Kinetic Behavior of Quaternary Ammonium Hydroxides in Mixed Methane and Carbon Dioxide Hydrates
Abstract
1. Introduction
2. Results and Discussion
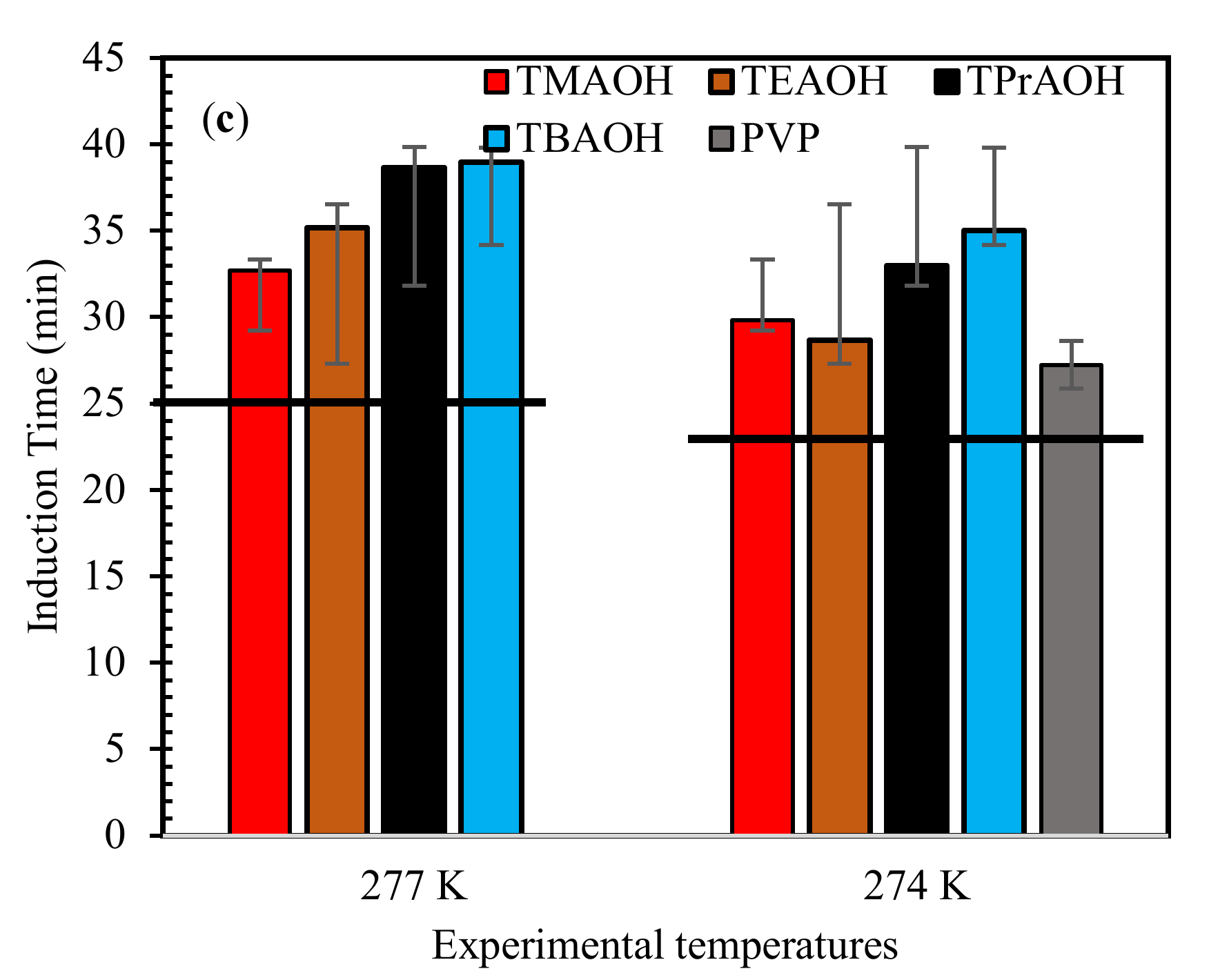
2.1. Influence of QAHs on Induction Time of Mixed Gas Hydrates
2.2. Influence of QAHs on Relative Inhibition Performance of Mixed Gas Hydrates
2.3. Influence of QAHs on Initial Formation Rate of Mixed Gas Hydrates
2.4. Influence of QAHs on Consumption of Mixed Gas Hydrates
2.5. QAHs Molar Concentration Effect on Hydrate Formation
3. Materials and Methods
3.1. Materials and Sample Preparation
3.2. Experimental Set-Up and Kinetic Measurement
3.3. Kinetic Measurements of Gas Hydrate Inhibitors
3.3.1. Induction Time Measurement
3.3.2. Relative Inhibition Performance
3.3.3. Gas Chromatography (GC) Analysis of Mixed Gas Hydrates
3.3.4. Total Mixed Gas Uptake
3.3.5. Initial Formation Rate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Nomenclature
| Abbreviation | Description |
| QACs | Quaternary Ammonium Compounds |
| QAHs | Quaternary Ammonium Hydroxides |
| CO2 | Carbon dioxide |
| CH4 | Methane |
| AAs | Anti-agglomerates |
| GC | Gas chromatography |
| HLVE | Hydrate liquid vapor equilibrium |
| KHIs | Kinetic hydrate inhibitors |
| LDHIs | Low-dosage hydrate inhibitors |
| ΔnH | Gas uptake in hydrate phase (moles) |
| PEO | Polyethylene oxide |
| PVCap | Polyvinyl caprolactum |
| PVP | Polyvinyl pyroledinium |
| RIPinduction | Relative inhibition performance |
| TMACl | Tetramethylammonium chloride |
| THIs | Thermodynamic hydrate inhibitor |
| TMAOH | Tetramethylammonium hydroxide |
| TPrAOH | Tetrapropylammonium hydroxide |
| TBAOH | Tetrabutylammonium hydroxide |
| tinduction | Induction time (min) |
| TEAOH | Tetraethylammonium hydroxide |
| V | Gas-phase volume |
| P | Pressure |
| R | Gas constant |
| z | Gas compressibility factor |
| T | Temperature |
| C2H5 | Ethane |
| C3H8 | Propane |
| ΔT | Subcooling temperature |
| subscripts | |
| f | Final |
| k | Rate constant |
| 0 | Time zero |
| H | Hydrates |
| Units | |
| K | Kelvin |
| MPa | Megapascal |
| wt% | Wight percent |
| mmol/mol | Millimoles of gas per moles of water |
| min | Minute |
| Mol/min | Moles per minute |
| mL | Milliliters |
| rpm | Revolutions per minute |
| h | Hours |
References
- U.S. Energy Information Administration (EIA). International Energy Outlook 2017 Overview; U.S. Energy Inf. Adm.: Washington, DC, USA, 2017; IEO2017, 143; Available online: https://www.iea.org/reports/world-energy-outlook-2017 (accessed on 10 October 2020).
- Mokhatab, S.; Poe, W.A.; Mak, J.Y. Natural Gas Treating, 3rd ed.; Gulf Professional Publishing: Houston, TX, USA, 2015; pp. 181–222. [Google Scholar]
- Khan, M.S.; Bavoh, C.B.; Rahman, M.A.; Lal, B.; Quainoo, A.K.; Maulud, A.S. Assessing the Alkyl Chain Effect of Ammonium Hydroxides Ionic Liquids on the Kinetics of Pure Methane and Carbon Dioxide Hydrates. Energies 2020, 13, 3272. [Google Scholar] [CrossRef]
- Khan, M.S.; Bavoh, C.B.; Partoon, B.; Nashed, O.; Lal, B.; Mellon, N.B. Impacts of ammonium based ionic liquids alkyl chain on thermodynamic hydrate inhibition for carbon dioxide rich binary gas. J. Mol. Liq. 2018, 261, 283–290. [Google Scholar] [CrossRef]
- Khan, M.S.; Cornelius, B.B.; Lal, B.; Bustam, M.A. Kinetic Assessment of Tetramethyl Ammonium Hydroxide (Ionic Liquid) for Carbon Dioxide, Methane and Binary Mix Gas Hydrates. In Recent Advances in Ionic Liquids; Rahman, M.M., Ed.; IntechOpen: London, UK, 2018; pp. 159–179. [Google Scholar]
- Khan, M.S.; Bavoh, C.B.; Partoon, B.; Lal, B.; Bustam, M.A.; Shariff, A.M. Thermodynamic effect of ammonium based ionic liquids on CO2 hydrates phase boundary. J. Mol. Liq. 2017, 238, 533–539. [Google Scholar] [CrossRef]
- Qasim, A.; Khan, M.S.; Lal, B.; Shariff, A.M. A perspective on dual purpose gas hydrate and corrosion inhibitors for flow assurance. J. Pet. Sci. Eng. 2019, 183, 106418. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B. Pre-Screening of Ionic Liquids as Gas Hydrate Inhibitor via Application of COSMO-RS for Methane Hydrate. In Solvents, Ionic Liquids and Solvent Effects, 1st ed.; Glossman-Mitnik, D., Maciejewska, M., Eds.; IntechOpen: London, UK, 2019; pp. 1–29. [Google Scholar]
- Qasim, A.; Khan, M.S.; Lal, B.; Shariff, A.M. Phase equilibrium measurement and modeling approach to quaternary ammonium salts with and without monoethylene glycol for carbon dioxide hydrates. J. Mol. Liq. 2019, 282, 106–114. [Google Scholar] [CrossRef]
- Parrish, W.R.; Prausnitz, J.M. Dissociation Pressures of Gas Hydrates Formed by Gas Mixtures. Ind. Eng. Chem. Process Des. Dev. 1972, 11, 26–35. [Google Scholar] [CrossRef]
- Khan, M.S.; Partoon, B.; Bavoh, C.B.; Lal, B.; Mellon, N.B. Influence of tetramethylammonium hydroxide on methane and carbon dioxide gas hydrate phase equilibrium conditions. Fluid Phase Equilib. 2017, 440, 1–8. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Ofei, T.N.; Lal, B. Investigating the Potential Cuttings Transport Behavior of Ionic Liquids in Drilling Mud in the Presence of sII Hydrates. Energy Fuels 2020, 34, 2903–2915. [Google Scholar] [CrossRef]
- Yuha, Y.B.M.; Bavoh, C.B.; Lal, B.; Broni-Bediako, E. Methane Hydrate Phase Behaviour in EMIM-Cl Water Based Mud (WBM): An Experimental and Modelling Study. South Afr. J. Chem. Eng. 2020, 34, 47–56. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Ntow, T.; Lal, B.; Sharif, A.M.; Shahpin, M.H.B.A.; Sundramoorthy, J.D. Assessing the impact of an ionic liquid on NaCl/KCl/polymer water-based mud (WBM) for drilling gas hydrate-bearing sediments. J. Mol. Liq. 2019, 294, 111643. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Yuha, Y.B.; Tay, W.H.; Ofei, T.N.; Lal, B.; Mukhtar, H. Experimental and Modelling of the impact of Quaternary Ammonium Salts/Ionic Liquid on the rheological and hydrate inhibition properties of Xanthan gum water-based muds for Drilling Gas Hydrate-Bearing Rocks. J. Pet. Sci. Eng. 2019, 106468. [Google Scholar] [CrossRef]
- Carroll, J. Natural Gas Hydrates: A Guide for Engineers, 2nd ed.; Gulf Professional Publishing: Houston, TX, USA, 2009; pp. 1–277. [Google Scholar]
- Demirbas, A. Methane Gas Hydrate (Green Energy and Technology), 1st ed.; Springer: London, UK, 2010; pp. 1–181. [Google Scholar]
- Øian, J.B. Deep Water and Long Distances-Flow Assurance Challenges and Solutions. 2011. Available online: https://wenku.baidu.com/view/9e1797c6e2bd960591c677e2.html?re=view (accessed on 25 December 2020).
- Sloan, D.; Koh, C.; Sum, A.K.; Ballard, A.L.; Creek, J.; Eaton, M.; Lachance, J.; Mcmullen, N.; Palermo, T.; Shoup, G.; et al. Natural Gas Hydrates in Flow Assurance, 1st ed.; Gulf Professional Publishing: Houston, TX, USA, 2010; pp. 1–224. [Google Scholar]
- Sloan, E.D. A changing hydrate paradigm-From apprehension to avoidance to risk management. Fluid Phase Equilib. 2005, 228–229, 67–74. [Google Scholar] [CrossRef]
- Håvard, D. Oil and Gas Production Handbook-An Introduction to Oil and Gas Production, Transport, Refining and Petrochemical Industry, 1st ed.; ABB AS: Oslo, Norway, 2013; pp. 1–152. [Google Scholar]
- Koh, C.A.; Sloan, E.D.; Sum, A.K.; Wu, D.T. Fundamentals and Applications of Gas Hydrates. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Azarinezhad-mohammadi, R. A Chemical Based Wet Cold Flow Approach for Addressing Hydrate Flow Assurance Problems. Ph.D. Thesis, Heriot-Watt University, Edinburgh, UK, September 2010. [Google Scholar]
- Mokhatab, S.; Wilkens, R.J.; Leontaritis, K.J. A Review of Strategies for Solving Gas-Hydrate Problems in Subsea Pipelines. Energy Sourcespart A Recover. Util. Environ. Eff. 2007, 29, 39–45. [Google Scholar] [CrossRef]
- Lal, B.; Nashed, O. Introduction to Gas Hydrates. In Chemical Additives for Gas Hydrates (Green Energy and Technology), 1st ed.; Springer: Cham, Switzerland, 2020; pp. 1–20, 27–46. [Google Scholar]
- Tariq, M.; Rooney, D.; Othman, E.; Aparicio, S.; Atilhan, M.; Khraisheh, M. Gas Hydrate Inhibition: A Review of the Role of Ionic Liquids. Ind. Eng. Chem. Res. 2014, 53, 17855–17868. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Lal, B.; Ben-Awuah, J.; Khan, M.S.; Ofori-Sarpong, G. Kinetics of Mixed Amino Acid and Ionic Liquid on CO2 Hydrate Formation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 495, 012073. [Google Scholar] [CrossRef]
- Frostman, L.M. Anti-agglomerant hydrate inhibitors for prevention of hydrate plugs in deepwater systems. SPE Annu. Tech. Conf. Exhib. 2000, 23–24. [Google Scholar]
- Kelland, M.A.; Svartås, T.M.; Andersen, L.D. Gas hydrate anti-agglomerant properties of polypropoxylates and some other demulsifiers. J. Pet. Sci. Eng. 2009, 64, 1–10. [Google Scholar] [CrossRef]
- Kelland, M.A.; Svartaas, T.M.; Dybvik, L.A. Control of Hydrate Formation by Surfactants and Polymers. SPE Annu. Tech. Conf. Exhib. 1994, 431–438. [Google Scholar]
- Lal, B.; Qasim, A.; Shariff, A.M. Ionic Liquids Usage in Oil and Gas Industry. In Ionic Liquids in Flow Assurance, 1st ed.; Springer: Cham, Switzerland, 2021; pp. 1–16. [Google Scholar]
- Karaaslan, U.; Parlaktuna, M. Kinetic inhibition of methane hydrate by polymers. ACS Div. Fuel Chem. Prepr. 2002, 47, 355–358. [Google Scholar]
- Li, X.S.; Liu, Y.J.; Zeng, Z.Y.; Chen, Z.Y.; Li, G.; Wu, H.J. Equilibrium hydrate formation conditions for the mixtures of methane + ionic liquids + water. J. Chem. Eng. Data 2011, 56, 119–123. [Google Scholar] [CrossRef]
- Tariq, M.; Connor, E.; Thompson, J.; Khraisheh, M.; Atilhan, M.; Rooney, D. Doubly dual nature of ammonium-based ionic liquids for methane hydrates probed by rocking-rig assembly. RSC Adv. 2016, 6, 23827–23836. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Keong, L.K.; Sabil, K.M. Experimental evaluation and thermodynamic modelling of AILs alkyl chain elongation on methane riched gas hydrate system. Fluid Phase Equilib. 2018, 473, 300–309. [Google Scholar] [CrossRef]
- Nashed, O.; Dadebayev, D.; Khan, M.S.M.S.; Bavoh, C.B.C.B.; Lal, B.; Shariff, A.M. Experimental and modelling studies on thermodynamic methane hydrate inhibition in the presence of ionic liquids. J. Mol. Liq. 2018, 249, 886–891. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Bavoh, C.B.; Keong, L.K.; Bustam, A. Influence of Ammonium based Compounds for Gas Hydrate Mitigation: A Short Review. Indian J. Sci. Technol. 2017, 10, 1–6. [Google Scholar] [CrossRef]
- Khan, M.S.; Liew, C.S.; Kurnia, K.A.; Cornelius, B.; Lal, B. Application of COSMO-RS in Investigating Ionic Liquid as Thermodynamic Hydrate Inhibitor for Methane Hydrate. Procedia Eng. 2016, 148, 862–869. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Lal, B.; Nashed, O.; Khan, M.S.; Keong, L.K.; Bustam, M.A. COSMO-RS: An ionic liquid prescreening tool for gas hydrate mitigation. Chinese J. Chem. Eng. 2016, 11, 1619–1624. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Sabil, K.M.; Ahmed, I. Desalination of Seawater through Gas Hydrate Process: An Overview. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 55, 65–73. [Google Scholar]
- Khan, M.S.; Bavoh, C.B.; Lal, B.; Keong, L.K.; Mellon, N.B.; Bustam, M.A.; Shariff, A.M. Application of Electrolyte Based Model on Ionic Liquids-Methane Hydrates Phase Boundary. IOP Conf. Ser. Mater. Sci. Eng. 2018, 458, 012073. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Keong, L.K.; Ahmed, I. Tetramethyl ammonium chloride as dual functional inhibitor for methane and carbon dioxide hydrates. Fuel 2019, 236, 251–263. [Google Scholar] [CrossRef]
- Arjmandi, M.; Tohidi, B.; Danesh, A.; Todd, A.C. Is subcooling the right driving force for testing low-dosage hydrate inhibitors? Chem. Eng. Sci. 2005, 60, 1313–1321. [Google Scholar] [CrossRef]
- Cha, M.; Shin, K.; Seo, Y.; Shin, J.Y.; Kang, S.P. Catastrophic growth of gas hydrates in the presence of kinetic hydrate inhibitors. J. Phys. Chem. A 2013, 117, 13988–13995. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.D.; Hillis, B.G.; Hou, F. Transition Zone Theory Compared to Standard Models: Reexamining the Theory of Crystal Growth from Melts. J. Phys. Chem. C 2020, 124, 18724–18740. [Google Scholar] [CrossRef]
- Kamal, M.S.; Hussein, I.A.; Sultan, A.S.; Von Solms, N. Application of various water soluble polymers in gas hydrate inhibition. Renew. Sustain. Energy Rev. 2016, 60, 206–225. [Google Scholar] [CrossRef]
- Erfani, A.; Varaminian, F.; Muhammadi, M. Gas hydrate formation inhibition using low dosage hydrate inhibitors. In Proceedings of the 2nd National Iranian Conference on Gas Hydrate (NICGH), Babul, Iran, 14–15 May 2013. [Google Scholar]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press Taylor Francis: New York, NY, USA, 2008; Volume 87. [Google Scholar]
- Nashed, O.; Koh, J.C.H.; Lal, B. Physical-chemical Properties of Aqueous TBAOH Solution for Gas Hydrates Promotion. Procedia Eng. 2016, 148, 1351–1356. [Google Scholar] [CrossRef][Green Version]
- Kumar, A.; Bhattacharjee, G.; Kulkarni, B.D.; Kumar, R. Role of Surfactants in Promoting Gas Hydrate Formation. Ind. Eng. Chem. Res. 2015, 54, 12217–12232. [Google Scholar] [CrossRef]
- Sun, M.; Firoozabadi, A. Gas hydrate powder formation—Ultimate solution in natural gas flow assurance. Fuel 2015, 146, 1–5. [Google Scholar] [CrossRef]
- Kartikawati, N.A.; Safdar, R.; Lal, B.; Mutalib, M.I.B.A.; Shariff, A.M. Measurement and correlation of the physical properties of aqueous solutions of ammonium based ionic liquids. J. Mol. Liq. 2018, 253, 250–258. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Shariff, A.M.; Mukhtar, H. Ammonium hydroxide ILs as dual-functional gas hydrate inhibitors for binary mixed gas (carbon dioxide and methane) hydrates. J. Mol. Liq. 2019, 274, 33–44. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Partoon, B.; Keong, L.K.; Bustam, M.A.; Mellon, N.B. Experimental Evaluation of a Novel Thermodynamic Inhibitor for CH4 and CO2 Hydrates. Procedia Eng. 2016, 148, 932–940. [Google Scholar] [CrossRef]
- Sa, J.H.; Lee, B.R.; Park, D.H.; Lee, K.H.; Han, K.; Chun, H.D.; Lee, K.H. Amino acids as natural inhibitors for hydrate formation in CO2 sequestration. Environ. Sci. Technol. 2011, 45, 5885–5891. [Google Scholar] [CrossRef] [PubMed]
- Sa, J.-H.; Kwak, G.-H.; Han, K.; Ahn, D.; Cho, S.J.; Lee, J.D.; Lee, K.-H. Inhibition of methane and natural gas hydrate formation by altering the structure of water with amino acids. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bavoh, C.B.; Lal, B.; Osei, H.; Sabil, K.M.; Mukhtar, H. A review on the role of amino acids in gas hydrate inhibition, CO2 capture and sequestration, and natural gas storage. J. Nat. Gas Sci. Eng. 2019, 64, 52–71. [Google Scholar] [CrossRef]
- Mech, D.; Pandey, G.; Sangwai, J.S. Effect of Molecular Weight of Polyethylene Glycol on the Equilibrium Dissociation Pressures of Methane Hydrate System. J. Chem. Eng. Data 2015, 60, 1878–1885. [Google Scholar] [CrossRef]
- Khan, M.S.; Yaqub, S.; Manner, N.; Karthwathi, N.A.; Qasim, A.; Mellon, N.B.; Lal, B. Experimental Equipment Validation for Methane (CH4) and Carbon Dioxide (CO2) Hydrates. IOP Conf. Ser. Mater. Sci. Eng. 2018, 344, 1–10. [Google Scholar]
- Bavoh, C.B.; Khan, M.S.; Lal, B.; Bt Abdul Ghaniri, N.I.; Sabil, K.M. New methane hydrate phase boundary data in the presence of aqueous amino acids. Fluid Phase Equilib. 2018, 478, 129–133. [Google Scholar] [CrossRef]
- Foo, K.S.; Khan, M.S.; Lal, B.; Sufian, S. Semi-clathratic impact of tetrabutylammonium hydroxide on the carbon dioxide hydrates. IOP Conf. Ser. Mater. Sci. Eng. 2018, 458, 012060. [Google Scholar] [CrossRef]
- Nashed, O.; Sabil, K.M.; Ismail, L.; Japper-Jaafar, A.; Lal, B. Mean induction time and isothermal kinetic analysis of methane hydrate formation in water and imidazolium based ionic liquid solutions. J. Chem. Thermodyn. 2018, 117, 147–154. [Google Scholar] [CrossRef]
- Nashed, O.; Partoon, B.; Lal, B.; Sabil, K.M.; Shariff, A.M. Review the impact of nanoparticles on the thermodynamics and kinetics of gas hydrate formation. J. Nat. Gas Sci. Eng. 2018, 55, 452–465. [Google Scholar] [CrossRef]
- Kashchiev, D.; Firoozabadi, A. Induction time in crystallization of gas hydrates. J. Cryst. Growth 2003, 250, 499–515. [Google Scholar] [CrossRef]
- Kang, S.P.; Shin, J.Y.; Lim, J.S.; Lee, S. Experimental measurement of the induction time of natural gas Hydrate and its prediction with polymeric kinetic inhibitor. Chem. Eng. Sci. 2014, 116, 817–823. [Google Scholar] [CrossRef]
- Mohammadi, A.; Manteghian, M. The induction time of hydrate formation from a carbon dioxide-methane gas mixture. Pet. Sci. Technol. 2014, 32, 37–41. [Google Scholar] [CrossRef]
- Koh, C.A.; Westacott, R.E.; Zhang, W.; Hirachand, K.; Creek, J.L.; Soper, A.K. Mechanisms of gas hydrate formation and inhibition. Fluid Phase Equilib. 2002, 194–197, 143–151. [Google Scholar] [CrossRef]
- Babaee, S.; Hashemi, H.; Mohammadi, A.H.; Naidoo, P.; Ramjugernath, D. Kinetic and thermodynamic behaviour of CF4 clathrate hydrates. J. Chem. Thermodyn. 2015, 81, 52–59. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Lal, B.; Keong, L.K.; Binti Jasamai, M.; Binti Idress, M. Synergic Kinetic Inhibition Effect of EMIM-Cl + PVP on CO2 Hydrate Formation. Procedia Eng. 2016, 148, 1232–1238. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Wong, A.J.H.; Babu, P.; Kumar, R.; Kulprathipanja, S.; Rangsunvigit, P.; Linga, P. Rapid methane hydrate formation to develop a cost effective large scale energy storage system. Chem. Eng. J. 2016, 290, 161–173. [Google Scholar] [CrossRef]
- Partoon, B.; Sabil, K.M.; Roslan, H.; Lal, B.; Keong, L.K. Impact of acetone on phase boundary of methane and carbon dioxide mixed hydrates. Fluid Phase Equilib. 2016, 412, 51–56. [Google Scholar] [CrossRef]
- Sabil, K.M.; Nashed, O.; Lal, B.; Ismail, L.; Japper-Jaafar, A.; Japper-, A.; Nashed, O.; Lal, B.; Ismail, L.; Japper-Jaafar, A. Experimental investigation on the dissociation conditions of methane hydrate in the presence of imidazolium-based ionic liquids. J. Chem. Thermodyn. 2015, 84, 7–13. [Google Scholar] [CrossRef]
- Partoon, B.; Javanmardi, J. Effect of mixed thermodynamic and kinetic hydrate promoters on methane hydrate phase boundary and formation kinetics. J. Chem. Eng. Data 2013, 58, 501–509. [Google Scholar] [CrossRef]






| Chemical | Purity (wt%) | MW (g mol−1) | Formula | Supplier |
|---|---|---|---|---|
| Water | Deionized | 18.02 | H2O | Self-prepared |
| Mixed gas (30% CO2 + 70% CH4) | 99.00% | 24.43 | - | Gas Walker Sdn Bhd |
| Mixed gas (50% CO2 + 50% CH4) | 99.00% | 30.02 | - | Gas Walker Sdn Bhd |
| Mixed gas (70% CO2 + 30% CH4) | 99.00% | 35.62 | - | Gas Walker Sdn Bhd |
| Tetrabutylammonium hydroxide | 99.00% | 259.47 | TBAOH | Merck Millipore |
| Tetraethylammonium hydroxide | 99.00% | 147.26 | TEAOH | Merck Millipore |
| Tetrapropylammonium hydroxide | 99.00% | 203.36 | TPrAOH | Merck Millipore |
| Tetramethylammonium hydroxide | 99.00% | 91.15 | TMAOH | Merck Millipore |
| Polyvinylpyrrolidone | 99.00% | 160,000 | PVP | Merck Millipore |
| Mixed Gas Composition | Temperature (K) | Pressure Range (MPa) |
|---|---|---|
| 30% CO2 + 70% CH4 | 274.0 and 277.0 | 7.50 |
| 50% CO2 + 50% CH4 | 274.0 and 277.0 | 6.50 |
| 70% CO2 + 30% CH4 | 274.0 and 277.0 | 5.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.S.; Bavoh, C.B.; Foo, K.S.; Shariff, A.M.; Kassim, Z.; Othman, N.A.B.; Lal, B.; Ahmed, I.; Rahman, M.A.; Gomari, S.R. Kinetic Behavior of Quaternary Ammonium Hydroxides in Mixed Methane and Carbon Dioxide Hydrates. Molecules 2021, 26, 275. https://doi.org/10.3390/molecules26020275
Khan MS, Bavoh CB, Foo KS, Shariff AM, Kassim Z, Othman NAB, Lal B, Ahmed I, Rahman MA, Gomari SR. Kinetic Behavior of Quaternary Ammonium Hydroxides in Mixed Methane and Carbon Dioxide Hydrates. Molecules. 2021; 26(2):275. https://doi.org/10.3390/molecules26020275
Chicago/Turabian StyleKhan, Muhammad Saad, Cornelius Borecho Bavoh, Khor Siak Foo, Azmi Mohd Shariff, Zamzila Kassim, Nurzatil Aqmar Bt Othman, Bhajan Lal, Iqbal Ahmed, Mohammad Azizur Rahman, and Sina Rezaei Gomari. 2021. "Kinetic Behavior of Quaternary Ammonium Hydroxides in Mixed Methane and Carbon Dioxide Hydrates" Molecules 26, no. 2: 275. https://doi.org/10.3390/molecules26020275
APA StyleKhan, M. S., Bavoh, C. B., Foo, K. S., Shariff, A. M., Kassim, Z., Othman, N. A. B., Lal, B., Ahmed, I., Rahman, M. A., & Gomari, S. R. (2021). Kinetic Behavior of Quaternary Ammonium Hydroxides in Mixed Methane and Carbon Dioxide Hydrates. Molecules, 26(2), 275. https://doi.org/10.3390/molecules26020275









