; Yoon, D.K.; Choi, S.-W. Photomodulated Supramolecular Chirality in Achiral Photoresponsive Rodlike Compounds Nanosegregated from the Helical Nanofilaments of Achiral Bent-Core Molecules. ACS Appl. Mater. Interfaces 2015, 7, 22686–22691. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-W.; Choi, H.-J.; Bae, J.-H.; Kim, B.-C.; Choi, S.-W. Photomodulating Chiroptic Behaviors in Nanosegregated Mesophase from a Mixture System Consisting of Nonchiral Bent-Core and Photo-Responsive Rod-Like Mesogens. J. Inf. Disp. 2018, 19, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-J.; Kim, B.-C.; Choi, H.-J.; Bae, S.; Araoka, F.; Choi, S.-W. Inverse Helical Nanofilament Networks Serving as a Chiral Nanotemplate. ACS Nano 2020, 14, 5243–5250. [Google Scholar] [CrossRef]
- Kim, B.-C.; Choi, H.-J.; Lee, J.-J.; Araoka, F.; Choi, S.-W. Circularly Polarized Luminescence Induced by Chiral Super Nanospaces. Adv. Funct. Mater. 2019, 29, 1903246. [Google Scholar] [CrossRef]
- Lee, J.-J.; Choi, S.-W. Enhancement of Luminescence Dissymmetry Factor in Nano-Segregated Phase Generated by Phase Separation between Helical Nanofilaments and Liquid-Crystalline Smectic a Phase. Crystals 2020, 10, 952. [Google Scholar] [CrossRef]
- Lee, J.-J.; Choi, S.-W. Preferential Circularly Polarized Luminescence from a Nano-Segregated Liquid Crystalline Phase Using a Polymerized Twisted Nematic Platform. Polymers 2020, 12, 2529. [Google Scholar] [CrossRef]
- Takezoe, H.; Takanishi, Y. Bent-Core Liquid Crystals: Their Mysterious and Attractive World. Jpn. J. Appl. Phys. 2006, 45, 597–625. [Google Scholar] [CrossRef]
- Reddy, R.A.; Tschierske, C. Bent-Core Liquid Crystals: Polar Order, Superstructural Chirality and Spontaneous Desymmetrisation in Soft Matter Systems. J. Mater. Chem. 2006, 16, 907–961. [Google Scholar] [CrossRef]
- Shadpour, S.; Nemati, A.; Liu, J.; Hegmann, T. Directing the Handedness of Helical Nanofilaments Confined in Nanochannels Using Axially Chiral Binaphthyl Dopants. ACS Appl. Mater. Interfaces 2020, 12, 13456–13463. [Google Scholar] [CrossRef] [PubMed]
- Le, K.V.; Takezoe, H.; Araoka, F. Chiral Superstructure Mesophases of Achiral Bent-Shaped Molecules—Hierarchical Chirality Amplification and Physical Properties. Adv. Mater. 2017, 29, 1602737. [Google Scholar] [CrossRef]
- Hough, L.; Jung, H.-T.; Kruüerke, D.; Heberling, M.; Nakata, M.; Jones, C.; Chen, D.; Link, D.R.; Zasadzinski, J.; Heppke, G.; et al. Helical nanofilament phases. Science 2009, 325, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Tsai, E.; Richardson, J.M.; Korblova, E.; Nakata, M.; Chen, D.; Shen, Y.; Shao, R.; Clark, N.A.; Walba, D.M. A modulated helical nanofilament phase. Angew. Chem. Int. Ed. 2013, 52, 5254–5257. [Google Scholar] [CrossRef] [PubMed]
- Araoka, F.; Sugiyama, G.; Ishikawa, K.; Takezoe, H. Highly Ordered Helical Nanofilament Assembly Aligned by a Nematic Director Field. Adv. Funct. Mater. 2013, 23, 2701–2707. [Google Scholar] [CrossRef]
- Thisayukta, J.; Niwano, H.; Takezoe, H.; Watanabe, J. Effect of chiral dopant on a helical Sm1 phase of banana-shaped NnO-PIMB molecules. J. Mater. Chem. 2001, 11, 2717–2721. [Google Scholar] [CrossRef]
- Shiromo, K.; Sahade, D.A.; Oda, T.; Nihira, T.; Takanishi, Y.; Ishikawa, K.; Takezoe, H. Finite enantiomeric excess nucleated in an achiral banana mesogen by chiral alignment surfaces. Angew. Chem. Int. Ed. 2005, 44, 1948–1951. [Google Scholar] [CrossRef]
- Choi, S.W.; Izumi, T.; Hoshino, Y.; Takanishi, Y.; Ishikawa, K.; Watanabe, J.; Takezoe, H. Circular-polarization-induced enantiomeric excess in liquid crystals of an achiral, bent-shaped mesogen. Angew. Chem. Int. Ed. 2006, 45, 1382–1385. [Google Scholar] [CrossRef]
- Choi, S.W.; Kang, S.; Takanishi, Y.; Ishikawa, K.; Watanabe, J.; Takezoe, H. Intrinsic chirality in a bent-core mesogen induced by extrinsic chiral structures. Angew. Chem. Int. Ed. 2006, 45, 6503–6506. [Google Scholar] [CrossRef]
- Ueda, T.; Masuko, S.; Araoka, F.; Ishikawa, K.; Takezoe, H. A General Method for the Enantioselective Formation of Helical Nanofilaments. Angew. Chem. Int. Ed. 2013, 52, 6863–6866. [Google Scholar] [CrossRef]
- Lee, G.; Carlton, R.J.; Araoka, F.; Abbott, N.L.; Takezoe, H. Amplification of the stereochemistry of biomolecular adsorbates by deracemization of chiral domains in bent-core liquid crystals. Adv. Mater. 2013, 25, 245–249. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Tuchband, M.R.; Horanyi, B.; Korblova, E.; Walba, D.M.; Glaser, M.A.; Maclennan, J.E.; Clark, N.A. Diastereomeric liquid crystal domains at the mesoscale. Nat. Commun. 2015, 6, 7763. [Google Scholar] [CrossRef] [Green Version]
- Takanishi, Y.; Shin, G.J.; Jung, J.C.; Choi, S.-W.; Ishikawa, K.; Watanabe, J.; Takezoe, H.; Toledano, P. Observation of very large chiral domains in a liquid crystal phase formed by mixtures of achiral bent-core and rod molecules. J. Mater. Chem. 2005, 15, 4020–4024. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, D.; Shen, Y.; Jones, C.D.; Glaser, M.A.; Maclennan, J.E.; Clark, N.A. Nanophase segregation in binary mixtures of a bent-core and a rodlike liquid-crystal molecule. Phys. Rev. E 2010, 81, 011704. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, L.; Nemati, H.; Yang, H.; Bunning, T.; Yang, D.-K. Effects of polymer network on electrically induced reflection band broadening of cholesteric liquid crystals. J. Polym. Sci. B Polym. Phys. 2017, 55, 835–846. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Lee, J.-J.; Park, J.-S.; Choi, Y.-J.; Choi, S.-W. Control of the Induced Handedness of Helical Nanofilaments Employing Cholesteric Liquid Crystal Fields. Molecules 2021, 26, 6055. https://doi.org/10.3390/molecules26196055
Kim J-Y, Lee J-J, Park J-S, Choi Y-J, Choi S-W. Control of the Induced Handedness of Helical Nanofilaments Employing Cholesteric Liquid Crystal Fields. Molecules. 2021; 26(19):6055. https://doi.org/10.3390/molecules26196055
Chicago/Turabian StyleKim, Ju-Yong, Jae-Jin Lee, Jun-Sung Park, Yong-Jun Choi, and Suk-Won Choi. 2021. "Control of the Induced Handedness of Helical Nanofilaments Employing Cholesteric Liquid Crystal Fields" Molecules 26, no. 19: 6055. https://doi.org/10.3390/molecules26196055






