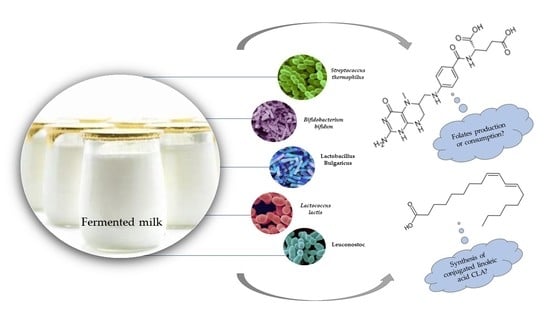
Changes in the Folate Content and Fatty Acid Profile in Fermented Milk Produced with Different Starter Cultures during Storage
Abstract
1. Introduction
2. Results and Discussion
2.1. Folates
2.2. Fatty Acid Composition
3. Materials and Methods
3.1. Samples
3.2. Folate Analysis
3.2.1. Chemicals, Enzymes, and Standards
3.2.2. Sample Preparation
3.2.3. Folate Quantification
3.3. Fatty Acid Composition
3.3.1. Fat Extraction
3.3.2. Preparation of Fatty Acid Methyl Esters
3.3.3. Gas Chromatography (GC) Analysis
3.3.4. Identification and Calculation of Fatty Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Blancquaert, D.; De Steur, H.; Gellynck, X.; Van Der Straeten, D. Present and Future of Folate Biofortification of Crop Plants. J. Exp. Bot. 2014, 65, 895–906. [Google Scholar] [CrossRef]
- Najgebauer-Lejko, D.; Liszka, K.; Tabaszewska, M.; Domagała, J. Probiotic Yoghurts with Sea Buckthorn, Elderberry, and Sloe Fruit Purees. Molecules 2021, 26, 2345. [Google Scholar] [CrossRef]
- Vora, A.; Riga, A.; Dollimore, D.; Alexander, K.S. Thermal Stability of Folic Acid. Thermochim. Acta 2002, 392–393, 209–220. [Google Scholar] [CrossRef]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef]
- Rad, A.H.; Khosroushahi, A.Y.; Khalili, M.; Jafarzadeh, S. Folate Bio-Fortification of Yoghurt and Fermented Milk: A Review. Dairy Sci. Technol. 2016, 96, 427–441. [Google Scholar] [CrossRef]
- Rampersaud, G.C.; Kauwell, G.P.; Bailey, L.B. Folate: A Key to Optimizing Health and Reducing Disease Risk in the Elderly. J. Am. Coll. Nutr. 2003, 22, 1–8. [Google Scholar] [CrossRef]
- Wright, A.; Finglas, P.; Southon, S. Proposed Mandatory Fortification of the UK Diet with Folic Acid: Have Potential Risks been Underestimated? Trends Food Sci. Technol. 2001, 12, 313–321. [Google Scholar] [CrossRef]
- Buttriss, J. Strategies Designed to Increase Awareness about Folates and Health, and to Increase Folate Intake: A Review. Trends Food Sci. Technol. 2005, 16, 246–252. [Google Scholar] [CrossRef]
- Saubade, F.; Hemery, Y.; Guyot, J.-P.; Humblot, C. Lactic Acid Fermentation as a Tool for Increasing the Folate Content of Foods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3894–3910. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guideline: Daily Iron and Folic Acid Supplementation in Pregnant Women; World Health Organization: Geneva, Switzerland, 2012; Available online: http://www.who.int/nutrition/publications/micronutrients/guidelines/daily_ifa_supp_pregnant_women/en/ (accessed on 30 August 2021).
- FAO/WHO. Vitamin and Mineral Requirements in Human Nutrition, Second Edition. 2005. Available online: http://www.who.int/nutrition/publications/micronutrients/9241546123/en/ (accessed on 30 August 2021).
- LeBlanc, J.G.; De Giori, G.S.; Smid, E.J.; Hugenholtz, J.; Sesma, F. Folate Production by Lactic Acid Bacteria and Other Food-Grade Microorganisms. Comm. Curr. Res. Educ. Top. Trends. Appl. Microbiol. 2007, 1, 329–339. [Google Scholar]
- Aryana, K.J.; Olson, D.W. A 100-Year Review: Yogurt and Other Cultured Dairy Products. J. Dairy Sci. 2017, 100, 9987–10013. [Google Scholar] [CrossRef]
- Divya, J.B.; Nampoothiri, K.M. Folate Fortification of Skim Milk by a Probiotic Lactococcus Lactis CM28 and Evaluation of Its Stability in Fermented Milk on Cold Storage. J. Food Sci. Technol. 2015, 52, 3513–3519. [Google Scholar] [CrossRef][Green Version]
- Sybesma, W.; Starrenburg, M.; Tijsseling, L.; Hoefnagel, M.H.N.; Hugenholtz, J. Effects of Cultivation Conditions on Folate Production by Lactic Acid Bacteria. Appl. Environ. Microbiol. 2003, 69, 4542–4548. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Young, C. Folate Levels in Cultures of Lactic Acid Bacteria. Int. Dairy J. 2000, 10, 409–413. [Google Scholar] [CrossRef]
- Crittenden, R.; Martinez, N.; Playne, M. Synthesis and Utilisation of Folate by Yoghurt Starter Cultures and Probiotic Bacteria. Int. J. Food Microbiol. 2003, 80, 217–222. [Google Scholar] [CrossRef]
- Chin, S.; Liu, W.; Storkson, J.; Ha, Y.; Pariza, M. Dietary Sources of Conjugated Dienoic Isomers of Linoleic Acid, a Newly Recognized Class of Anticarcinogens. J. Food Compos. Anal. 1992, 5, 185–197. [Google Scholar] [CrossRef]
- Jiang, J.; Björck, L.; Fondén, R. Production of Conjugated Linoleic Acid by Dairy Starter Cultures. J. Appl. Microbiol. 1998, 85, 95–102. [Google Scholar] [CrossRef]
- Lin, H.; Boylston, T.D.; Luedecke, L.O.; Shultz, T.D. Factors Affecting the Conjugated Linoleic Acid Content of Cheddar Cheese. J. Agric. Food Chem. 1998, 46, 801–807. [Google Scholar] [CrossRef]
- AkalIn, A.S.; Tokusoglu, O. A Potential Anticarcinogenic Agent: Conjugated Linoleic Acid (CLA). Pak. J. Nutr. 2003, 2, 109–110. [Google Scholar] [CrossRef][Green Version]
- Parodi, P.W. Anti-Cancer Agents in Milkfat. Aust. J. Dairy Technol. 2003, 58, 114–118. [Google Scholar]
- Aydin, R. Conjugated Linoleic Acid: Structure, Sources and Biological Properties. Turk. J. Vet. Anim. Sci. 2003, 29, 189–195. [Google Scholar]
- Park, Y. Conjugated Linoleic Acid (CLA): Good or Bad Trans Fat? J. Food Compos. Anal. 2009, 22, 4–12. [Google Scholar] [CrossRef]
- Kee, J.-I.; Ganesan, P.; Kwak, H.-S. Bioactive Conjugated Linoleic Acid (CLA) in Milk. Food Sci. Anim. Resour. 2010, 30, 879–885. [Google Scholar] [CrossRef]
- Precht, D.; Molkentin, J. Effect of Feeding on Trans Positional Isomers of Octadecenoic Acid in Milk Fats. Milchwissenschaft 1997, 52, 564–568. [Google Scholar]
- Collomb, M.; Schmid, A.; Sieber, R.; Wechsler, D.; Ryhänen, E.-L. Conjugated Linoleic Acids in Milk Fat: Variation and Physiological Effects. Int. Dairy J. 2006, 16, 1347–1361. [Google Scholar] [CrossRef]
- Frelich, J.; Šlachta, M.; Hanuš, O.; Špička, J.; Samková, E.; Węglarz, A.; Zapletal, P. Seasonal Variation in Fatty Acid Composition of Cow Milk in Relation to the Feeding System. Anim. Sci. Pap. Rep. 2012, 30, 219–229. [Google Scholar]
- Kelsey, J.; Corl, B.; Collier, R.; Bauman, D. The Effect of Breed, Parity, and Stage of Lactation on Conjugated Linoleic Acid (CLA) in Milk Fat from Dairy Cows. J. Dairy Sci. 2003, 86, 2588–2597. [Google Scholar] [CrossRef]
- Chilliard, Y.; Ferlay, A.; Mansbridge, R.M.; Doreau, M. Ruminant Milk Fat Plasticity: Nutritional Control of Saturated, Polyunsaturated, Trans and Conjugated Fatty Acids. Anim. Res. 2000, 49, 181–205. [Google Scholar] [CrossRef]
- Zegarska, Z.; Paszczyk, B.; Rafałowski, R.; Borejszo, Z. Annual Changes in the Content of Unsaturated Fatty Acids with 18 Carbon Atoms, Including Cis9trans11 C18:2 (CLA) Acid, in Milk Fat. Pol. J. Food Nutr. Sci. 2006, 56, 409–414. [Google Scholar]
- Hanuš, O.; Křížová, L.; Samková, E.; Špička, J.; Kučera, J.; Klimešová, M.; Roubal, P.; Jedelská, R. The Effect of Cattle Breed, Season and Type of Diet on the Fatty Acid Profile of Raw Milk. Arch. Anim. Breed. 2016, 59, 373–380. [Google Scholar] [CrossRef]
- Shantha, N.C.; Decker, E.A.; Ustunol, Z. Conjugated Linoleic Acid Concentration in Processed Cheese. J. Am. Oil Chem. Soc. 1992, 69, 425–428. [Google Scholar] [CrossRef]
- Bisig, W.; Eberhard, P.; Collomb, M.; Rehberger, B. Influence of Processing on the Fatty Acid Composition and the Content of Conjugated Linoleic Acid in Organic and Conventional Dairy Products—A Review. Le Lait 2007, 87, 1–19. [Google Scholar] [CrossRef]
- Salamon, R.V.; Lóki, K.; Varga-Visi, E.; Mándoki, Z.; Csapó, J. Increase of Conjugated Linoleic Acid Content of Dairy Products by Adding Sunflower Oil. Krmiva 2009, 51, 99–103. [Google Scholar]
- Junior, O.O.S.; Pedrao, M.R.; Dias, L.F.; Paula, L.N.; Coro, F.A.; De Souza, N.E. Fatty Acid Content of Bovine Milkfat from Raw Milk to Yoghurt. Am. J. Appl. Sci. 2012, 9, 1300–1306. [Google Scholar] [CrossRef]
- Seçkin, A.K.; Gursoy, O.; Kinik, O.; Akbulut, N. Conjugated Linoleic Acid (CLA) Concentration, Fatty Acid Composition and Cholesterol Content of Some Turkish Dairy Products. LWT 2005, 38, 909–915. [Google Scholar] [CrossRef]
- Prandini, A.; Sigolo, S.; Tansini, G.; Brogna, N.; Piva, G. Different Level of Conjugated Linoleic Acid (CLA) in Dairy Products from Italy. J. Food Compos. Anal. 2007, 20, 472–479. [Google Scholar] [CrossRef]
- Alonso, L.; Cuesta, E.P.; Gilliland, S.E. Production of free conjugated linoleic acid by Lactobacillus acidophilus and Lactobacillus casei of human intestinal origin. J. Dairy Sci. 2003, 86, 1941–1946. [Google Scholar] [CrossRef]
- Kim, Y.; Liu, R. Increase of Conjugated Linoleic Acid Content in Milk by Fermentation with Lactic Acid Bacteria. J. Food Sci. 2002, 67, 1731–1737. [Google Scholar] [CrossRef]
- Sieber, R.; Collomb, M.; Aeschlimann, A.; Jelen, P.; Eyer, H. Impact of Microbial Cultures on Conjugated Linoleic Acid in Dairy Products—A Review. Int. Dairy J. 2004, 14, 1–15. [Google Scholar] [CrossRef]
- Domagała, J.; Sady, M.; Najgebauer-Lejko, D.; Czernicka, M.; Wieteska, I. The Content of Conjugated Linoleic Acid (CLA) in Cream Fermented Using Different Starter Cultures. Biotechnol. Anim. Husb. 2009, 25, 745–751. [Google Scholar]
- Hennessy, A.; Ross, R.; Devery, R.; Stanton, C. Optimization of a Reconstituted Skim Milk Based Medium for Enhanced CLA Production by Bifidobacteria. J. Appl. Microbiol. 2009, 106, 1315–1327. [Google Scholar] [CrossRef]
- Ogawa, J.; Kishino, S.; Ando, A.; Sugimoto, S.; Mihara, K.; Shimizu, S. Production of Conjugated Fatty Acids by Lactic Acid Bacteria. J. Biosci. Bioeng. 2005, 100, 355–364. [Google Scholar] [CrossRef]
- Hugenschmidt, S.; Schwenninger, S.M.; Gnehm, N.; Lacroix, C. Screening of a Natural Biodiversity of Lactic and Propionic Acid Bacteria for Folate and Vitamin B12 Production in Supplemented Whey Permeate. Int. Dairy J. 2010, 20, 852–857. [Google Scholar] [CrossRef]
- Laiño, J.E.; del Valle, M.J.; de Giori, G.S.; LeBlanc, J.G.J. Applicability of a Lactobacillus Amylovorus Strain as Co-Culture for Natural Folate Bio-Enrichment of Fermented Milk. Int. J. Food Microbiol. 2014, 191, 10–16. [Google Scholar] [CrossRef]
- Gujska, E.; Czarnowska, M.; Michalak, J. Content of Folates in Fresh and Cold Stored Kefirs and Yoghurts. Zywnosc Nauka Technol. Jakosc/Food Sci. Technol. Qual. 2014, 5, 124–133. [Google Scholar] [CrossRef]
- Strandler, H.S.; Patring, J.; Jägerstad, M.; Jastrebova, J. Challenges in the Determination of Unsubstituted Food Folates: Impact of Stabilities and Conversions on Analytical Results. J. Agric. Food Chem. 2015, 63, 2367–2377. [Google Scholar] [CrossRef]
- Holasová, M.; Fiedlerová, V.; Roubal, P.; Pechačová, M. Possibility of Increasing Natural Folate Content in Fermented Milk Products by Fermentation and Fruit Component Addition. Czech J. Food Sci. 2005, 23, 196–201. [Google Scholar] [CrossRef]
- Holasova, M.; Fiedlerova, V.; Roubal, P.; Pechacova, M. Biosynthesis of Folates by Lactic Acid Bacteria and Propionibacteria in Fermented Milk. Czech J. Food Sci. 2004, 22, 175–181. [Google Scholar] [CrossRef]
- Jagerstad, M.; Jastrebova, J.; Svensson, U. Folates in Fermented Vegetables—A Pilot Study. LWT 2004, 37, 603–611. [Google Scholar] [CrossRef]
- Kariluoto, S.; Aittamaa, M.; Korhola, M.; Salovaara, H.; Vahteristo, L.; Piironen, V. Effects of Yeasts and Bacteria on the Levels of Folates in Rye Sourdoughs. Int. J. Food Microbiol. 2006, 106, 137–143. [Google Scholar] [CrossRef]
- Gangadharan, D.; Nampoothiri, K.M.; Gangadharan, D.; Nampoothiri, K.M. Folate Production Using Lactococcus lactis ssp. cremoris with Implications for Fortification of Skim Milk and Fruit Juices. LWT 2011, 44, 1859–1864. [Google Scholar] [CrossRef]
- Wigertz, K.; Jägerstad, M. Comparison of a HPLC and Radioprotein-Binding Assay for the Determination of Folates in Milk and Blood Samples. Food Chem. 1995, 54, 429–436. [Google Scholar] [CrossRef]
- Iyer, R.; Tomar, S.K.; Mohanty, A.K.; Singh, P.; Singh, R. Bioprospecting of Strains of Streptococcus Thermophilus from Indian Fermented Milk Products for Folate Production. Dairy Sci. Technol. 2011, 91, 237–246. [Google Scholar] [CrossRef]
- Padalino, M.; Perez-Conesa, D.; López-Nicolás, R.; Frontela-Saseta, C.; Ros, G. Effect of Fructooligosaccharides and Galactooligosaccharides on the Folate Production of some Folate-Producing Bacteria in Media Cultures or Milk. Int. Dairy J. 2012, 27, 27–33. [Google Scholar] [CrossRef]
- Lin, M.Y.; Young, C.M. Biosynthesis of Folates by Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus. J. Food Drug Anal. 2000, 8, 195–199. [Google Scholar]
- Laiño, J.E.; del Valle, M.J.; de Giori, G.S.; LeBlanc, J.G. Development of a High Folate Concentration Yogurt Naturally Bio-Enriched Using Selected Lactic Acid Bacteria. LWT 2013, 54, 1–5. [Google Scholar] [CrossRef]
- Ayad, E.H. Starter Culture Development for Improving Safety and Quality of Domiati Cheese. Food Microbiol. 2009, 26, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Stoś, K.; Przygoda, B.; Matczuk, E.; Stolińska-Fiedorowicz, H.; Kłys, W. Witaminy. In Normy dla populacji Polski; Jarosz, M., Ed.; IŻŻ: Warsaw, Poland, 2017; pp. 166–170. Available online: https://ncez.pl/upload/normy-net-1.pdf (accessed on 30 August 2021).
- Korhola, M.; Hakonen, R.; Juuti, K.; Edelmann, M.; Kariluoto, S.; Nyström, L.; Sontag-Strohm, T.; Piironen, V. Production of Folate in Oat Bran Fermentation by Yeasts Isolated from Barley and Diverse Foods. J. Appl. Microbiol. 2014, 117, 679–689. [Google Scholar] [CrossRef]
- Paszczyk, B.; Łuczyńska, J.; Polak-Śliwińska, M. The Effect of Storage on the Yogurt Fatty Acid Profile. Mljekarstvo 2020, 70, 59–70. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Wijendran, V.; Hayes, K. Dietary N-6 and N-3 Fatty Acid Balance and Cardiovascular Health. Annu. Rev. Nutr. 2004, 24, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Serafeimidou, A.; Zlatanos, S.; Kritikos, G.; Tourianis, A. Change of Fatty Acid Profile, Including Conjugated Linoleic Acid (CLA) Content, during Refrigerated Storage of Yogurt Made of Cow and Sheep Milk. J. Food Compos. Anal. 2013, 31, 24–30. [Google Scholar] [CrossRef]
- Florence, A.C.R.; Béal, C.; Silva, R.C.; Bogsan, C.S.; Pilleggi, A.L.O.; Gioielli, L.A.; Oliveira, M.N. Fatty Acid Profile, Trans-Octadecenoic, A-Linolenic and Conjugated Linoleic Acid Contents Differing in Certified Organic and Conventional Probiotic Fermented Milks. Food Chem. 2012, 135, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Paszczyk, B.; Brandt, W.; Łuczyńska, J. Isomers of c18:1 and c18:2 Acids in Fresh and Stored Fermented Milks Produced with Selected Starter Cultures. Czech J. Food Sci. 2016, 34, 391–396. [Google Scholar] [CrossRef]
- Patring, J.; Wandel, M.; Jägerstad, M.; Frølich, W. Folate content of Norwegian and Swedish Flours and Bread Analysed by Use of Liquid Chromatography—Mass Spectrometry. J. Food Compos. Anal. 2009, 22, 649–656. [Google Scholar] [CrossRef]
- Konings, E.J.M. A Validated Liquid Chromatographic Method for Determining Folates in Vegetables, Milk Powder, Liver, and Flour. J. AOAC Int. 1999, 82, 119–127. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Rogers, L.; Gregory, J.F. Determination of Folate in Cereal-Grain Food Products Using Trienzyme Extraction and Combined Affinity and Reversed-Phase Liquid Chromatography. J. Agric. Food Chem. 1997, 45, 407–413. [Google Scholar] [CrossRef]
- Jastrebova, J.; Witthöft, C.; Grahn, A.; Svensson, U.; Jägerstad, M. HPLC Determination of Folates in Raw and Processed Beetroots. Food Chem. 2003, 80, 579–588. [Google Scholar] [CrossRef]
- Czarnowska, M.; Gujska, E. Effect of Freezing Technology and Storage Conditions on Folate Content in Selected Vegetables. Plant Foods Hum. Nutr. 2012, 67, 401–406. [Google Scholar] [CrossRef]
- Blakley, R.L. The Biochemistry of Folic Acid and Related Pteridines. In North-Holland Research Monographs; North-Holland Publishing Company: Amsterdam, The Netherlands, 1969; pp. 1–570. [Google Scholar]
- STATISTICA, version 13.1; Statsoft: Kraków, Poland, 2013.
- Christie, W.W. The Isolation of Lipids from Tissues. Recommended Procedures. Chloroform-Methanol (2:1, V/V) Extraction and “Folch” Wash. In Lipid Analysis. Isolation, Separation, Identification and Structural Analysis of Lipids; Christie, W.W., Ed.; Pergamon Press Oxford: New York, NY, USA; Toronto, Japan; Sydney, Australia; Braunschweig, Germany, 1973; pp. 39–40. [Google Scholar]
- Milkfat: Preparation of Fatty Acid Methyl Esters; ISO 15884:2002 (IDF 182:2002); International Organization for Standardization: Geneva, Switzerland, 2002.
- Roach, J.A.; Mossoba, M.M.; Yurawecz, M.; Kramer, J.K. Chromatographic Separation and Identification of Conjugated Linoleic Acid Isomers. Anal. Chim. Acta 2002, 465, 207–226. [Google Scholar] [CrossRef]
- Kramer, J.K.G.; Cruz-Hernandez, C.; Deng, Z.; Zhou, J.; Jahreis, G.; Dugan, M.E.R. Analysis of Conjugated Linoleic Acid and Trans 18:1 Isomers in Synthetic and Animal Products. Am. J. Clin. Nutr. 2004, 79, 1137S–1145S. [Google Scholar] [CrossRef]
- Ledoux, M.; Chardigny, J.-M.; Darbois, M.; Soustre, Y.; Sébédio, J.-L.; Laloux, L. Fatty Acid Composition of French Butters, with Special Emphasis on Conjugated Linoleic Acid (CLA) Isomers. J. Food Compos. Anal. 2005, 18, 409–425. [Google Scholar] [CrossRef]
| Fermented Milk (FM) | Days of Storage at 8 °C | H4folate (µg/kg) | 5-CH3-H4folate(µg/kg) | Total Folates (Sum as Folic Acid) (µg/kg) | Folates Losses during Storage (%) |
|---|---|---|---|---|---|
| FM 1 Lactobacillus delbrueckii subsp. bulgaricus, and Streptococcus thermophilus | 0 | 22.1 1 ± 1.1 | 24.4 ± 1.3 | 45.3 a2 ± 0.3 | - |
| 7 | 12.3 ± 0.8 | 15.2 ± 0.8 | 27.1 b ± 0.8 | 40 | |
| 14 | 8.4 ± 0.2 | 14.2 ± 0.5 | 22.3 c ± 0.8 | 50 | |
| 21 | 11.3 ± 0.6 | 10.3 ± 0.3 | 20.3 d ± 0.9 | 55 | |
| FM 2 Lactococcus lactis subsp. cremoris, Leuconostoc, Lactococcus lactis subsp. lactis, and Lactococcus lactis subsp. lactis diacetylactis | 0 | 12.2 ± 0.2 | 61.2 ± 3.1 | 71.1 a ± 3.4 | - |
| 7 | 9.4 ± 0.4 | 25.2 ± 2.2 | 33.2 b ± 2.1 | 53 | |
| 14 | 12.3 ± 0.1 | 20.0 ± 2.4 | 31.2 b ± 2.3 | 56 | |
| 21 | 9.2 ± 0.2 | 15.3 ± 1.1 | 23.3 c ± 1.1 | 67 | |
| FM 3 Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus delbrueckii subsp. lactis, Streptococcus thermophilus, and Bifidobacterium bifidum | 0 | 27.5 ± 2.3 | 84.0 ± 2.7 | 105.4 a ± 6.1 | - |
| 7 | 17.8 ± 1.1 | 63.3 ± 0.9 | 77.2 b ± 3.6 | 27 | |
| 14 | 15.2 ± 0.8 | 34.8 ± 3.2 | 49.4 c ± 4.8 | 53 | |
| 21 | 11.3 ± 0.6 | 19.2 ± 1.8 | 30.3 d ± 2.6 | 71 | |
| FM 4 Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus, and Bifidobacterium bifidum | 0 | 23.4 ± 0.2 | 41.1 ± 2.1 | 60.3 b ± 1.4 | - |
| 7 | 16.2 ± 0.7 | 65.4 ± 5.2 | 79.2 a ± 6.3 | +31 | |
| 14 | 13.3 ± 1.4 | 39.0 ± 3.3 | 51.1 c ± 4.1 | 15 | |
| 21 | 8.4 ± 0.6 | 23.1 ± 2.4 | 31.2 d ± 2.1 | 48 |
| Folate Content 1 in Fermented Milk | Coverage of the Daily Demand for Folate (%) 2 | Producing Microorganisms | Ref. |
|---|---|---|---|
| 5–50 µg/kg | 1–12 | S. thermophilus | [50] |
| 20–50 µg/L | 5–12 | S. thermophilus | [55] |
| 50–200 µg/kg | 12–50 | S. thermophilus | [56] |
| 250–280 µg/L | 63–70 | L. amylovorus, S. thermophilus and L. delbruecki subsp. bulgaricus | [46] |
| 10–70 µg/L | 3–18 | L. delbruecki subsp. bulgaricus | [57] |
| 80–180 µg/L | 20–45 | L. delbruecki subsp. bulgaricus, S. thermophilus | [58] |
| 27–35 µg/kg | 7–9 | L. delbruecki subsp. bulgaricus, S. thermophilus | [47] |
| 45 µg/kg | 11 | L. delbruecki subsp. bulgaricus, S. thermophilus | Own study |
| 50–100 µg/L | 13–25 | B. longum | [16] |
| 60–90 µg/kg | 15–23 | S. thermophilus, B. animalis | [17] |
| 30–60 µg/kg | 8–15 | S. thermophilus, B. longum | [49] |
| 60 | 15 | L. delbrueckii subsp. bulgaricus, S. thermophilus, and B. bifidum | Own study |
| 105 µg/kg | 26 | L. delbruecki subsp. bulgaricus, L. lactis, S. thermophilus, and B. bifidum | Own study |
| 120–130 µg/kg | 30–33 | Lc. lactis subsp. lactis, Lc. lactis subsp. cremoris | [59] |
| 2–20 µg/L | 1–5 | Lc. lactis subsp. cremoris | [53] |
| 71 µg/kg | 18 | Lc. lactis subsp. cremoris, Leuconostoc, Lc. lactis subsp. lactis, and L. lactis subsp. lactis diacetylactis | Own study |
| Fermented Milk (FM) | Fatty Acids | Days of Storage at 8 °C | |||
|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | ||
| FM 1 Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus | SCFA 1 | 98.58 ± 8.34 a | 74.76 ± 4.16 b | 68.97 ± 7.79 b | 94.02 ± 9.97 a |
| SFA | 481.28 ± 17.06 b | 541.03 ± 20.45 a | 546.80 ± 22.96 a | 562.27 ± 19.44 a | |
| MUFA | 58.33 ± 5.39 a | 53.81 ± 2.73 a | 56.48 ± 5.06 a | 58.30 ± 8.41 a | |
| PUFA | 21.78 ± 2.40 a2 | 20.26 ± 2.44 a | 21.29 ± 2.63 a | 22.27 ± 3.05 a | |
| n-3 | 2.05 ± 0.01 a | 2.03 ± 0.11 a | 2.14 ± 0.18 a | 2.37 ± 0.39 a | |
| n-6 | 15.21 ± 0.27 a | 15.25 ± 0.63 a | 15.93 ± 0.88 a | 16.76 ± 2.60 a | |
| n-6/n-3 | 7.41 ± 0.14 a | 7.53 ± 0.12 a | 7.47 ± 0.25 a | 7.07 ± 0.11 a | |
| CLA | 3.51 ± 0.22 a | 3.38 ± 0.11 a | 3.59 ± 0.31 a | 3.73 ± 0.67 a | |
| FM 2 Lactococcus lactis subsp. cremoris, Leuconostoc, Lactococcus lactis subsp. lactis, and Lactococcus lactis subsp. lactis diacetylactis | SCFA | 99.92 ± 2.92 a | 96.31 ± 13.94 a | 73.51 ± 7.24 b | 83.12 ± 5.47 b |
| SFA | 489.77 ± 9.15 a | 496.57 ± 25.64 a | 477.98 ± 20.01 a | 502.29 ± 8.33 a | |
| MUFA | 52.44 ± 3.21 c | 58.13 ± 3.03 b | 53.40 ± 3.88 bc | 63.49 ± 3.71 a | |
| PUFA | 20.17 ± 2.58 a | 19.03 ± 2.03 a | 18.70 ± 2.17 a | 20.73 ± 3.95 a | |
| n-3 | 2.03 ± 0.03 a | 2.05 ± 0.03 a | 2.06 ± 0.02 a | 2.06 ± 0.03 a | |
| n-6 | 13.97 ± 0.60 a | 12.97 ± 0.47 a | 12.90 ± 0.49 a | 13.86 ± 1.01 a | |
| n-6/n-3 | 6.87 ± 0.39 a | 6.32 ± 0.14 b | 6.25 ± 0.30 b | 6.71 ± 0.40 ab | |
| CLA | 3.61 ± 0.17 ab | 3.84 ± 0.16 a | 3.43 ± 0.38 b | 3.93 ± 0.09 a | |
| FM 3 Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus delbrueckii subsp. lactis, Streptococcus thermophilus, and Bifidobacterium bifidum | SCFA | 67.96 ± 5.52 c | 65.32 ± 6.94 c | 78.14 ± 4.27 b | 88.37 ± 3.49 a |
| SFA | 607.54 ± 20.84 a | 468.87 ± 12.05 b | 478.63 ± 9.23 b | 612.00 ± 25.36 a | |
| MUFA | 52.15 ± 13.09 ab | 43.24 ± 4.99 b | 48.79 ± 2.76 b | 62.75 ± 2.50 a | |
| PUFA | 18.02 ± 4.60 a | 16.59 ± 1.40 a | 17.63 ± 2.85 a | 17.83 ± 2.00 a | |
| n-3 | 1.86 ± 0.57 a | 1.65 ± 0.22 a | 1.80 ± 0.11 a | 1.68 ± 0.12 a | |
| n-6 | 13.70 ± 3.98 a | 12.29 ± 1.11 a | 13.35 ± 1.04 a | 12.76 ± 0.46 a | |
| n-6/n-3 | 7.39 ± 0.16 a | 7.49 ± 0.39 a | 7.43 ± 0.32 a | 7.63 ± 0.78 a | |
| CLA | 2.54 ± 0.28 c | 2.71 ± 0.30 bc | 3.06 ± 0.18 ab | 3.42 ± 0.23 a | |
| FM 4 Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus, and Bifidobacterium bifidum | SCFA | 70.46 ± 15.24 a | 64.76 ± 4.16 a | 74.27 ± 7.45 a | 75.02 ± 4.19 a |
| SFA | 570.24 ± 9.96 a | 538.53 ± 22.74 a | 563.05 ± 22.10 a | 567.52 ± 24.15 a | |
| MUFA | 52.15 ± 16.95 a | 53.81 ± 2.73 a | 56.48 ± 5.06 a | 58.30 ± 8.41 a | |
| PUFA | 18.07 ± 4.58 a | 20.26 ± 2.44 a | 20.04 ± 2.67 a | 20.82 ± 2.08 a | |
| n-3 | 1.86 ± 0.53 a | 2.03 ± 0.11 a | 2.14 ± 0.18 a | 2.24 ± 0.13 a | |
| n-6 | 13.59 ± 3.93 a | 15.25 ± 0.63 a | 15.68 ± 0.69 a | 15.44 ± 0.65 a | |
| n-6/n-3 | 7.33 ± 0.15 ab | 7.53 ± 0.12 a | 7.36 ± 0.46 a | 6.89 ± 0.28 b | |
| CLA | 2.88 ± 0.65 b | 3.38 ± 0.11 ab | 3.59 ± 0.31 ab | 3.89 ± 0.65 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czarnowska-Kujawska, M.; Paszczyk, B. Changes in the Folate Content and Fatty Acid Profile in Fermented Milk Produced with Different Starter Cultures during Storage. Molecules 2021, 26, 6063. https://doi.org/10.3390/molecules26196063
Czarnowska-Kujawska M, Paszczyk B. Changes in the Folate Content and Fatty Acid Profile in Fermented Milk Produced with Different Starter Cultures during Storage. Molecules. 2021; 26(19):6063. https://doi.org/10.3390/molecules26196063
Chicago/Turabian StyleCzarnowska-Kujawska, Marta, and Beata Paszczyk. 2021. "Changes in the Folate Content and Fatty Acid Profile in Fermented Milk Produced with Different Starter Cultures during Storage" Molecules 26, no. 19: 6063. https://doi.org/10.3390/molecules26196063
APA StyleCzarnowska-Kujawska, M., & Paszczyk, B. (2021). Changes in the Folate Content and Fatty Acid Profile in Fermented Milk Produced with Different Starter Cultures during Storage. Molecules, 26(19), 6063. https://doi.org/10.3390/molecules26196063