Physico-Chemical Characterization and Antimicrobial Properties of Hybrid Film Based on Saponite and Phloxine B
Abstract
:1. Introduction
2. Results and Discussion
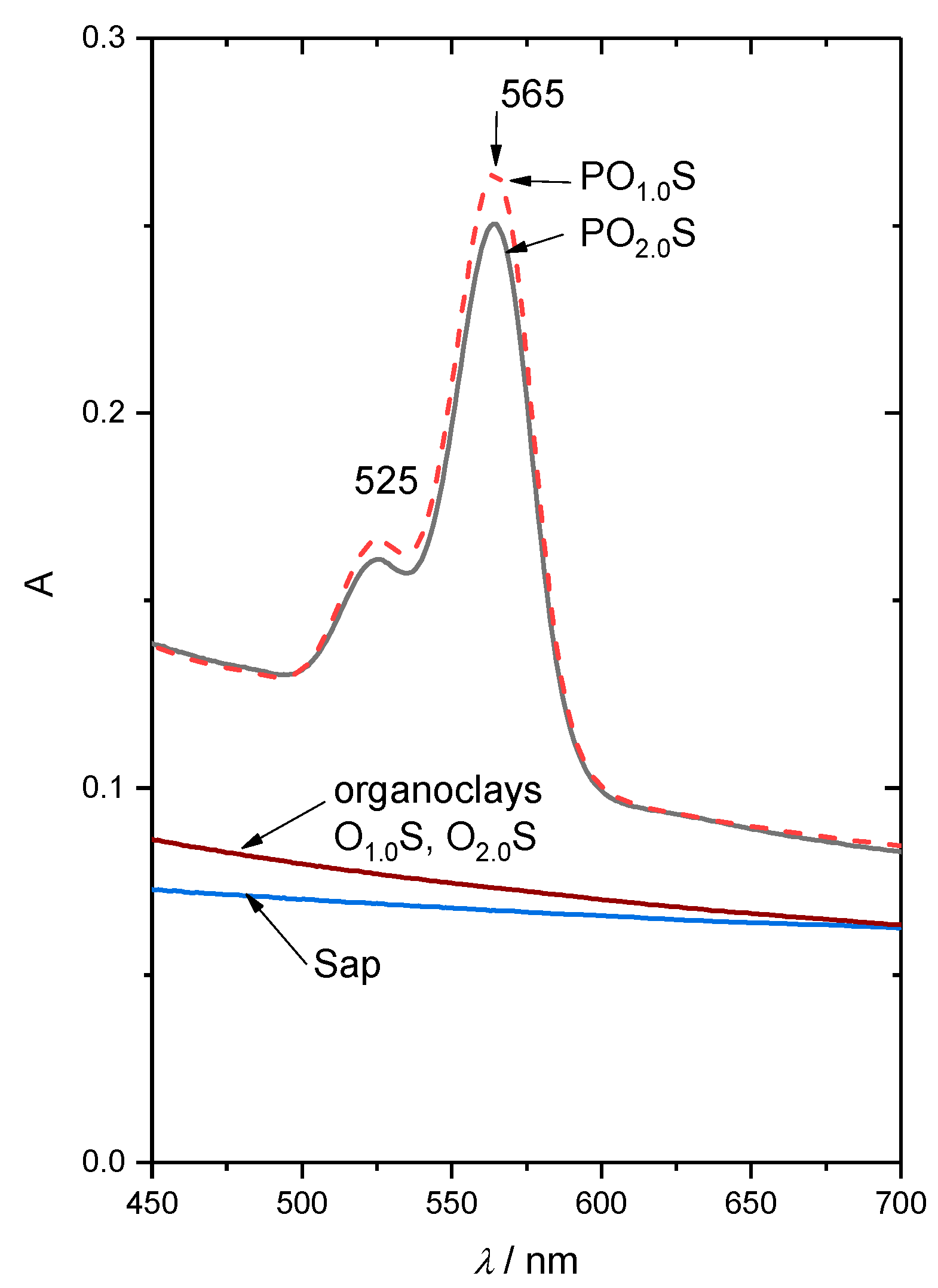
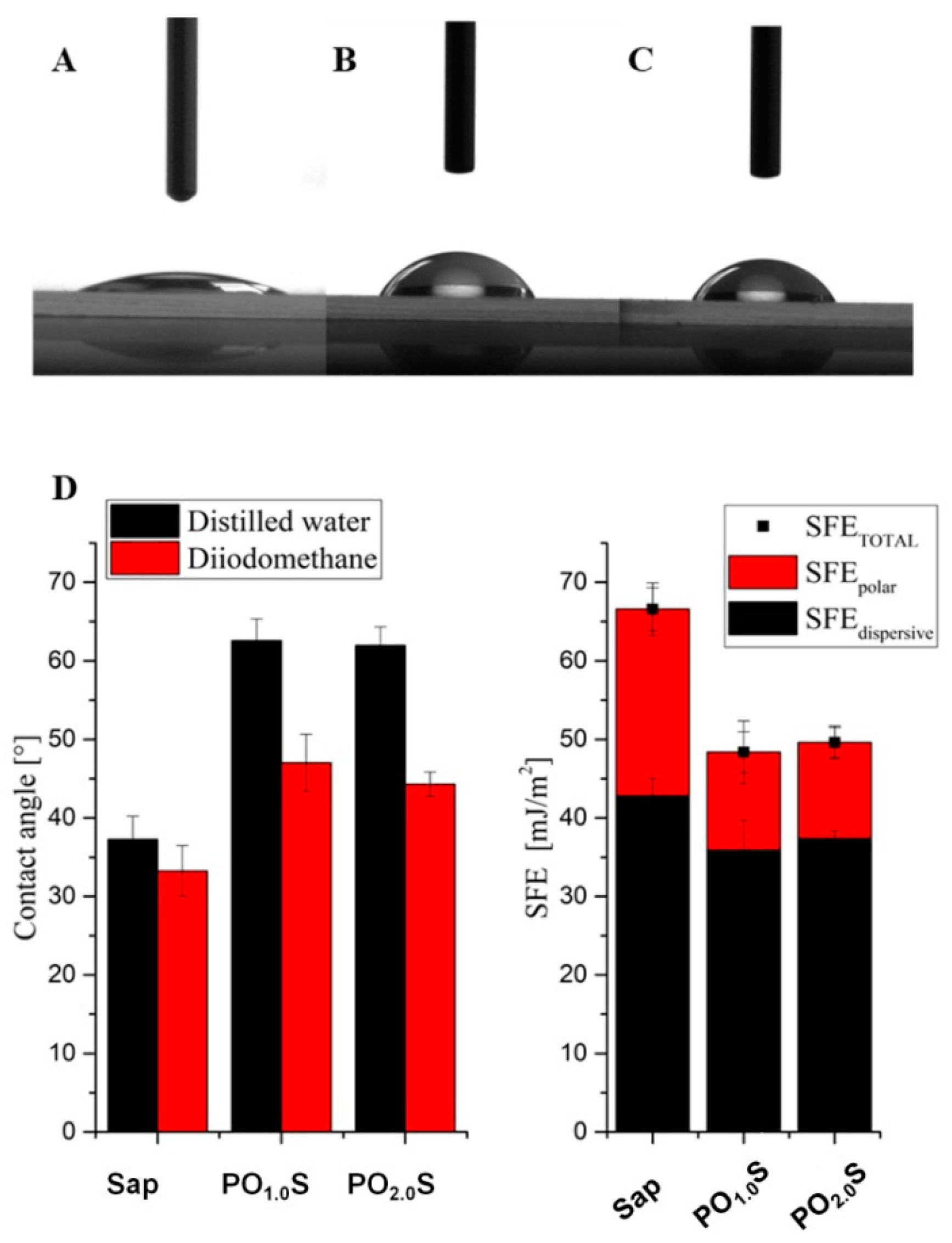
2.1. Physico-Chemical Characterization
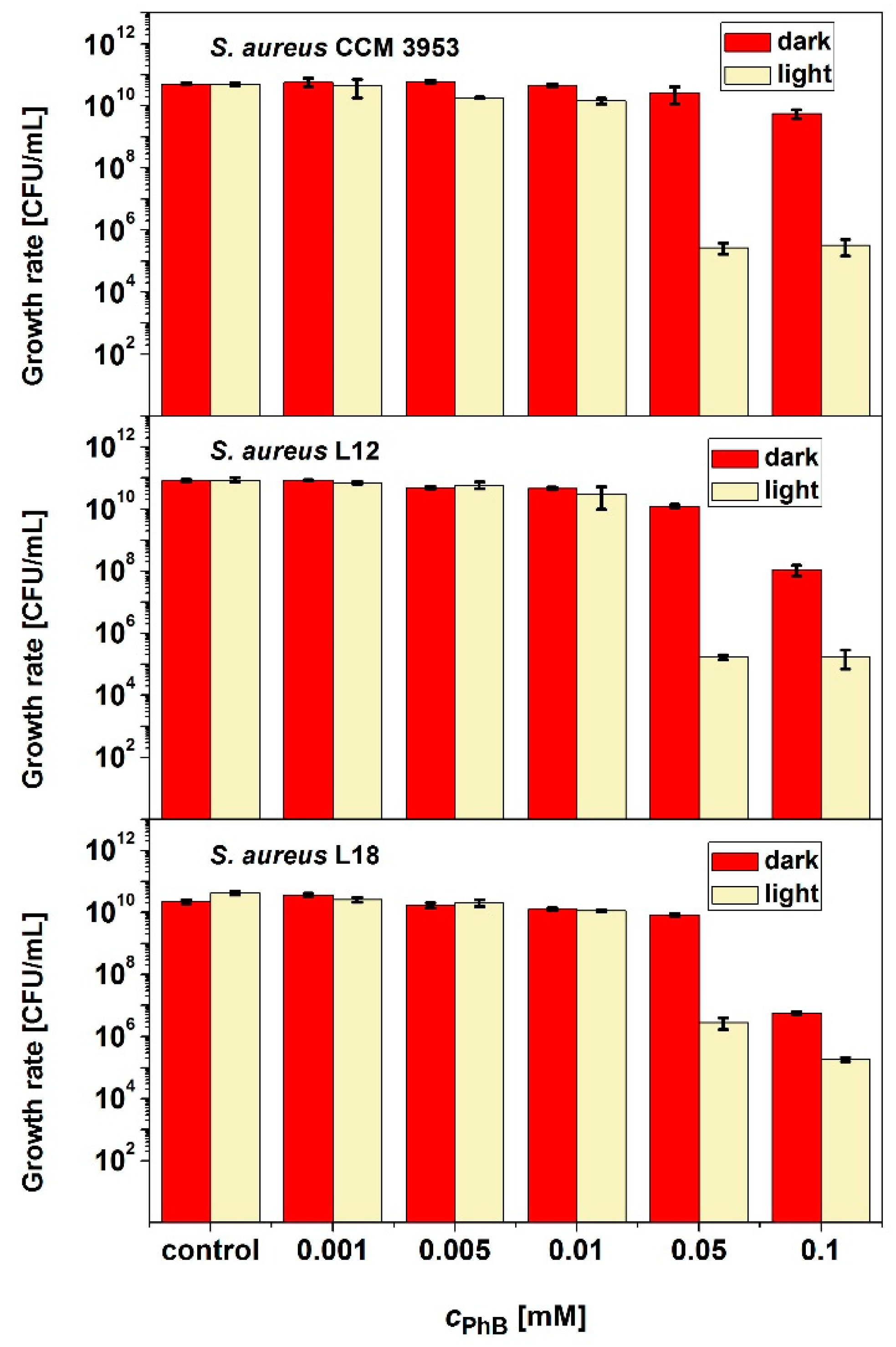
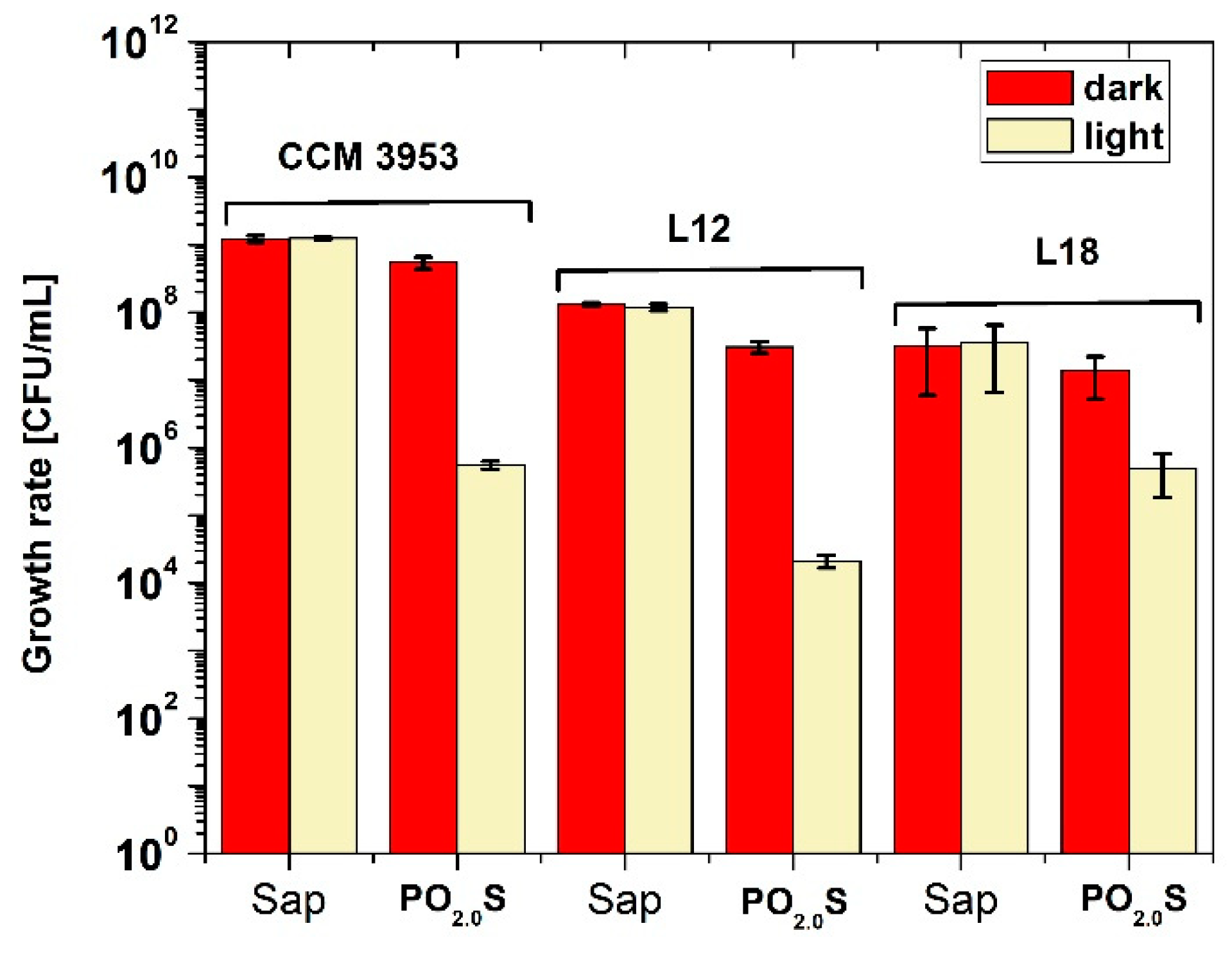
2.2. Antimicrobial Activity of PDI
2.3. Anti-Biofilm Effectiveness of PDI with Hybrid Film
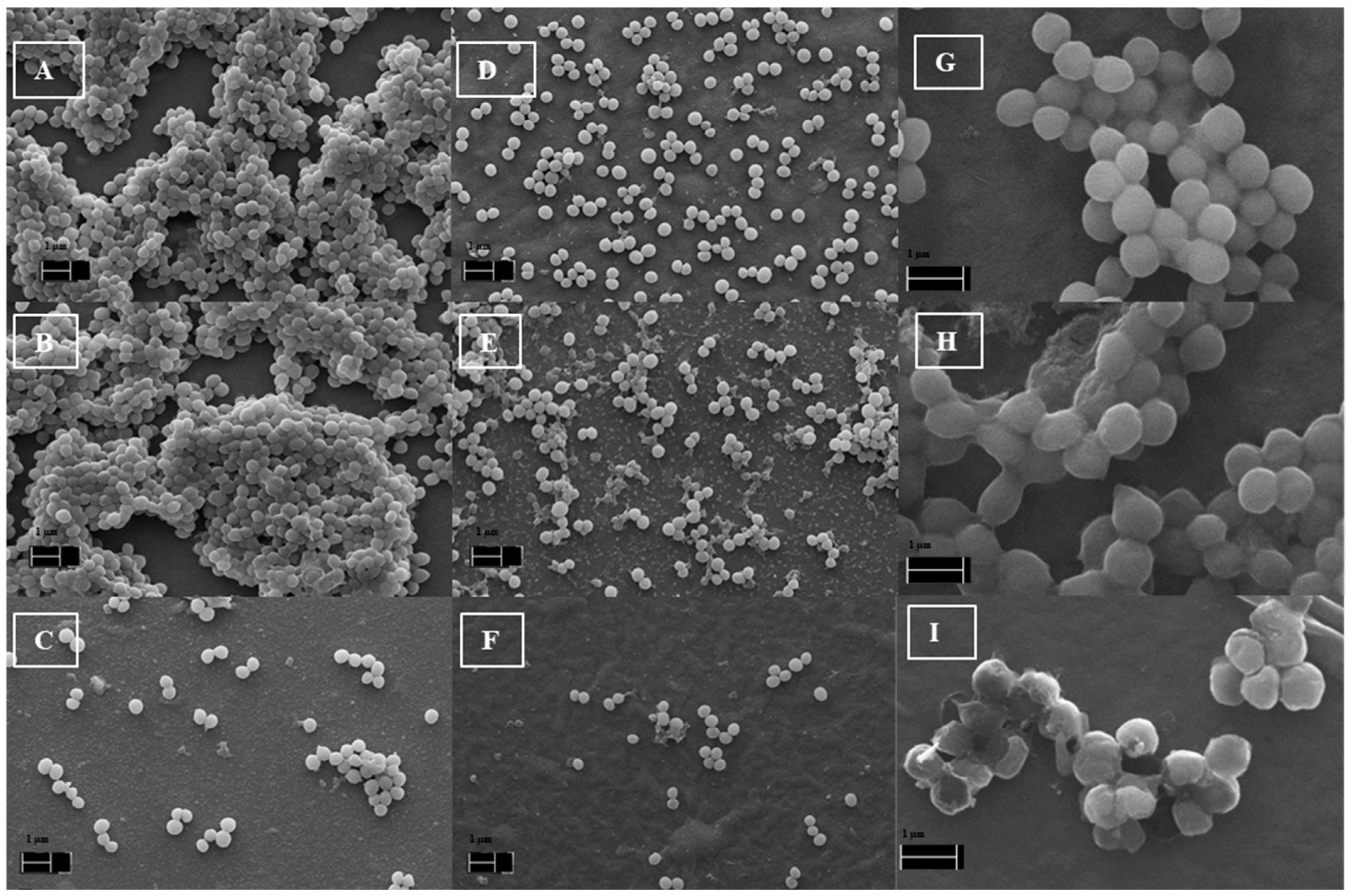
2.4. SEM of S. aureus Biofilm on the Hybrid Films
3. Material and Methods
3.1. Bacterial Strains and Antimicrobial Effectiveness of PhB
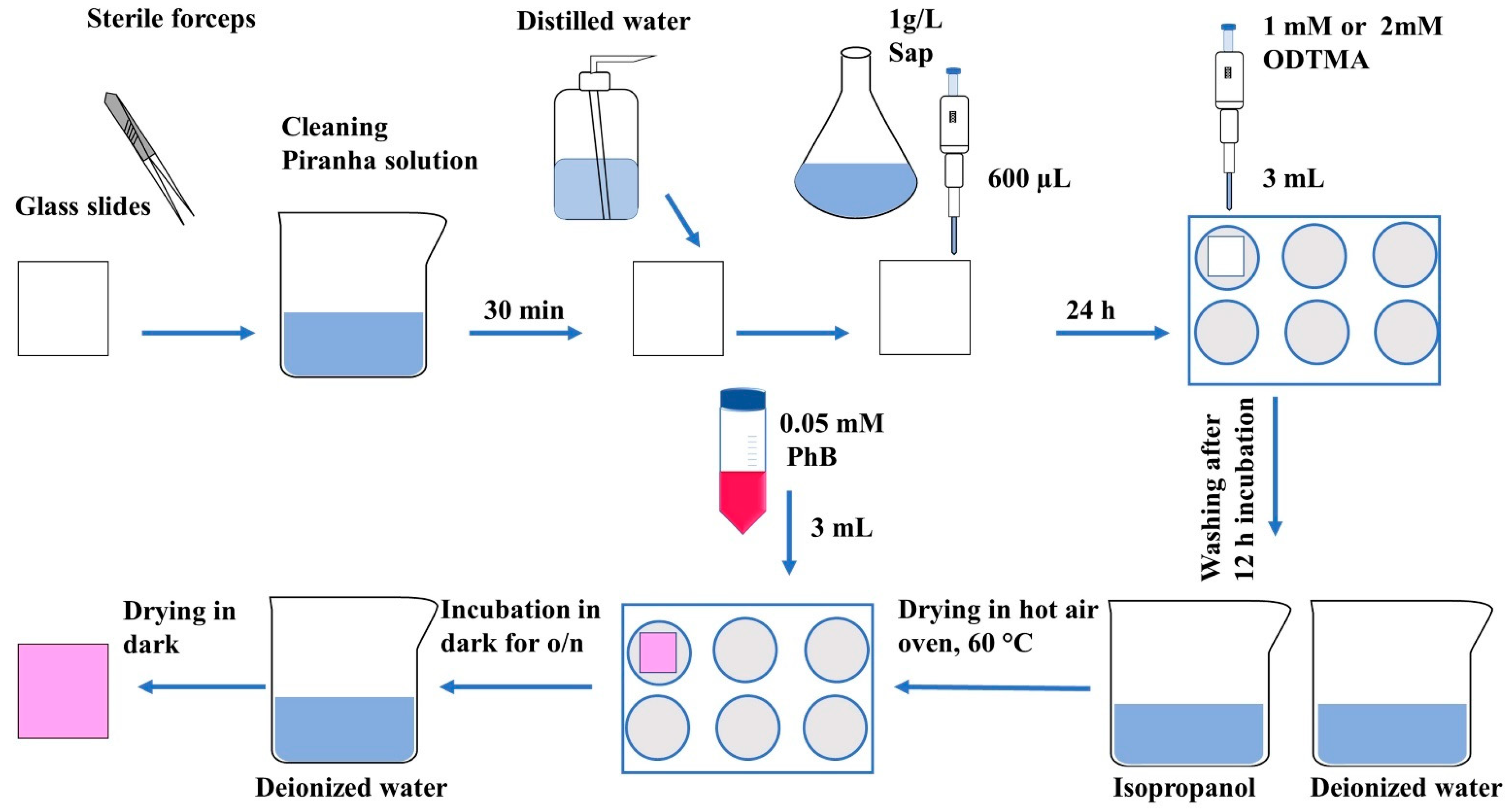
3.2. Preparation of Hybrid Film
3.3. Characterization of Hybrid Film
3.4. Photodynamic Anti-Biofilm Effectiveness
3.5. Scanning Electron Microscopy of S. aureus Biofilm
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Walter, J.; Haller, S.; Quinten, C.; Kärki, T.; Zacher, B.; Eckmanns, T.; Abu Sin, M.; Plachouras, D.; Kinross, P.; Suetens, C. Healthcare-Associated Pneumonia in Acute Care Hospitals in European Union/European Economic Area Countries: An Analysis of Data from a Point Prevalence Survey, 2011 to 2012. Eurosurveillance 2018, 23. [Google Scholar] [CrossRef]
- Hagel, S.; Ludewig, K.; Pletz, M.W.; Frosinski, J.; Moeser, A.; Wolkewitz, M.; Gastmeier, P.; Harbarth, S.; Brunkhorst, F.M.; Kesselmeier, M.; et al. Effectiveness of a Hospital-Wide Infection Control Programme on the Incidence of Healthcare-Associated Infections and Associated Severe Sepsis and Septic Shock: A Prospective Interventional Study. Clin. Microbiol. Infect. 2019, 25, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Monegro, A.F.M.; Regunath, H. Hospital Acquired Infections. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Hawkey, P.M.; Warren, R.E.; Livermore, D.M.; McNulty, C.A.M.; Enoch, D.A.; Otter, J.A.; Wilson, A.P.R. Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacteria: Report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J. Antimicrob. Chemother. 2018, 73, iii2–iii78. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, B.G.; Hall, L.; White, N.; Barnett, A.G.; Halton, K.; Paterson, D.L.; Riley, T.V.; Gardner, A.; Page, K.; Farrington, A.; et al. An Environmental Cleaning Bundle and Health-Care-Associated Infections in Hospitals (REACH): A Multicentre, Randomised Trial. Lancet Infect. Dis. 2019, 19, 410–418. [Google Scholar] [CrossRef]
- Smith, D.R.M.; Pouwels, K.B.; Hopkins, S.; Naylor, N.R.; Smieszek, T.; Robotham, J.V. Epidemiology and Health-Economic Burden of Urinary-Catheter-Associated Infection in English NHS Hospitals: A Probabilistic Modelling Study. J. Hosp. Infect. 2019, 103, 44–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wozniak, T.M.; Bailey, E.J.; Graves, N. Health and Economic Burden of Antimicrobial-Resistant Infections in Australian Hospitals: A Population-Based Model. Infect. Control Hosp. Epidemiol. 2019, 40, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Ielapi, N.; Nicoletti, E.; Lorè, C.; Guasticchi, G.; Avenoso, T.; Barbetta, A.; de Franciscis, S.; Andreucci, M.; Sapienza, P.; Serra, R. The Role of Biofilm in Central Venous Catheter Related Bloodstream Infections. Rev. Recent Clin. Trials 2020, 15, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Mandakhalikar, K.D.; Rahmat, J.N.; Chiong, E.; Neoh, K.G.; Shen, L.; Tambyah, P.A. Extraction and Quantification of Biofilm Bacteria: Method Optimized for Urinary Catheters. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Johnson, C.T.; Wroe, J.A.; Agarwal, R.; Martin, K.E.; Guldberg, R.E.; Donlan, R.M.; Westblade, L.F.; García, A.J. Hydrogel Delivery of Lysostaphin Eliminates Orthopedic Implant Infection by Staphylococcus Aureus and Supports Fracture Healing. PNAS 2018, 115, E4960–E4969. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, S.; Wang, F.; Wilson, T.G.; Palmer, K.; Valderrama, P.; Rodrigues, D.C. The Role of Bacterial Biofilm and Mechanical Forces in Modulating Dental Implant Failures. J. Mech. Behav. Biomed. Mater. 2019, 92, 118–127. [Google Scholar] [CrossRef]
- Wilson, A.P.R. The Role of the Environment in the Spread of Healthcare Associated Infections. J. Hosp. Infect. 2018, 100, 363–364. [Google Scholar] [CrossRef] [PubMed]
- Afle, F.C.D.; Agbankpe, A.J.; Johnson, R.C.; Houngbégnon, O.; Houssou, S.C.; Bankole, H.S. Healthcare-Associated Infections: Bacteriological Characterization of the Hospital Surfaces in the University Hospital of Abomey-Calavi/so-Ava in South Benin (West Africa). BMC Infect. Dis. 2019, 19, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Gopal, A.; Karaosmanoglu, S.; Lin, J.; Munshi, T.; Zhang, W.; Chen, X.; Yan, L. Photosensitizer Doped Zeolitic Imidazolate Framework-8 Nanocomposites for Combined Antibacterial Therapy to Overcome Methicillin-Resistant Staphylococcus Aureus (MRSA). Colloids Surf. B Biointerfaces 2020, 190, 110900. [Google Scholar] [CrossRef] [PubMed]
- Lineback, C.B.; Nkemngong, C.A.; Wu, S.T.; Li, X.; Teska, P.J.; Oliver, H.F. Hydrogen Peroxide and Sodium Hypochlorite Disinfectants Are More Effective against Staphylococcus Aureus and Pseudomonas Aeruginosa Biofilms than Quaternary Ammonium Compounds. Antimicrob. Resist. Infect. Control 2018, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Mi, G.; Shi, D.; Wang, M.; Webster, T.J. Reducing Bacterial Infections and Biofilm Formation Using Nanoparticles and Nanostructured Antibacterial Surfaces. Adv. Healthc. Mater. 2018, 7, 1800103. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, R.; Feng, Y.; Ouyang, H.; Zhou, N.; Zhang, X.; Wei, Y. Recent Development and Prospects of Surface Modification and Biomedical Applications of MXenes. Nanoscale 2020, 12, 1325–1338. [Google Scholar] [CrossRef]
- Ahmadabadi, H.Y.; Yu, K.; Kizhakkedathu, J.N. Surface Modification Approaches for Prevention of Implant Associated Infections. Colloids Surf. B Biointerfaces 2020, 193, 111116. [Google Scholar] [CrossRef]
- Perni, S.; Caserta, S.; Pasquino, R.; Jones, S.A.; Prokopovich, P. Prolonged Antimicrobial Activity of PMMA Bone Cement with Embedded Gentamicin-Releasing Silica Nanocarriers. ACS Appl. Bio Mater. 2019, 2, 1850–1861. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, Y.; Li, Y.; Su, L.; Zhang, Y.; Huang, F.; Liu, J.; Liu, J.; van Kooten, T.G.; An, Y.; et al. Nanocarriers with Conjugated Antimicrobials to Eradicate Pathogenic Biofilms Evaluated in Murine in Vivo and Human Ex Vivo Infection Models. Acta Biomater. 2018, 79, 331–343. [Google Scholar] [CrossRef]
- Subbiahdoss, G.; Sharifi, S.; Grijpma, D.W.; Laurent, S.; van der Mei, H.C.; Mahmoudi, M.; Busscher, H.J. Magnetic Targeting of Surface-Modified Superparamagnetic Iron Oxide Nanoparticles Yields Antibacterial Efficacy against Biofilms of Gentamicin-Resistant Staphylococci. Acta Biomater. 2012, 8, 2047–2055. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial Photodynamic Inactivation: A Bright New Technique to Kill Resistant Microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maisch, T. A New Strategy to Destroy Antibiotic Resistant Microorganisms: Antimicrobial Photodynamic Treatment. Mini-Rev. Med. Chem. 2009, 9, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.; Hall, L.; Halton, K.; Graves, N. Improving Hospital Environmental Hygiene with the Use of a Targeted Multi-Modal Bundle Strategy. Infect. Dis. Health 2018, 23, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Hendricks, G.L.; Tori, K.; Fuchs, B.B.; Mylonakis, E. Strategies against Methicillin-Resistant Staphylococcus Aureus Persisters. Future Med. Chem. 2018, 10, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Planas, O.; Bresolí-Obach, R.; Nos, J.; Gallavardin, T.; Ruiz-González, R.; Agut, M.; Nonell, S. Synthesis, Photophysical Characterization, and Photoinduced Antibacterial Activity of Methylene Blue-Loaded Amino- and Mannose-Targeted Mesoporous Silica Nanoparticles. Molecules 2015, 20, 6284–6298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kossakowska-Zwierucho, M.; Szewczyk, G.; Sarna, T.; Nakonieczna, J. Farnesol Potentiates Photodynamic Inactivation of Staphylococcus Aureus with the Use of Red Light-Activated Porphyrin TMPyP. J. Photochem. Photobiol. B Biol. 2020, 206, 111863. [Google Scholar] [CrossRef] [PubMed]
- Bornhütter, T.; Pohl, J.; Fischer, C.; Saltsman, I.; Mahammed, A.; Gross, Z.; Röder, B. Development of Singlet Oxygen Luminescence Kinetics during the Photodynamic Inactivation of Green Algae. Molecules 2016, 21, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caires, C.S.A.; Silva, C.M.; Lima, A.R.; Alves, L.M.; Lima, T.H.N.; Rodrigues, A.C.S.; Chang, M.R.; Oliveira, S.L.; Whitby, C.; Nascimento, V.A.; et al. Photodynamic Inactivation of Methicillin-Resistant Staphylococcus Aureus by a Natural Food Colorant (E-141ii). Molecules 2020, 25, 4464. [Google Scholar] [CrossRef]
- Parasuraman, P.; Antony, A.P.; Lal S.B., S.; Sharan, A.; Siddhardha, B.; Kasinathan, K.; Bahkali, N.A.; Dawoud, T.M.S.; Syed, A. Antimicrobial Photodynamic Activity of Toluidine Blue Encapsulated in Mesoporous Silica Nanoparticles against Pseudomonas Aeruginosa and Staphylococcus Aureus. Biofouling 2019, 35, 89–103. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-H.; Chien, H.-F.; Lin, M.-H.; Chen, C.-P.; Shen, M.; Chen, C.-T. Chitosan Inhibits the Rehabilitation of Damaged Microbes Induced by Photodynamic Inactivation. Int. J. Mol. Sci. 2018, 19, 2598. [Google Scholar] [CrossRef]
- Anju, V.; Paramanantham, P.; Siddhardha, B.; Lal S.B., S.; Sharan, A.; Alyousef, A.A.; Arshad, M.; Syed, A. Malachite Green-Conjugated Multi-Walled Carbon Nanotubes Potentiate Antimicrobial Photodynamic Inactivation of Planktonic Cells and Biofilms of Pseudomonas Aeruginosa and Staphylococcus Aureus. Int. J. Nanomed. 2019, 14, 3861–3874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, A.; Kaduskar, D.V. Comparative Study on Photodynamic Activation of Ortho-Toluidine Blue and Methylene Blue Loaded Mesoporous Silica Nanoparticles Against Resistant Microorganisms. Available online: https://www.ingentaconnect.com/contentone/ben/ddf/2018/00000012/00000003/art00003 (accessed on 15 April 2020).
- S., K.; Rama Pawar, R.; D. Kevadiya, B.; C. Bajaj, H. Synthesis of Saponite Based Nanocomposites to Improve the Controlled Oral Drug Release of Model Drug Quinine Hydrochloride Dihydrate. Pharmaceuticals 2019, 12, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lackovičová, M.; Baranyaiová, T.; Bujdák, J. The Chemical Stabilization of Methylene Blue in Colloidal Dispersions of Smectites. Appl. Clay Sci. 2019, 181, 105222. [Google Scholar] [CrossRef]
- Gaálová, B.; Vyletelová, I.; Pokorná, K.; Kikhney, J.; Moter, A.; Bujdák, J.; Bujdáková, H. Decreased Vitality and Viability of Escherichia Coli Isolates by Adherence to Saponite Particles. Appl. Clay Sci. 2019, 183, 105316. [Google Scholar] [CrossRef]
- Rasooly, R. Expanding the Bactericidal Action of the Food Color Additive Phloxine B to Gram-Negative Bacteria. FEMS Immunol. Med. Microbiol. 2005, 45, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Rasooly, R. Phloxine B, a Versatile Bacterial Stain. FEMS Immunol. Med. Microbiol. 2007, 49, 261–265. [Google Scholar] [CrossRef]
- Shiotsu-Ogura, Y.; Yoshida, A.; Kan, P.; Sasaki, H.; Toyama, T.; Izukuri, K.; Hamada, N.; Yoshino, F. Antimicrobial Photodynamic Therapy Using a Plaque Disclosing Solution on Streptococcus Mutans. Photodiagn. Photodyn. Ther. 2019, 26, 252–257. [Google Scholar] [CrossRef]
- Nakonechny, F.; Nisnevitch, M. Aspects of Photodynamic Inactivation of Bacteria. Microorganisms 2019. [Google Scholar] [CrossRef] [Green Version]
- Kureková, V.B.; Belušáková, S.; Boháč, P.; Bujdák, J. Resonance Energy Transfer in the Systems of Smectite Modified with a Fluorescent Cationic Polymer and a Photosensitizer. Appl. Clay Sci. 2019, 183, 105326. [Google Scholar] [CrossRef]
- Bhatt, A.S.; Sakaria, P.L.; Vasudevan, M.; Pawar, R.R.; Sudheesh, N.; Bajaj, H.C.; Mody, H.M. Adsorption of an Anionic Dye from Aqueous Medium by Organoclays: Equilibrium Modeling, Kinetic and Thermodynamic Exploration. RSC Adv. 2012, 2, 8663–8671. [Google Scholar] [CrossRef]
- Shin, W.S. Competitive Sorption of Anionic and Cationic Dyes onto Cetylpyridinium-Modified Montmorillonite. J. Environ. Sci. Health Part A 2008, 43, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Donauerová, A.; Bujdák, J.; Smolinská, M.; Bujdáková, H. Photophysical and Antibacterial Properties of Complex Systems Based on Smectite, a Cationic Surfactant and Methylene Blue. J. Photochem. Photobiol. B Biol. 2015, 151, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Chi, M.; Sun, X.; Xie, X.; Weir, M.D.; Oates, T.W.; Zhou, Y.; Wang, L.; Bai, Y.; Xu, H.H. Novel Nanomaterial-Based Antibacterial Photodynamic Therapies to Combat Oral Bacterial Biofilms and Infectious Diseases. Int. J. Nanomed. 2019, 14, 6937–6956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.-H.; Lee, J.-H.; Ryu, H.-J.; Elzatahry, A.A.; Alothman, Z.A.; Choy, J.-H. Drug–Clay Nanohybrids as Sustained Delivery Systems. Appl. Clay Sci. 2016, 130, 20–32. [Google Scholar] [CrossRef]
- Zhou, C.H.; Zhou, Q.; Wu, Q.Q.; Petit, S.; Jiang, X.C.; Xia, S.T.; Li, C.S.; Yu, W.H. Modification, Hybridization and Applications of Saponite: An Overview. Appl. Clay Sci. 2019, 168, 136–154. [Google Scholar] [CrossRef]
- Murugesan, S.; Scheibel, T. Copolymer/Clay Nanocomposites for Biomedical Applications. Adv. Funct. Mater. 2020, 30, 1908101. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, T.; Shimada, T.; Takagi, S. Photophysical Properties and Adsorption Behaviors of Novel Tri-Cationic Boron(III) Subporphyrin on Anionic Clay Surface. ACS Appl. Mater. Interfaces 2016, 8, 7522–7528. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S. Database of Absorption and Fluorescence Spectra of >300 Common Compounds for Use in PhotochemCAD. Photochem. Photobiol. 2018, 94, 290–327. [Google Scholar] [CrossRef] [Green Version]
- Davachi, S.M.; Shekarabi, A.S. Preparation and Characterization of Antibacterial, Eco-Friendly Edible Nanocomposite Films Containing Salvia Macrosiphon and Nanoclay. Int. J. Biol. Macromol. 2018, 113, 66–72. [Google Scholar] [CrossRef]
- Croes, S.; Stobberingh, E.E.; Stevens, K.N.J.; Knetsch, M.L.W.; Koole, L.H. Antimicrobial and Anti-Thrombogenic Features Combined in Hydrophilic Surface Coatings for Skin-Penetrating Catheters. Synergy of Co-Embedded Silver Particles and Heparin. ACS Appl. Mater. Interfaces 2011, 3, 2543–2550. [Google Scholar] [CrossRef]
- Viola, G.M.; Rosenblatt, J.; Raad, I.I.; Darouiche, R.O. Comparison of Bacterial Adherence to Titanium Versus Polyurethane for Cardiac Implantable Electronic Devices. Am. J. Cardiol. 2013, 111, 1764–1766. [Google Scholar] [CrossRef] [PubMed]
- Mirani, Z.A.; Fatima, A.; Urooj, S.; Aziz, M.; Khan, M.N.; Abbas, T. Relationship of Cell Surface Hydrophobicity with Biofilm Formation and Growth Rate: A Study on Pseudomonas Aeruginosa, Staphylococcus Aureus, and Escherichia Coli. Iran. J. Basic Med. Sci. 2018, 21, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, C.; Hilgarth, M.; Ehrmann, M.; Vogel, R.F. Bap and Cell Surface Hydrophobicity Are Important Factors in Staphylococcus Xylosus Biofilm Formation. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Rasooly, A.; Weisz, A. In Vitro Antibacterial Activities of Phloxine B and Other Halogenated Fluoresceins against Methicillin-Resistant Staphylococcus Aureus. Antimicrob. Agents Chemother. 2002, 46, 3650–3653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, S.; Kasotakis, E.; Maher, S.; Cavanagh, B.; O’Gara, J.P.; Pandit, A.; Keyes, T.E.; Devocelle, M.; O’Neill, E. A Novel Medical Device Coating Prevents Staphylococcus Aureus Biofilm Formation on Medical Device Surfaces. FEMS Microbiol. Lett. 2019, 366. [Google Scholar] [CrossRef]
- Braoios, A.; Fluminhan, A.; Pizzolitto, A.C. UNESP Multiplex PCR Use for Staphylococcus Aureus Identification and Oxacillin and Mupirocin Resistance Evaluation. Rev. Ciências Farm. Básica Apl. 2009, 303–307. [Google Scholar]
- Rudawska, A.; Jacniacka, E. Analysis for Determining Surface Free Energy Uncertainty by the Owen–Wendt Method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dadi, N.C.t.; Dohál, M.; Medvecká, V.; Bujdák, J.; Koči, K.; Zahoranová, A.; Bujdáková, H. Physico-Chemical Characterization and Antimicrobial Properties of Hybrid Film Based on Saponite and Phloxine B. Molecules 2021, 26, 325. https://doi.org/10.3390/molecules26020325
Dadi NCt, Dohál M, Medvecká V, Bujdák J, Koči K, Zahoranová A, Bujdáková H. Physico-Chemical Characterization and Antimicrobial Properties of Hybrid Film Based on Saponite and Phloxine B. Molecules. 2021; 26(2):325. https://doi.org/10.3390/molecules26020325
Chicago/Turabian StyleDadi, Nitin Chandra teja, Matúš Dohál, Veronika Medvecká, Juraj Bujdák, Kamila Koči, Anna Zahoranová, and Helena Bujdáková. 2021. "Physico-Chemical Characterization and Antimicrobial Properties of Hybrid Film Based on Saponite and Phloxine B" Molecules 26, no. 2: 325. https://doi.org/10.3390/molecules26020325
APA StyleDadi, N. C. t., Dohál, M., Medvecká, V., Bujdák, J., Koči, K., Zahoranová, A., & Bujdáková, H. (2021). Physico-Chemical Characterization and Antimicrobial Properties of Hybrid Film Based on Saponite and Phloxine B. Molecules, 26(2), 325. https://doi.org/10.3390/molecules26020325








