Herein, novel derivatives of folic acid were prepared by direct reactions of different reagents, with folic acid, or by reaction of some derivatives prepared with the same (or another) reagent. The study was directed to find new derivatives of folic acid having a promising biological activity, but, from the study carried out and the results obtained, all of the derivatives prepared were inactive against fungi, while some of these derivatives had a moderate antibacterial activity. The conclusion of this study is that the reactions done on the NH2 group of folic acid, either substituted groups formed or fused systems, are not valuable as antifungal or antibacterial agents. Future work will be directed to the carboxylic groups of folic acid to synthesize a new isolated or fused system from folic acid in hopes of getting a more promising drug.
4.1. Experimental Chemistry
All chemicals used were supplied by Sigma (New York, NY, USA). Digital Electro thermal IA 9100 Series used for measuring melting points and they were uncorrected. Infra-red spectra were examined on ATRAlpha FTIR spectrophotometer (Billerica, MA, USA).
1H-NMR and
13C-NMR spectra examined on a Bruker AC-850 MHz apparatus (Bruker, Billerica, Massachusetts). Chemical shifts expressed as (ppm) relative to internal standard (TMS), and DMSO-d6 used as the solvent and in
13C-NMR the solvent was CDCl
3 and DMSO mixture. CHN analyses and biological activity were achieved in Cairo University at Micro-Analytical Center. Spectral data of all compounds are available in
Supplementary Materials.
4.1.1. “N-(4-{[(7-methyl-8,10-dioxo-8,10-dihydroimidazo[2,1-b]pteridin-2-yl)methyl]amino}-benzoyl)glutamic acid” (2)
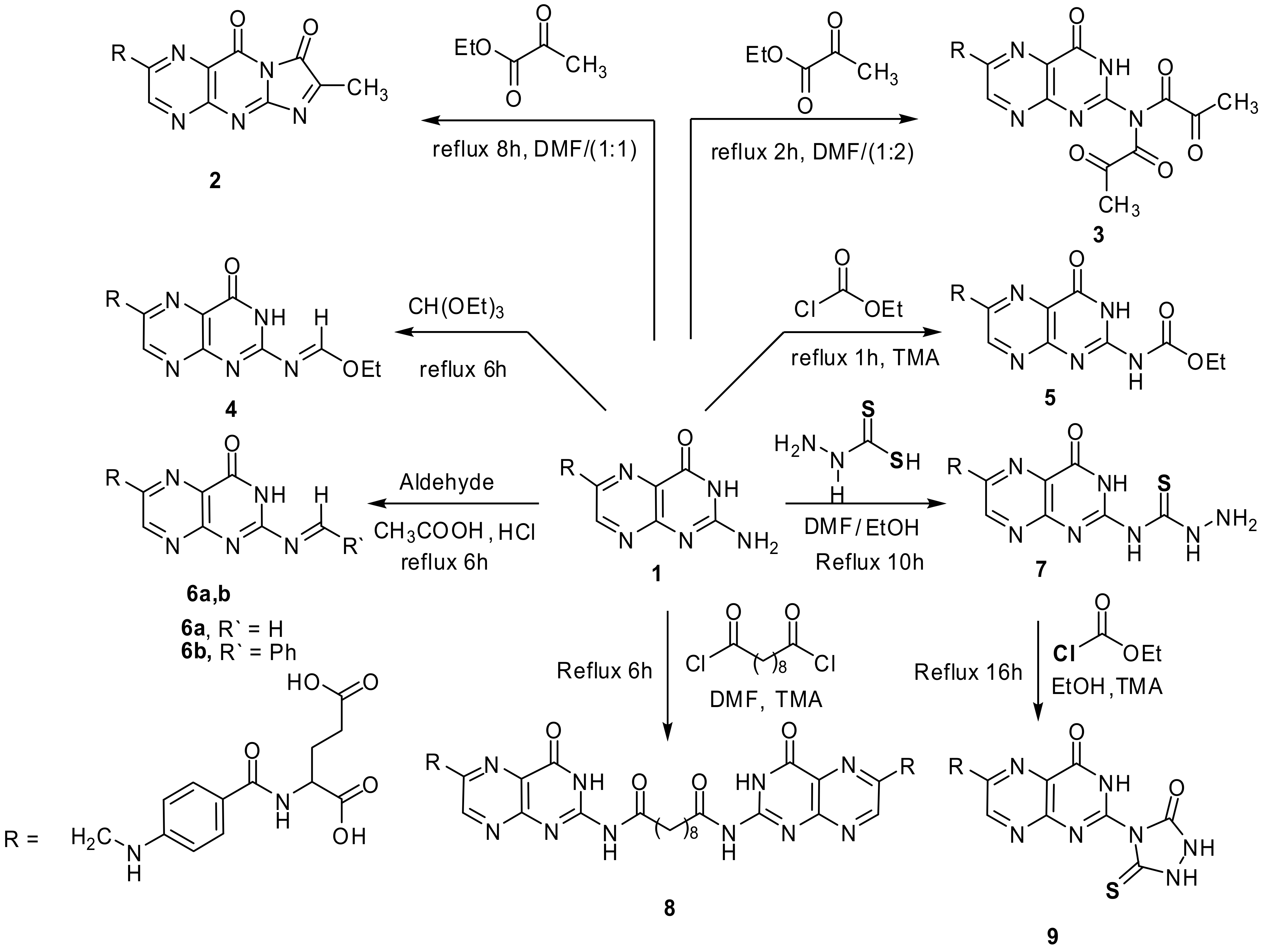
A mixture of folic acid (0.001 mol, 0.44 g) and ethyl pyruvate (0.001 mol, 0.11 g) dissolved in (15 mL) DMF and refluxed. After 8 hr (TLC, RF = 0.6, eluent: CH2Cl2) the solvent evaporated under vacuum, the semisolid product formed, poured onto ice, the solid resulted filtered off and crystallized from DMF-ethanol mixture (1:1) to give yellowish brown product. Yield, 87%, m.p. 223–225 °C. IR, 3437, 3407 cm−1 (2OH), 3239–3071 cm−1 (2NH), 2941 cm−1 (Ar-H), 2759 cm−1 (Aliphatic-H), 1689–1599 cm−1 (5C=O and C=N), 1495 cm−1 (C=C). 1H-NMR (DMSO d6, 850 MHz): δ = 1.30 (s, 3H, CH3), 1.89–2.01 (m, 2H, CH2CH2COOH), 2.72 (t, 2H, CH2CH2COOH), 4.25 (t, 1H, NHCHCOOH), 4.46 (s, 2H, pteridine-CH2-N), 6.63 (d, 2H, N-Ph-(H)ortho), 7.24 (s, 1H, HN-Ph), 7.57 (d, 2H, N-Ph-(H)meta), 7.94 (s, 1H, NHCO), 8.63 (s,1H, pteridine-C7H), 11.48 (s,1H, CH2CH2COOH), 12.36 (s,1H, CH2COOH). 13C-NMR (DMSO d6, 200 MHz): δ = 24.51 (CH3), 30.89 (CH2CH2COOH), 33.21 (CH2CH2COOH), 45.95 (NHCH2), 50.15 (NHCH), 112.42 (N-Ph-(C)ortho), 113.45 (N-Ph-(C)Para), 121.76 (pteridine C4a), 128.00 (N-Ph-(C)meta), 147.28 (pteridine C6), 151.73 (pteridine C7), 152.77 (N-Ph-(C)), 154.88 (pteridine C8a), 157.47 (pteridine C2), 162.99 (pteridine C4), 167.65 NHCO, 174.25 (NHCHCOOH), 174.87 (CH2CH2COOH). Anal. Calcd. for C22H19N7O7 (493.13): C, 53.55; H, 3.88; N, 19.87; found: C, 53.21; H, 3.62; N, 19.77.
4.1.2. “N-[4-({[2-(dipyruvoylamino)-4-oxo-3,4-dihydropteridin-6-yl]methyl}-amino)benzoyl]-glutamic acid” (3)
A mixture of folic acid (0.001 mol, 0.44 g) and ethyl pyruvate (0.001 mol, 0.23 g) in DMF (20 mL) was refluxed for 2 h until the reaction completed (TLC, RF = 0.5, eluent: CH2Cl2). The solvent evaporated under vacuum. The precipitate formed, crystallized from EtOH to give yellow product. Yield, 52%, m.p. 188–190 °C. IR, 3541, 3408 cm−1 (2OH), 3225–3161 cm−1 (3NH), 3022–2941 cm−1 (Ar-H), 2791 cm−1 (Aliphatic-H), 1684–1599 cm−1 (8C=O and C=N), 1496 cm−1 (C=C). 1HNMR (DMSO d6, 850 MHz): δ = 1.89-2.01 (m, 2H, CH2CH2COOH), 2.21 (s, 6H, 2CH3), 2.72 (t, 2H, CH2CH2COOH), 4.25 (t, 1H, NHCHCOOH), 4.46 (s, 2H, pteridine-CH2-N), 6.63 (d, 2H, N-Ph-(H)ortho), 7.24 (s, 1H, HN-Ph), 7.57 (d, 2H, N-Ph-(H)meta), 7.94 (s, 1H, NHCO), 8.63 (s,1H, pteridine-C7H), 10.15 (s, 1H, pteridine-NH), 11.45 (s,1H, CH2CH2COOH), 12.38 (s,1H, CH2COOH). 13C-NMR (DMSO d6, 200 MHz): δ = 26.79 (2CH3), 30.80 (CH2CH2COOH), 34.28 (CH2CH2COOH), 45.97 (NHCH2), 52.35 (NHCH), 111.26 (N-Ph-(C)ortho), 112.56 (N-Ph-(C)Para), 121.61 (pteridine C4a), 128.85 (N-Ph-(C)meta), 148.38 (pteridine C6), 150.74 (pteridine C7), 151.73 (N-Ph-(C)), 154.28 (pteridine C8a), 156.28 (pteridine C2), 161.39 (NCOCOCH3, 162.35 (pteridine C4), 166.06 NHCO, 174.33 (NHCHCOOH), 174.41 (CH2CH2COOH), 183.35 NCOCOCH3). Anal. Calcd. for C25H23N7O10 (581.49): C, 51.64; H, 3.99; N, 16.86; found: C, 51.51; H, 3.82; N, 16.77.
4.1.3. “N-(4-{[(2-{[1-ethoxymethylene]amino}-4-oxo-3,4-dihydropteridin-6-yl)methyl]amino}-benzoyl)glutamic acid” (4)
Folic acid (0.01 mol, 4.4 g) added to triethyl orthoformate (8 mL, excess) and stirred under boiling for 6 h (TLC, RF = 0.8, eluent: CH2Cl2). The precipitate formed after cooling filtered and crystallized from EtOH to give yellow-brown powder. Yield, 85%, m.p. 220–222 °C. IR, 3470–3400 cm−1 (2OH), 3274–3115 cm−1 (3NH), 2974 cm−1 (Ar-H), 2803 cm−1 (Aliphatic-H), 1693–1601 cm−1 (4C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.15 (t, 3H, CH3), 1.91–2.03 (m, 2H, CH2CH2COOH), 2.51 (t, 2H, CH2CH2COOH), 3.39 (q, 2H, CH2), 4.31 (t, 1H, NHCHCOOH), 4.48 (s, 2H, pteridine-CH2-N), 6.64 (d, 2H, N-Ph-(H)ortho), 6.93 (s, 1H, HN-Ph), 7.64 (m, 2H, N-Ph-(H) (meta)), 7.65 (s, 1H, N=CH), 8.12 (s, 1H, NHCO), 8.79 (s,1H, pteridine-C7H), 10.38 (s, 1H, pteridine NH), 11.42 (s, 1H, CH2CH2COOH), 12.40 (s,1H, CH2COOH). Anal. Calcd. for C22H23N7O7 (497.46): C, 53.12; H, 4.66; N, 19.71; found: C, 52.89; H, 4.47; N, 19.54.
4.1.4. “N-{4-[({2-[(ethoxycarbonyl)amino]-4-oxo-3,4-dihydropteridin-6-yl}methyl)amino]-benzoyl}glutamic acid” (5)
Folic acid (0.001 mol, 0.44 g), ethyl chloroformate (0.001 mol,0.11 g) and drops of TMA were mixed together in EtOH (12 mL) and refluxed for 1 h until the reaction completed (TLC, RF = 0.65, eluent: CH2Cl2). The solvent evaporated and the precipitate formed crystallized from EtOH to give yellow product. Yield, 87%, m.p. 218–220 °C. IR, 3438, 3422 cm−1 (2OH), 3251–3108 cm−1 (4NH), 3079–2939 cm−1 (Ar-H), 2782–2724 cm−1 (Aliphatic-H), 1690–1598 cm−1 (5C=O and C=N), 1496 cm−1 (C=C). 1H-NMR (DMSO d6, 850 MHz): δ = 1.14 (t, 3H, CH2CH3), 1.90–2.05 (m, 2H, CH2CH2COOH), 2.31 (t, 2H, CH2CH2COOH), 4.02 (q, 2H, CH2CH3), 4.08 (t, 1H, NHCHCOOH), 4.53 (s, 2H, pteridine-CH2-N), 6.64 (d, 2H, N-Ph-(H)ortho), 7.64 (s, 1H, HN-Ph), 7.65 (d, 2H, N-Ph-(H)meta), 8.15 (s, 1H, NHCO), 8.21 (s,1H, pteridine-C7H), 8.72 (s, 1H, NHCOOEt), 10.31 (s, 1H, pteridine NH), 11.51 (s,1H, CH2CH2COOH), 12.40 (s,1H, CH2COOH). 13C-NMR (DMSO d6, 200 MHz): δ = 25.09 (CH3CH2), 30.87 (CH2CH2COOH), 34.11 (CH2CH2COOH), 41.20 (CH3CH2), 45.23 (NHCH2), 52.10 (NHCH), 111.44 (N-Ph-(C)ortho), 112.56 (N-Ph-(C)Para), 121.61 (pteridine C4a), 128.85 (N-Ph-(C)meta), 148.52 (pteridine C6), 150.70 (pteridine C7), 151.74 (N-Ph-(C)), 154.27 (pteridine C8a), 156.41 (pteridine C2), 161.89 (NCOOCH2CH3), 162.35 (pteridine C4), 166.06 (NHCO), 174.33 (NHCHCOOH), 174.41 (CH2CH2COOH). Anal. Calcd. for C22H23N7O8 (513.46): C, 51.46; H, 4.51; N, 19.10; found: C, 51.25; H, 4.35; N, 18.84.
4.2. Synthesis of Derivatives 6a,b (General Procedure)
Folic acid (0.001 mol, 0.4 g) and appropriate aldehyde (0.001 mol) mixed in glacial acetic acid (10 mL) and drops of hydrochloric acid (0.4 mL) were added, then, the mixture refluxed for 4 h (TLC, RF = 0.5–0.6, eluent: CH2Cl2). The formed precipitate filtered and crystallized from EtOH.
4.2.1. “N-[4-({[2-(methyleneamino)-4-oxo-3,4-dihydropteridin-6-yl]methyl}-amino)benzoyl]glutamic acid” (6a)
Green powder. Yield, 81%, m.p. 210–212 °C. IR, 3418, 3403 cm−1 (2OH), 3284–3179 cm−1 (3NH), 2934 cm−1 (Ar-H), 2832 cm−1 (Aliphatic-H), 1690–1618 cm−1 (4C=O and C=N), 1590 cm−1 (C=C). 1H-NMR (DMSO d6, 850 MHz): δ = 1.88–2.07 (m, 2H, CH2CH2COOH), 2.71 (t, 2H, CH2CH2COOH), 4.27 (t, 1H, NHCHCOOH), 4.48 (s, 2H, pteridine-CH2-N), 6.59 (d, 2H, N-Ph-(H)ortho), 7.23 (s, 1H, HN-Ph), 7.55 (m, 2H, N-Ph-(H) (meta)), 7.94 (s, 1H, NHCO), 7.99 (s, 2H, N=CH2), 8.64 (s,1H, pteridine-C7H), 10.33 (s, 1H, pteridine NH), 11.37 (s, 1H, CH2CH2COOH), 12.41 (s,1H, CH2COOH). 13C-NMR (DMSO d6, 200 MHz): δ = 28.15 (CH2CH2COOH), 33.15 (CH2CH2COOH), 43.89 (NHCH2), 54.56 (NHCH), 112.25 (N-Ph-(C)ortho), 114.01 (N-Ph-(C)Para), 122.47 (pteridine C4a), 128.25 (N-Ph-(C)meta), 139.75 (N=CH2), 146.78 (pteridine C6), 151.20 (pteridine C7), 152.41 (N-Ph-(C)), 154.44 (pteridine C8a), 157.61 (pteridine C2), 163.36 (pteridine CO), 166.49 (NHCO), 175.21 (NHCHCOOH), 175.15 (CH2CH2COOH). Anal. Calcd. for C20H19N7O6 (453.41): C, 52.98; H, 4.22; N, 21.62; found: C, 52.71; H, 4.01; N, 21.41.
4.2.2. “N-[4-(((2-(Benzylideneamino)-4-oxo-3,4-dihydropteridin-6-yl)methyl)-amino)benzoyl)-glutamic acid” (6b)
Yellow powder. Yield, 62%, m.p. 224–226 °C. IR, 3412, 3399 cm−1 (2OH), 3279–3176 cm−1 (3NH), 2951 cm−1 (Ar-H), 2850 cm−1 (Aliphatic-H), 1687–1610 cm−1 (4C=O and C=N), 1550 cm−1 (C=C). 1H-NMR (DMSO d6, 850 MHz): δ = 1.85-2.04 (m, 2H, CH2CH2COOH), 2.73 (t, 2H, CH2CH2COOH), 4.22 (t, 1H, NHCHCOOH), 4.45 (s, 2H, pteridine-CH2-N), 6.61 (d, 2H, N-Ph-(H)ortho), 7.22 (s, 1H, HN-Ph), 7.57 (m, 2H, N-Ph-(H) meta), 7.63 (d, 1H, benzylidene Ph-(H)para), 7.86 (d, 2H, benzylidene Ph-(H)ortho), 7.90 (m, 2H, benzylidene Ph-(H)meta), 7.93 (s, 1H, NHCO), 8.03 (s, 1H, benzylidene N=C-H), 8.60 (s,1H, pteridine-C7H), 10.24 (s, 1H, pteridine NH), 11.38 (s, 1H, CH2CH2COOH), 12.30 (s,1H, CH2COOH). 13C-NMR (DMSO d6, 200 MHz): δ = 30.75 (CH2CH2COOH), 33.10 (CH2CH2COOH), 45.94 (NHCH2), 50.07 (NHCH), 113.20 (N-Ph-(C)ortho), 114.23 (N-Ph-(C)Para), 121.36 (pteridine C4a), 128.22 (N-Ph-(C)meta), 128.09 (benzylidene Ph(meta)), 129.25 (benzylidene Ph(ortho)), 131.70 (benzylidene Ph(para)), 133.01 (benzylidene CH-Ph), 146.20 (pteridine C6), 151.52 (pteridine C7), 152.44 (N-Ph-(C)), 154.40 (pteridine C8a), 157.13 (pteridine C2), 163.36 (pteridine CO), 164.21 (benzylidene N=C-H), 167.89 NHCO, 174.25 (NHCHCOOH), 174.54 (CH2CH2COOH). Anal. Calcd. for C26H23N7O6 (529.50): C, 58.98; H, 4.38; N, 18.52; found: C, 58.74; H, 4.22; N, 18.46.
4.2.3. “N-{4-[({2-[(hydrazinocarbonothioyl)amino]-4-oxo-3,4-dihydropteridin-6-yl}methyl)-amino]benzoyl}glutamic acid” (7)
Folic acid (0.001 mol, 0.44 g) dissolved with thioformic acid hydrazide (0.001 mol, 0.1 g) in EtOH/DMF (2:1–15 mL) and stirred under reflux for 10 h. (TLC, RF = 0.4, eluent: CH2Cl2). The precipitate formed filtered and crystallized from EtOH to give yellow powder. Yield 91%, m.p. over 300 °C. IR, 3450–3342 cm−1 (2OH), 3342–3049 cm−1 (NH2 and 5NH), 2940 cm−1 (Ar-H), 2875 cm−1 (Aliphatic-H), 1685–1600 cm−1 (4C=O and C=N), 1338 cm−1 (C=S). 1H-NMR (DMSO d6, 850 MHz): δ = 1.89–2.01 (m, 2H, CH2CH2COOH), 2.49 (t, 2H, CH2CH2COOH), 4.09 (t, 1H, NHCHCOOH), 4.44 (s, 2H, pteridine-CH2-N), 5.69 (s, 2H, NH2), 6.91(d, 2H, N-Ph-(H)ortho), 6.99 (s, 1H, HN-Ph), 7.60 (m, 6H, N-Ph-(H) (meta)), 8.10 (s, 1H, NHCO), 8.63 (s,1H, pteridine-C7H), 10.31 (s, 1H, pteridine NH), 11.31 (s, 1H, NHNH2), 11.32 (s, 1H, CH2CH2COOH), 12.18 (s,1H, CH2COOH), 12.66 (s, 1H, NH-pyrimidine), 12.89 (s, 1H, NH-C=S). Calcd. for C20H21N9O6S (515.50): C, 46.60; H, 4.11; N, 24.45; found: C, 46.43; H, 4.01; N, 24.18.
4.2.4. “N,N’-bis[6-({[4-(N-glutamincarbonyl)phenyl]amino}-methyl)-4-oxo-3,4-dihydropteridin-2-yl]decanediamide” (8)
Compound 1 (0.001 mol, 0.88 g) and sebacoyl chloride (0.001 mol, 0.24 g) and drops of TMA in DMF (10 mL) was refluxed for 6 h (TLC, RF = 0.6, eluent: CH2Cl2). The mixture was poured into ice-cold water, the precipitate obtained crystallized from EtOH to give orange crystals. Yield, 72%, m.p. 184–186 °C. IR, 3432, 3415 cm−1 (2OH), 3270–3160 cm−1 (4NH), 2951 cm−1 (Ar-H), 2850 cm−1 (Aliphatic-H), 1688–1621 cm−1 (5C=O and C=N), 1347 cm−1 (C=S). 1H-NMR (DMSO d6, 850 MHz): δ = 1.03 (m, 4H, 2CH2, CH2CH2CH2CH2CONH), 1.26 (m, 4H, 2CH2, CH2CH2CH2CH2CONH), 1.90–2.04 (t, 2H, CH2CH2COOH), 2.23 (m, 4H, 2CH2, CH2CH2CH2CH2CONH), 2.71 (t, 2H, CH2CH2COOH), 3.34 (t, 4H, 2CH2, CH2CH2CH2CH2CONH), 4.24 (t, 1H, NHCHCOOH), 4.51 (s, 2H, pteridine-CH2-N), 6.61 (d, 2H, N-Ph-(H)ortho), 7.21 (s, 1H, HN-Ph), 7.49 (m, 2H, N-Ph-(H) (meta)), 7.90 (s, 1H, NHCO), 8.61 (s,1H, pteridine-C7H), 10.37 (s, 1H, pteridine NH), 11.12 (s, 2H, 2NH, CH2CH2CH2CH2CONH), 11.43 (s, 1H, CH2CH2COOH), 12.34 (s,1H, CH2COOH). Anal. Calcd. for C48H52N14O14 (1049.01): C, 54.96; H, 5.00; N, 18.69; found: C, 54.78; H, 4.81; N, 18.52.
4.2.5. “N-[4-({[4-oxo-2-(3-oxo-5-thioxo-1,2,4-triazolidin-4-yl)-3,4-dihydro-pteridin-6-yl]methyl}amino)benzoyl]glutamic acid” (9)
Compound 7 (0.001 mol, 0.51 g) was refluxed for 16 h with ethyl chloroformate (0.001 mol, 0.11 g) and TMA (3 drops) in EtOH (15 mL) (TLC, RF = 0.8, eluent: CH2Cl2). A yellow powder formed which filtered and crystallized from EtOH. Yield, 78%, m.p. over 300 °C. IR, 3412–3378 cm−1 (2OH), 3331–3149 cm−1 (5NH), 2961 cm−1 (Ar-H), 2867 cm−1 (Aliphatic-H), 1681–1618 cm−1 (5C=O and C=N), 1350 cm−1 (C=S). 1H-NMR (DMSO d6, 850 MHz): δ = 1.90–2.03 (m, 2H, CH2CH2COOH), 2.497 (t, 2H, CH2CH2COOH), 4.04 (N-CHCOOH), 4.48 (s, 2H, pteridine-CH2-N), 6.931(d, 2H, N-Ph-(H)ortho), 6.95 (s, 1H, HN-Ph), 7.63 (m, 6H, N-Ph-(H) (meta)), 8.12 (s, 1H, NHCO), 8.63 (s,1H, pteridine-C7H), 10.13 (s, 1H, triazolidine NHC=S), 10.37 (s, 1H, pteridine NH), 11.31 (s, 1H, CH2CH2COOH), 11.60 (s, 1H, triazolidine NHC=O), 12.21 (s,1H, CH2COOH), 13.01 (s, 1H, NH-pyrimidine). 13C-NMR (DMSO d6, 200 MHz): δ = 29.66 (CH2CH2COOH), 31.23 (CH2CH2COOH), 45.18 (NHCH2), 53.41 (NHCH), 112.25 (N-Ph-(C)ortho), 114.01 (N-Ph-(C)Para), 122.44 (pteridine C4a), 127.90 (N-Ph-(C)meta), 148.02 (pteridine C6), 151.48 (pteridine C7), 153.14 (N-Ph-(C)), 154.22 (pteridine C8a), 156.32 (triazolidine C=O), 161.01 (pteridine C2), 165.90 (pteridine CO), 166.05 (NHCO), 174.45 (NHCHCOOH), 174.58 (CH2CH2COOH), 182.37 (triazolidine C=S). Calcd. for C21H19N9O7S (541.50): C, 46.58; H, 3.54; N, 23.28; found: C, 46.33; H, 3.40; N, 23.12.
4.2.6. “N-(4-{[(9-amino-8-cyano-11-oxo-7-phenyl-11H-pyrimido[2,1-b]pteridin-2-yl)methyl]-amino}benzoyl)glutamic acid” (10)
A mixture of folic acid (0.001 mol, 0.44 g), malononitrile (0.001 mol, 0.66 g), benzaldehyde (0.11 g, 1 mmol), and drops of TMA in ethanol (15 mL) was stirred under reflux for 4 h (TLC, RF = 0.4, eluent: CH2Cl2). The reaction cooled at rt (room temperature). then, the precipitate formed filtered and crystallized from EtOH to give orange crystals. Yield 80%, m.p. 228–230 °C. IR, 3420–3400 cm−1 (2OH), 3250–3071 cm−1 (NH2 and 2NH), 2950 cm−1 (Ar-H), 2850 cm−1 (Aliphatic-H), 2217 cm−1 (CN), 1682–1598 cm−1 (4C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.89–2.02 (m, 2H, CH2CH2COOH), 2.50 (t, 2H, CH2CH2COOH), 4.02 (t, 1H, NHCHCOOH), 4.48 (s, 2H, pteridine-CH2-N), 6.65 (s, 2H, NH2), 6.93 (d, 2H, N-Ph-(H)ortho), 6.95 (s, 1H, HN-Ph), 7.62–7.96 (m, 7H, N-Ph-(H) (meta) and pyrimidine-4-Ph), 8.12 (s, 1H, NHCO), 8.65 (s,1H, pteridine-C7H), 11.45 (s, 1H, CH2CH2COOH), 12.18 (s,1H, CH2COOH). Calcd. for C29H23N9O6 (593.55): C, 58.68; H, 3.91; N, 21.24; found: C, 58.42; H, 3.68; N, 21.01.
4.2.7. “N-(4-{[(7,9-dimethyl-11-oxo-11H-pyrimido[2,1-b]pteridin-2-yl)methyl]-amino}benzoyl) glutamic acid” (11)
Folic acid (0.001 mol, 0.44 g) added to acetylacetone (0.001 mol, 0.1 g) in DMF (12 mL) and stirred under reflux for 4 h (TLC, RF = 0.8, eluent: CH2Cl2). The precipitate formed crystallized from EtOH to give reddish-brown product. Yield, 93%, m.p. 252–254 °C. IR, 3421–3403 cm−1 (2OH), 3266–3214 cm−1 (2NH), 2952 cm−1 (Ar-H), 2853 cm−1 (Aliphatic-H), 1681–1621 cm−1 (4C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.91–2.03 (m, 2H, CH2CH2COOH), 2.26 (s, 3H, CH3), 2.40 (s, 3H, CH3), 2.53 (t, 2H, CH2CH2COOH), 4.24 (t, 1H, NHCHCOOH), 4.46 (s, 2H, pteridine-CH2-N), 6.53 (d, 2H, N-Ph-(H)ortho), 6.90 (s, 1H, HN-Ph), 7.27 (s, 1H, pyrimidine CH), 7.56 (m, 2H, N-Ph-(H) (meta)), 8.01 (s, 1H, NHCO), 8.62 (s,1H, pteridine-C7H), 11.27 (s, 1H, CH2CH2COOH), 12.06 (s,1H, CH2COOH). 13CNMR (DMSO d6, 200 MHz): δ = 24.30 (CH3), 26.45 (CH3), 29.28 (CH2CH2COOH), 31.11 (CH2CH2COOH), 45.04 (NHCH2), 48.47 (pyrimidine CH2), 52.49 (NHCH), 111.27 (N-Ph-(C)ortho), 112.58 (N-Ph-(C)Para), 120.95 (pyrimidine C5), 121.68 (pteridine C4a), 127.98 (N-Ph-(C)meta), 148.36 (pteridine C6), 150.72 (pteridine C7), 151.71 (N-Ph-(C)), 154.35 (pteridine C8a), 161.44 (pteridine C2), 165.95 (pteridine CO), 166.05 (NHCO), 171.79 (pyrimidine N=CH), 174.45 (NHCHCOOH), 174.58 (CH2CH2COOH). Anal. Calcd. for C24H23N7O6 (505.48): C, 57.03; H, 4.59; N, 19.40; found: C, 56.71; H, 4.26; N, 19.11.
4.2.8. “N-(4-{[(13a-hydroxy-5,12-dioxo-12,13a-dihydro-5H-indeno-[2′,1′:4,5]imidazo[2,1-b]pteridin-10-yl)methyl]amino}benzoyl)-glutamic acid” (12)
Folic acid (0.001 mol, 0.44 g) and ninhydrin (0.001 mol, 0.18 g) in EtOH (12 mL) were refluxed for 4 h (TLC, RF = 0.75, eluent: CH2Cl2). A yellowish orange crystal formed on hot which, filtered and washed with EtOH. Yield 74%, m.p. 260–262 °C. IR, 3448–3343 cm−1 (3OH), 3248–3073 cm−1 (2NH), 2938 cm−1 (Ar-H), 2788 cm−1 (Aliphatic-H), 1682–1599 cm−1 (5C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.94–2.04 (m, 2H, CH2CH2COOH), 2.51 (t, 2H, CH2CH2COOH), 4.04 (t, 1H, NHCHCOOH), 4.47 (s, 2H, pteridine-CH2-N), 5.36 (s, 1H, imidazoloindene OH), 6.91(d, 2H, N-Ph-(H)ortho), 6.95 (s, 1H, HN-Ph), 7.62-7.96 (m, 6H, N-Ph-(H) (meta) and indene-Ph), 8.11 (s, 1H, NHCO), 8.63 (s,1H, pteridine-C7H), 11.37 (s, 1H, CH2CH2COOH), 12.21 (s,1H, CH2COOH). Calcd. for C28H21N7O8 (583.51): C, 57.63; H, 3.63; N, 16.80; found: C, 57.47; H, 3.41; N, 16.53.
4.2.9. “N-(4-{[(7-methyl-9,11-dioxo-6,11-dihydro-9H-pyrimido[2,1-b]pteridin-2-yl)methyl]-amino}benzoyl)glutamic acid” (13)
Folic acid (0.001 mol, 0.44 g) and ethyl acetoacetate (0.001 mol, 0.13 g) in DMF (13 mL) stirred under reflux for 5 h (TLC, RF = 0.75, eluent: CH2Cl2). The precipitate separated after cooling crystallized from ethanol to give orange crystals. Yield, 81%, m.p. 246–248 °C. IR, 3418–3412 cm−1 (2OH), 3271–3223 cm−1 (3NH), 2956 cm−1 (Ar-H), 2850 cm−1 (Aliphatic-H), 1677–1620 cm−1 (5C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.90–2.00 (m, 2H, CH2CH2COOH), 2.25 (s, 3H, CH3), 2.55 (t, 2H, CH2CH2COOH), 4.20 (t, 1H, N-CHCOOH), 4.43 (s, 2H, pteridine-CH2-N), 6.51 (d, 2H, N-Ph-(H)ortho), 6.93 (s, 1H, HN-Ph), 7.21 (s, 1H, pyrimidine CH), 7.58 (m, 2H, N-Ph-(H) (meta)), 8.04 (s, 1H, NHCO), 8.66 (s,1H, pteridine-C7H), 10.95 (s, 1H, pyrimidine NH), 11.24 (s, 1H, CH2CH2COOH), 12.14 (s,1H, CH2COOH). Anal. Calcd. for C23H21N7O7 (507.46): C, 54.44; H, 4.17; N, 19.32; found: C, 54.13; H, 4.08; N, 19.01.
4.2.10. “N-(4-{[(7-amino-9,11-dioxo-8,11-dihydro-9H-pyrimido[2,1-b]pteridin-2-yl)methyl]-amino}benzoyl)glutamic acid” (14)
Folic acid (0.001 mol, 0.44 g) and ethyl cyanoacetate (0.001 mol, 0.13 g) in DMF (15 mL) stirred at boiling point for 4 h until the reaction finished (TLC, RF = 0.7, eluent: CH2Cl2). The product obtained after solvent evaporation crystallized from EtOH-DMF to give reddish brown product. Yield, 79%, m.p. 231–2330 °C. IR, 3424, 3412 cm−1 (2OH), 3278–3189 cm−1 (NH2 and 2NH), 2911 cm−1 (Ar-H), 2843 cm−1 (Aliphatic-H), 1679–1624 cm−1 (5C=O and C=N), 1592 cm−1 (C=C). 1H-NMR (DMSO d6, 850 MHz): δ = 1.74–2.10 (m, 2H, CH2CH2COOH), 2.73 (t, 2H, CH2CH2COOH), 2.89 (s, 2H, pyrimidine CH2), 4.22 (t, 1H, NHCHCOOH), 4.51 (s, 2H, pteridine-CH2-N), 6.63 (d, 2H, N-Ph-(H) ortho), 7.23 (s, 1H, HN-Ph), 7.56 (m, 2H, N-Ph-(H)(meta)), 7.98 (s, 1H, NHCO), 8.06 (s, 2H, NH2), 8.61 (s,1H, pteridine-C7H), 11.46 (s, 1H, CH2CH2COOH), 12.47 (s,1H, CH2COOH). 13C-NMR (DMSO d6, 200 MHz): δ = 28.10 (CH2CH2COOH), 33.19 (CH2CH2COOH), 43.44 (NHCH2), 43.05 (pyrimidine CH2), 54.50 (NHCH), 113.22 (N-Ph-(C)ortho), 114.61 (N-Ph-(C)Para), 123.34 (pteridine C4a), 128.24 (N-Ph-(C)meta), 146.32 (pteridine C6), 151.22 (pteridine C7), 152.11 (N-Ph-(C)), 154.79 (pteridine C8a), 160.09 (pteridine C2), 165.36 (pteridine CO), 166.22 (NHCO), 171.03 (pyrimidine N=CH), 174.12 (NHCHCOOH), 175.00 (CH2CH2COOH). Anal. Calcd. for C22H20N8O7 (508.44): C, 51.97; H, 3.96; N, 22.04; found: C, 51.79; H, 3.81; N, 21.91.
4.2.11. “N-(4-{[(7,10-dioxo-6,7,8,10-tetrahydroimidazo[2,1-b]pteridin-2-yl)methyl]-amino}-benzoyl)glutamic acid” (15)
Ethyl chloroacetate (0.001 mol, 0.12 g) and folic acid (0.001 mol, 0.44 g) in DMF (11 mL) stirred under reflux for 4.5 h (TLC, RF = 0.8, eluent: CH2Cl2). The precipitate separated after cooling crystallized from EtOH to yield orange crystals. Yield, 88%, m.p. 221–224 °C. IR, 3423–3401 cm−1 (2OH), 3251–3204 cm−1 (3NH), 2927 cm−1 (Ar-H), 2854 cm−1 (Aliphatic-H), 1668–1618 cm−1 (5C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.93–2.04 (m, 2H, CH2CH2COOH), 2.55 (t, 2H, CH2CH2COOH), 3.51 (s, 2H, imidazolidinone CH2), 4.26 (t, 1H, NHCHCOOH), 4.48 (s, 2H, pteridine-CH2-N), 6.53 (d, 2H, N-Ph-(H)ortho), 6.92 (s, 1H, HN-Ph), 7.57 (m, 2H, N-Ph-(H) (meta)), 8.03 (s, 1H, NHCO), 8.64 (s,1H, pteridine-C7H), 10.87 (s, 1H, imidazole NH), 11.28 (s, 1H, CH2CH2COOH), 12.11 (s,1H, CH2COOH). Anal. Calcd. for C21H19N7O7 (481.42): C, 52.39; H, 3.98; N, 20.37; found: C, 52.00; H, 3.71; N, 20.19.
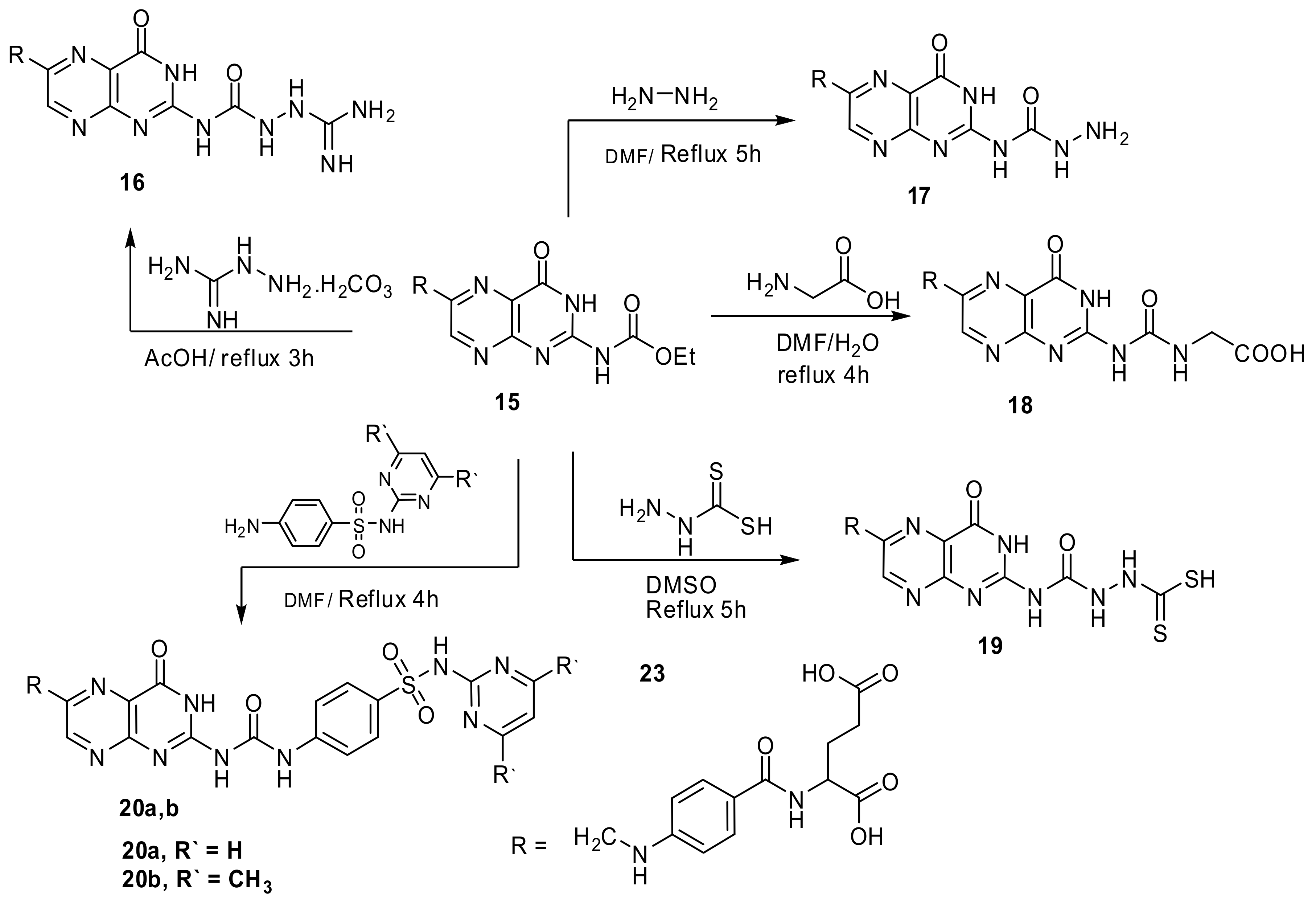
4.2.12. “N-{4-[({2-[({2-[amino(imino)methyl]hydrazino}-carbonyl)amino]-4-oxo-3,4-dihydropteridin-6-yl}methyl)amino]benzoyl}-glutamic acid” (16)
Compound 15 (0.001 mol, 0.51 g) and aminoguanidinium hydrocarbonate (0.001 mol, 0.14 g) in glacial acetic acid (15 mL) was stirred under reflux for 3 h (TLC, RF = 0.6, eluent: CH2Cl2). A brownish powder formed on hot, the precipitate filtered while hot and washed with ethanol. Yield 87%, m.p. 274–276 °C. IR, 3439–3410 cm−1 (2OH), 3319–3161 cm−1 (NH2 and 7 NH), 2954 cm−1 (Ar-H), 2851 cm−1 (Aliphatic-H), 1678–1620 cm−1 (5C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.93–2.05 (m, 2H, CH2CH2COOH), 2.55 (t, 2H, CH2CH2COOH), 4.31 (t, 1H, NHCHCOOH), 4.54 (s, 2H, pteridine-CH2-N), 6.27 (s, 2H, NH2), 6.63 (d, 2H, N-Ph-(H)ortho), 6.91 (s, 1H, HN-Ph), 7.65 (m, 2H, N-Ph-(H) (meta)), 7.80 (s, 1H, C=NH), 8.14 (s, 1H, NHCO), 8.51 (s, 1H, pteridine-NHCONH), 8.82 (s,1H, pteridine-C7H), 10.17 (s, 1H, NH-NH), 10.31 (s, 1H, pteridine NH), 10.99 (s, 1H, NH-NH), 11.40 (s, 1H, CH2CH2COOH), 12.30 (s,1H, CH2COOH). Anal. Calcd. for C21H23N11O7 (541.48): C, 46.58; H, 4.28; N, 28.45; found: C, 46.36; H, 4.09; N, 28.31.
4.2.13. “N-{4-[({2-[(hydrazinocarbonyl)amino]-4-oxo-3,4-dihydropteridin-6-yl}methyl)amino]-benzoyl}glutamic acid” (17)
Compound 15 (0.51g, 1 mmol) and NH2NH2 (excess, 3 mL) in DMF (12 mL) were refluxed for 5 h (TLC, RF = 0.5, eluent: CH2Cl2). The solution concentrated under vacuum and poured onto crushed ice, an orange compound resulted, crystallized from DMF:EtOH mixture 1:3 to give yellowish powder. Yield, 65%, m.p. 231–233 °C. IR, 3411, 3386 cm−1 (2OH), 3309–3214 cm−1 (NH2 and 5NH), 3001 cm−1 (Ar-H), 2876 cm−1 (Aliphatic-H), 1686–1618 cm−1 (5C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.93–2.02 (m, 2H, CH2CH2COOH), 2.42 (t, 2H, CH2CH2COOH), 4.09 (t, 1H, NHCHCOOH), 4.33 (s, 2H, s, 1H, NHCONHNH2), 4.53 (s, 2H, pteridine-CH2-N), 6.61 (d, 2H, N-Ph-(H)ortho), 7.62 (s, 1H, HN-Ph), 7.68 (d, 2H, N-Ph-(H)meta), 8.11 (s, 1H, NHCO), 8.22 (s,1H, pteridine-C7H), 8.69 (s, 1H, NHCONHNH2), 9.61 (s, 1H, NHCONHNH2), 10.34 (s, 1H, pteridine NH), 11.41 (s,1H, CH2CH2COOH), 12.34 (s,1H, CH2COOH). 13C-NMR (DMSO d6, 200 MHz): δ = 31.18 (CH2CH2COOH), 35.41 (CH2CH2COOH), 47.13 (NHCH2), 51.99 (NHCH), 112.12 (N-Ph-(C)ortho), 114.43 (N-Ph-(C)Para), 121.67 (pteridine C4a), 129.11 (N-Ph-(C)meta), 147.71 (pteridine C6), 151.58 (pteridine C7), 151.78 (N-Ph-(C)), 155.41 (pteridine C8a), 158.18 (pteridine C2), 161.89 (NCONHNH2), 162.11 (pteridine C4), 166.43 (NHCO), 175.81 (NHCHCOOH), 177.71 (CH2CH2COOH). Anal. Calcd. for C20H21N9O7 (499.44): C, 48.10; H, 4.24; N, 25.24; found: C, 47.72; H, 4.11; N, 25.18.
4.2.14. “N-[4-({[2-({[(carboxymethyl)amino]carbonyl}amino)-4-oxo-3,4-dihydropteridin-6-yl]methyl}amino)benzoyl]glutamic acid” (18)
Compound 15 (0.001 mol, 0.5 g) and glycine (0.001 mol, 0.08 g) in DMF/ H2O mixture (9:1, 10 mL) was refluxed for 4 h (TLC, RF = 0.70, eluent: CH2Cl2). The solvent concentrated by evaporation. The brown solid formed after pouring on crushed ice crystallized from EtOH. Yield, 83%, m.p. 210–212 °C. IR, 3451–3410 cm−1 (3OH), 3233–3185 cm−1 (3NH), 2971 cm−1 (Ar-H), 2857 cm−1 (Aliphatic-H), 1674–1623 cm−1 (6C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.92–2.01 (m, 2H, CH2CH2COOH), 2.55 (t, 2H, CH2CH2COOH), 4.03 (s, 2H, NHCH2COOH), 4.33 (t, 1H, NHCHCOOH), 4.51 (s, 2H, pteridine-CH2-N), 6.58 (d, 2H, N-Ph-(H)ortho), 6.96 (s, 1H, HN-Ph), 7.61 (m, 2H, N-Ph-(H) (meta)), 8.01 (s, 1H, NHCO), 8.66 (s,1H, pteridine-C7H), 8.78 (s, 1H, NHCH2COOH), 10.31 (s, 1H, pteridine NH), 11.31 (s, 1H, CH2CH2COOH), 11.60 (s,1H, CH2COOH), 12.22 (s, 1H, NHCH2COOH). 13CNMR (DMSO d6, 200 MHz): δ = 25.08 (CH3), 30.19 (CH2CH2COOH), 34.13 (CH2CH2COOH), 41.10 (glycine CH2), 45.90 (NHCH2), 51.21 (NHCH), 111.18 (N-Ph-(C)ortho), 120.99 (N-Ph-(C)Para), 127.97 (pteridine C4a), 128.69 (N-Ph-(C)meta), 148.09 (pteridine C6), 150.67 (pteridine C7), 150.91 (N-Ph-(C)), 154.69 (pteridine C8a), 156.30 (pteridine C2), 160.91 (pteridine C4), 161.53 (NHCONH), 166.52 NHCO, 172.09 (NHCHCOOH), 173.17 (glycine CO), 174.51 (CH2CH2COOH). Anal. Calcd. for C22H22N8O9 (542.46): C, 48.71; H, 4.09; N, 20.66; found: C, 48.53; H, 3.78; N, 20.51.
4.2.15. “N-[4-({[2-({[2-(mercaptocarbonothioyl)hydrazino]-carbonyl}amino)-4-oxo-3,4-dihydropteridin-6-yl]methyl}amino)benzoyl]glutamic acid” (19)
Compound 15 (0.001 mol, 0.51 g) and thioformic acid hydrazide (0.001 mol, 0.1 g) in DMSO (12 mL) were refluxed for 5 h (TLC, RF = 0.4, eluent: CH2Cl2). The product formed after pouring onto crushed ice crystallized from DMF/EtOH 1:1 to yield brown powder. Yield, 84%, m.p. 281–283 °C. IR, 3423–3402 cm−1 (2OH), 3251–3147 cm−1 (6NH), 2928 cm−1 (Ar-H), 2850 cm−1 (Aliphatic-H), 2627 cm−1 (SH), 1678–1621 cm−1 (5C=O and C=N), 1351 cm−1 (C=S). 1H-NMR (DMSO d6, 850 MHz): δ = 1.89–2.04 (m, 2H, CH2CH2COOH), 2.50 (t, 2H, CH2CH2COOH), 4.08 (s, 2H, NHCH2COOH), 4.31 (t, 1H, NHCHCOOH), 4.54 (s, 2H, pteridine-CH2-N), 6.54 (d, 2H, N-Ph-(H)ortho), 6.96 (s, 1H, HN-Ph), 7.62 (m, 2H, N-Ph-(H) (meta)), 8.09 (s, 1H, NHCO), 8.61 (s,1H, pteridine-C7H), 10.36 (s, 1H, pteridine NH), 11.17 (s, 1H, NH-NH), 11.38 (s, 1H, NH-NH), 11.43 (s, 1H, CH2CH2COOH), 12.27 (s,1H, CH2COOH), 13.98 (s, 1H, SH). Anal. Calcd. for C21H21N9O7S2 (575.58): C, 43.82; H, 3.68; N, 21.90; S, 11.14; found: C, 43.57; H, 3.49; N, 21.77; S, 10.91.
4.3. Reaction of Compound 15 with Sulfa Drugs (20a,b): General Procedure
A mixture of 15 (0.001 mol, 0.51 g) and appropriate sulfa drug (0.001 mol) in DMF (15 mL) was stirred under reflux for 4h (TLC, RF = 0.45, eluent: CH2Cl2). The precipitate formed crystallized from EtOH to produce yellow to orange powder.
4.3.1. “N-(4-{[(4-oxo-2-{[({4-[(pyrimidin-2-ylamino)sulfonyl]-phenyl}amino)carbonyl]-amino}-3,4-dihydropteridin-6-yl)methyl]amino}-benzoyl)glutamic acid” (20a)
Yellowish orange, yield, 77%, m.p. 238–240 °C. IR, 3438–3417 cm−1 (2OH), 3331–3199 cm−1 (6NH), 2952 cm−1 (Ar-H), 2853 cm−1 (Aliphatic-H), 1684–1623 cm−1 (5C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.88–2.04 (m, 2H, CH2CH2COOH), 2.51 (t, 2H, CH2CH2COOH), 4.34 (t, 1H, NHCHCOOH), 4.55 (s, 2H, pteridine-CH2-N), 6.42 (d, 2H, J = 8.4 Hz, benzene C2H,C6H), 6.65 (d, 2H, N-Ph-(H)ortho), 6.81 (d, 2H, J = 8.4 Hz, benzene C3H,C5H), 6.92 (s, 1H, HN-Ph), 7.66 (m, 2H, N-Ph-(H) (meta)), 8.17 (s, 1H, NHCO), 8.41 (t, 1H, pyrimidine C3H), 8.53 (s, 1H, pteridine-NHCONH), 8.64 (d, 2H, pyrimidine C2H,C4H), 8.77 (s,1H, pteridine-C7H), 8.99 (s, 1H, pteridine-NHCONH), 10.31 (s, 1H, pteridine NH), 11.38 (s, 1H, CH2CH2COOH), 12.27 (s,1H, CH2COOH), 12.83 (s, 1H, SO2NH). Anal. Calcd. for C30H27N11O9S (717.67): C, 50.21; H, 3.79; N, 21.47; S, 4.47; found: C, 49.91; H, 3.58; N, 21.62; S, 4.30.
4.3.2. “N-[4-({[2-({[(4-{[(4,6-dimethylpyrimidin-2-yl)amino]-sulfonyl}phenyl)amino]-carbonyl}amino)-4-oxo-3,4-dihydropteridin-6-yl]methyl}amino) benzoyl]glutamic acid” (20b)
Yield, 72%, Orange powder, m.p. 246–248 °C. IR, 3442–3421 cm−1 (2OH), 3325–3212 cm−1 (6NH), 2975 cm−1 (Ar-H), 2857 cm−1 (Aliphatic-H), 1680–1621 cm−1 (5C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.91–2.05 (m, 2H, CH2CH2COOH), 2.54 (t, 2H, CH2CH2COOH), 2.43 (s, 6H, 2CH3), 4.37 (t, 1H, NHCHCOOH), 4.52 (s, 2H, pteridine-CH2-N), 6.45 (d, 2H, J = 8.4 Hz, benzene C2H,C6H), 6.68 (d, 2H, N-Ph-(H)ortho), 6.83 (d, 2H, J = 8.4 Hz, benzene C3H,C5H), 6.89 (s, 1H, HN-Ph), 7.61 (m, 2H, N-Ph-(H) (meta)), 8.13 (s, 1H, NHCO), 8.40 (t, 1H, pyrimidine C3H), 8.55 (s, 1H, pteridine-NHCONH), 8.61 (d, 2H, pyrimidine C2H,C4H), 8.79 (s,1H, pteridine-C7H), 8.91 (s, 1H, pteridine-NHCONH), 10.31 (s, 1H, pteridine NH), 11.31 (s, 1H, CH2CH2COOH), 12.34 (s,1H, CH2COOH), 12.85 (s, 1H, SO2NH). Anal. Calcd. for C32H31N11O9S (745.72): C, 51.54; H, 4.19; N, 20.66; S, 4.30; found: C, 51.21; H, 4.02; N, 20.49; S, 4.17.
4.3.3. “N-[4-({[2-({[2-(aminocarbonyl)hydrazino]methylene}-amino)-4-oxo-3,4-dihydropteridin-6-yl]methyl}amino)benzoyl]glutamic acid” (21)
Compound 4 (0.001 mol, 0.5 g) and semicarbazide HCl (0.001 mol, 0.11 g) and drops of TMA in DMF (14 mL) was stirred under reflux for 4 h (TLC, RF = 0.65, eluent: CH2Cl2). Brown powder formed after crystallization from EtOH. Yield 78%, m.p. 250–252 °C. IR, 3412–3389 cm−1 (2OH), 3329–33251 cm−1 (NH2 and 5 NH), 2949 cm−1 (Ar-H), 2850 cm−1 (Aliphatic-H), 1666–1623 cm−1 (5C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.91–2.05 (m, 2H, CH2CH2COOH), 2.54 (t, 2H, CH2CH2COOH), 4.31 (t, 1H, NHCHCOOH), 4.44 (s, 2H, pteridine-CH2-N), 6.64 (d, 2H, N-Ph-(H)ortho), 6.89 (s, 1H, HN-Ph), 6.98 (s, 2H, NH2), 7.61 (m, 2H, N-Ph-(H) (meta)), 7.65 (s, 1H, N=CH), 8.21 (s, 1H, NHCO), 8.84 (s,1H, pteridine-C7H), 10.31 (s, 1H, pteridine NH), 10.57 (s, 1H, NH-NH), 10.99 (s, 1H, NH-NH), 11.41 (s, 1H, CH2CH2COOH), 12.28 (s,1H, CH2COOH). Anal. Calcd. for C21H22N10O7 (526.46): C, 47.91; H, 4.21; N, 26.61; found: C, 47.63; H, 3.92; N, 26.47.
4.3.4. “N-{4-[({2-[({2-[amino(imino)methyl]hydrazino}-methylene)amino]-4-oxo-3,4-dihydropteridin-6-yl}methyl)amino]-benzoyl}glutamic acid” (22)
Compound 14 (0.001 mol, 0.5 g) and aminoguanidinium hydrocarbonate (0.001 mol, 0.14 g) in glacial AcOH (15 mL) was stirred under reflux (TLC, RF = 0.6, eluent: CH2Cl2), after 2 h a green precipitate formed, which crystallized from EtOH. Yield 96%, m.p. 278–280 °C. IR, 3442–3415 cm−1 (2OH), 3289–3151 cm−1 (NH2 and 6 NH), 2951 cm−1 (Ar-H), 2847 cm−1 (Aliphatic-H), 1672–1617 cm−1 (4C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.93–2.05 (m, 2H, CH2CH2COOH), 2.52 (t, 2H, CH2CH2COOH), 4.29 (t, 1H, NHCHCOOH), 4.51 (s, 2H, pteridine-CH2-N), 6.21 (s, 2H, NH2), 6.61 (d, 2H, N-Ph-(H)ortho), 6.90 (s, 1H, HN-Ph), 7.60 (m, 2H, N-Ph-(H) (meta)), 7.55 (s, 1H, N=CH), 7.82 (s, 1H, C=NH), 8.11 (s, 1H, NHCO), 8.82 (s,1H, pteridine-C7H), 10.13 (s, 1H, NH-NH), 10.33 (s, 1H, pteridine NH), 10.90 (s, 1H, NH-NH), 11.42 (s, 1H, CH2CH2COOH), 12.31 (s,1H, CH2COOH). Anal. Calcd. for C21H23N11O6 (525.48): C, 48.00; H, 4.41; N, 29.32; found: C, 47.61; H, 4.28; N, 29.10.
4.3.5. “N-[4-({[2-({[(carboxymethyl)amino]methylene}amino)-4-oxo-3,4-dihydro-pteridin-6-yl]methyl}amino)benzoyl]glutamic acid” (23)
A mixture of compound 14 (0.001 mol, 0.5 g) and glycine (0.001 mol, 0.08 g) in DMF/ H2O mixture (9:1, 10 mL) refluxed for 3h (TLC, RF = 0.75, eluent: CH2Cl2). The brown solid formed crystallized from EtOH to give crystals. Yield, 91%, m.p. 218–220 °C. IR, 3443–3405 cm−1 (3OH), 3317–3122 cm−1 (4NH), 2910 cm−1 (Ar-H), 2850 cm−1 (Aliphatic-H), 1688–1619 cm−1 (5C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.92–2.03 (m, 2H, CH2CH2COOH), 2.54 (t, 2H, CH2CH2COOH), 4.18 (s, 2H, NHCH2COOH), 4.33 (t, 1H, NHCHCOOH), 4.55 (s, 2H, pteridine-CH2-N), 6.63 (d, 2H, N-Ph-(H)ortho), 6.90 (s, 1H, HN-Ph), 7.44 (m, 2H, N-Ph-(H) (meta)), 7.52 (s, 1H, N=CH), 8.09 (s, 1H, NHCO), 8.77 (s,1H, pteridine-C7H), 8.96 (s, 1H, NHCH2COOH), 10.36 (s, 1H, pteridine NH), 11.34 (s, 1H, CH2CH2COOH), 11.58 (s,1H, CH2COOH), 12.28 (s, 1H, NHCH2COOH). Anal. Calcd. for C22H22N8O8 (526.46): C, 50.19; H, 4.21; N, 21.28; found: C, 49.84; H, 4.06; N, 21.01.
4.4. Reaction of Compound 14 and Sulfa Drugs (24a,b): General Procedure
A mixture of 14 (0.001 mol, 0.5 g) and appropriate sulfa drug (0.001 mol) in DMF (15 mL) was stirred under reflux for 3h (TLC, RF = 0.4, eluent: CH2Cl2). The precipitate formed crystallized from EtOH to yield brownish powder.
4.4.1. “N-(4-{[(4-oxo-2-{[({4-[(pyrimidin-2-ylamino)sulfonyl]-phenyl}amino) methylene]-amino}-3,4-dihydropteridin-6-yl)methyl]amino}-benzoyl)-glutamic acid” (24a)
Brownish powder, yield, 76%, m.p. 236–238 °C. IR, 3456–3412 cm−1 (2OH), 3321–3228 cm−1 (5NH), 2974 cm−1 (Ar-H), 2803 cm−1 (Aliphatic-H), 1687–1616 cm−1 (4C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.93–2.05 (m, 2H, CH2CH2COOH), 2.53 (t, 2H, CH2CH2COOH), 4.32 (t, 1H, NHCHCOOH), 4.52 (s, 2H, pteridine-CH2-N), 6.41 (d, 2H, J = 8.4 Hz, benzene C2H,C6H), 6.61 (d, 2H, N-Ph-(H)ortho), 6.84 (d, 2H, J = 8.4 Hz, benzene C3H,C5H), 6.91 (s, 1H, HN-Ph), 7.02 (s, 1H, N=CH-NH), 7.52 (s, 1H, N=CH), 7.64 (m, 2H, N-Ph-(H) (meta)), 8.11 (s, 1H, NHCO), 8.45 (t, 1H, pyrimidine C3H), 8.62 (d, 2H, pyrimidine C2H,C4H), 8.79 (s,1H, pteridine-C7H), 10.29 (s, 1H, pteridine NH), 11.44 (s, 1H, CH2CH2COOH), 12.28 (s,1H, CH2COOH), 12.87 (s, 1H, SO2NH). Anal. Calcd. for C30H27N11O8S (701.67): C, 51.35; H, 3.88; N, 21.96; S, 4.57; found: C, 51.02; H, 3.42; N, 21.71, S, 4.33.
4.4.2. “N-[4-({[2-({[(4-{[(4,6-dimethylpyrimidin-2-yl)amino]-sulfonyl}phenyl)amino]-methylene}amino)-4-oxo-3,4-dihydropteridin-6-yl]methyl}-amino)benzoyl]glutamic acid” (24b)
Brown powder, yield, 74%, m.p. 258–260 °C. IR, 3453–3405 cm−1 (2OH), 3314–3212 cm−1 (5NH), 2952 cm−1 (Ar-H), 2851 cm−1 (Aliphatic-H), 1692–1621 cm−1 (4C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.90–2.04 (m, 2H, CH2CH2COOH), 2.51 (t, 2H, CH2CH2COOH), 2.68 (s, 6H, 2CH3), 4. 30 (t, 1H, NHCHCOOH), 4.52 (s, 2H, pteridine-CH2-N), 6.41 (d, 2H, J = 8.4 Hz, benzene C2H,C6H), 6.64 (d, 2H, N-Ph-(H)ortho), 6.80 (d, 2H, J = 8.4 Hz, benzene C3H,C5H), 6.91 (s, 1H, HN-Ph), 7.03 (s, 1H, N=CH-NH), 7.53 (s, 1H, N=CH), 7.61 (m, 2H, N-Ph-(H) (meta)), 8.11 (s, 1H, NHCO), 8.44 (t, 1H, pyrimidine C3H), 8.61 (d, 2H, pyrimidine C2H,C4H), 8.80 (s,1H, pteridine-C7H), 10.33 (s, 1H, pteridine NH), 11.45 (s, 1H, CH2CH2COOH), 12.31 (s,1H, CH2COOH), 12.84 (s, 1H, SO2NH). Anal. Calcd. for C32H31N11O8S (729.72): C, 52.67; H, 4.28; N, 21.11; S, 4.39; found: C, 52.40; H, 4.10; N, 21.03; S, 4.13.
4.4.3. “N-(4-{[(4-amino-3-mercapto-2,12-dioxo-5-phenyl-1,12-dihydro-2H-pyrido[3′,2′:5,6]-pyrimido[2,1-b]pteridin-10-yl)methyl]-amino}benzoyl)-glutamic acid” (25)
Compound 10 (0.001 mol, 0.59 g), thioglycolic acid (0.001 mol, 0.09 g), and drops of TMA in EtOH (12 mL) were refluxed for 15 h (TLC, RF = 0.55, eluent: CH2Cl2). The precipitate formed crystallized from DMF/EtOH 1:1 to yield dark orange crystals. Yield, 76%, m.p. 251–253 °C. IR, 3401–3389 cm−1 (2OH), 3287–3165 cm−1 (NH2, 3NH), 2947 cm−1 (Ar-H), 2852 cm−1 (Aliphatic-H), 2657 cm−1 (SH), 1683–1622 cm−1 (5C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.90–2.03 (m, 2H, CH2CH2COOH), 2.55 (t, 2H, CH2CH2COOH), 4.06 (t, 1H, NHCHCOOH), 4.53 (s, 2H, pteridine-CH2-N), 6.92 (d, 2H, N-Ph-(H)ortho), 6.96 (s, 1H, HN-Ph), 7.68–7.90 (m, 7H, N-Ph-(H)(meta) and pyrimidine-4-Ph), 8.12 (s, 1H, NHCO), 8.67 (s,1H, pteridine-C7H), 11.38 (s, 1H, pyridine-NH), 11.44 (s, 1H, CH2CH2COOH), 12.31 (s,1H, CH2COOH), 14.01 (s, 1H, pyridine-SH). Anal. Calcd. for C31H25N9O7S (667.65): C, 55.77; H, 3.77; N, 18.88; S, 4.80; found: C, 55.54; H, 3.62; N, 18.64; S, 4.67.
4.4.4. “N-{4-[({8-cyano-9-[(mercaptoacetyl)amino]-11-oxo-7-phenyl-11H-pyrimido[2,1-b]pteridin-2-yl}methyl)amino]benzoyl}glutamic acid” (26)
Compound 10 (0.001 mol, 0.59 g) and thioglycolic acid (0.001 mol, 0.09 g) in EtOH (13 mL) were refluxed for 5 h (TLC, RF = 0.5, eluent: CH2Cl2). The precipitate formed on hot filtered and crystallized from DMF/EtOH 1:1 to produce yellowish brown crystals. Yield, 89%, m.p. 238–240 °C. IR, 3427–3412 cm−1 (2OH), 3248–3153 cm−1 (3NH), 2961 cm−1 (Ar-H), 2866 cm−1 (Aliphatic-H), 2634 cm−1 (SH), 2206 cm−1 (CN), 1678–1618 cm−1 (5C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.91–2.07 (m, 2H, CH2CH2COOH), 2.53 (t, 2H, CH2CH2COOH), 3.64 (s, 2H, NHCOCH2SH), 4.06 (t, 1H, NHCHCOOH), 4.52 (s, 2H, pteridine-CH2-N), 6.90 (d, 2H, N-Ph-(H)ortho), 6.99 (s, 1H, HN-Ph), 7.61-7.91 (m, 7H, N-Ph-(H)(meta) and pyrimidine-4-Ph), 8.19 (s, 1H, NHCO), 8.66 (s,1H, pteridine-C7H), 10.37 (s, 1H, NHCOCH2SH), 11.44 (s, 1H, CH2CH2COOH), 12.28 (s,1H, CH2COOH), 13.97 (s, 1H, NHCOCH2SH). Anal. Calcd. for C31H25N9O7S (667.65): C, 55.77; H, 3.77; N, 18.88; S, 4.80; found: C, 55.54; H, 3.62; N, 18.64; S, 4.67.
4.4.5. “N-{4-[({8-cyano-9-[(mercaptocarbonothioyl)amino]-11-oxo-7-phenyl-11H-pyrimido-[2,1-b]pteridin-2-yl}methyl)amino]benzoyl}-glutamic acid” (27)
Compound 10 (0.001 mol, 0.59 g), CS2 (excess, 1 mL) and KOH (0.003 mol, 0.17 g) in EtOH (20 mL) was refluxed for 5 h (TLC, RF = 0.35, eluent: CH2Cl2). The solution poured onto crushed ice after concentration then acidified with dilute HCl. The precipitate formed crystallized from EtOH to produce reddish brown powder. Yield, 57%, m.p. 240–242 °C. IR, 3418–3406 cm−1 (2OH), 3221–3170 cm−1 (3NH), 2950 cm−1 (Ar-H), 2838 cm−1 (Aliphatic-H), 2630 cm−1 (SH), 2202 cm−1 (CN), 1689–1621 cm−1 (4C=O and C=N), 1328 cm−1 (C=S). 1H-NMR (DMSO d6, 850 MHz): δ = 1.92–2.04 (m, 2H, CH2CH2COOH), 2.54 (t, 2H, CH2CH2COOH), 4.05 (t, 1H, NHCHCOOH), 4.41 (s, 2H, pteridine-CH2-N), 6.90 (d, 2H, N-Ph-(H)ortho), 6.95 (s, 1H, HN-Ph), 7.61-7.95 (m, 7H, N-Ph-(H) (meta) and pyrimidine-4-Ph), 8.18 (s, 1H, NHCO), 8.66 (s,1H, pteridine-C7H), 10.56 (s, 1H, NHC=S); 11.41 (s, 1H, CH2CH2COOH), 12.29 (s,1H, CH2COOH), 13.98 (s, 1H, SH). Calcd. for C30H23N9O6S2 (669.69): C, 53.80; H, 3.46; N, 18.82; S, 9.58; found: C, 53.66; H, 3.39; N, 18.71; S, 9.42.
4.4.6. “N-(4-{[(12-oxo-5-phenyl-2,4-dithioxo-1,3,4,12-tetrahydro-2H-pyrimido-[5′,4′:5,6]-pyrimido[2,1-b]pteridin-10-yl)methyl]- amino}benzoyl)glutamic acid” (28)
Compound 10 (0.001 mol, 0.59 g), CS2 (excess, 1 mL) and KOH (0.003 mol, 0.17 g, 3 mmol) dry pyridine (10 mL) was refluxed for 12 h (TLC, RF = 0.5, eluent: CH2Cl2). The solution poured onto crushed ice/dilute HCl. The product crystallized from EtOH to yield orange powder. Yield, 57%, m.p. 262–264 °C. IR, 3419–3410 cm−1 (2OH), 3234–3187 cm−1 (4NH), 2971 cm−1 (Ar-H), 2846 cm−1 (Aliphatic-H), 1675–1623 cm−1 (4C=O and C=N); 1350–1273 (2C=S). 1H-NMR (DMSO d6, 850 MHz): δ = 1.93–2.05 (m, 2H, CH2CH2COOH), 2.54 (t, 2H, CH2CH2COOH), 4.01 (t, 1H, NHCHCOOH), 4.44 (s, 2H, pteridine-CH2-N), 6.91 (d, 2H, N-Ph-(H)ortho), 6.97 (s, 1H, HN-Ph), 7.62–7.90 (m, 7H, N-Ph-(H) (meta) and pyrimidine-4-Ph), 8.22 (s, 1H, NHCO), 8.67 (s,1H, pteridine-C7H), 10.56 (s, 1H, N1HC=S); 11.41 (s, 1H, CH2CH2COOH), 12.29 (s,1H, CH2COOH), 12.56 (s, 1H, N2HC=S). Calcd. for C30H23N9O6S2 (669.69): C, 53.80; H, 3.46; N, 18.82; S, 9.58; found: C, 53.66; H, 3.39; N, 18.71; S, 9.42.
4.4.7. “N-(4-{[(2,4-diamino-3-cyano-12-oxo-5-phenyl-12H-pyrido[3′,2′:5,6]pyrimido[2,1-b]pteridin-10-yl)methyl]amino}benzoyl)-glutamic acid” (29)
Compound 10 (0.001 mol, 0.59 g) with malononitrile (0.001 mol, 0.07 g), and drops of TMA in EtOH (13 mL) refluxed for 13 h (TLC, RF = 0.6, eluent: CH2Cl2). The red product formed crystallized from EtOH to produce dark red powder. Yield, 69%, m.p. 236–238 °C. IR, 3423–3415 cm−1 (2OH), 3245–3162 cm−1 (2NH2, 2NH), 2944 cm−1 (Ar-H), 2852 cm−1 (Aliphatic-H), 2201 cm−1 (CN); 1678–1621 cm−1 (4C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.93–2.04 (m, 2H, CH2CH2COOH), 2.55 (t, 2H, CH2CH2COOH), 4.11 (t, 1H, NHCHCOOH), 4.42 (s, 2H, pteridine-CH2-N), 6.12 (s, 2H, C2NH2), 6.58 (s, 2H, C4NH2), 6.92 (d, 2H, N-Ph-(H)ortho), 6.99 (s, 1H, HN-Ph), 7.62–7.93 (m, 7H, N-Ph-(H) (meta) and pyrimidine-4-Ph), 8.21 (s, 1H, NHCO), 8.61 (s,1H, pteridine-C7H), 11.43 (s, 1H, CH2CH2COOH), 12.22 (s,1H, CH2COOH). Calcd. for C32H25N11O6 (659.61): C, 58.27; H, 3.82; N, 23.36; found: C, 58.05; H, 3.71; N, 23.23.
4.4.8. “N-(4-{[(4-amino-12-oxo-5-phenyl-12H-pyrimido-[5′,4′:5,6]pyrimido-[2,1-b]pteridin-10-yl)methyl]amino}benzoyl)glutamic acid” (30)
Compound 10 (0.001 mol, 0.59 g) refluxed in excess formamide (8 mL) for 9 h (TLC, RF = 0.7, eluent: CH2Cl2). The solution poured on ice-cold water and then extracted with CH2Cl2 (25 mL, two times) to give yellow product which crystallized from EtOH. Yield, 42%, m.p. 225–227 °C. IR, 3421–3411 cm−1 (2OH), 3221–3137 cm−1 (NH2, 2NH), 2957 cm−1 (Ar-H), 2850 cm−1 (Aliphatic-H), 1671–1623 cm−1 (4C=O and C=N). 1H-NMR (DMSO d6, 850 MHz): δ = 1.88–2.05 (m, 2H, CH2CH2COOH), 2.50 (t, 2H, CH2CH2COOH), 4.07 (t, 1H, NHCHCOOH), 4.45 (s, 2H, pteridine-CH2-N), 6.50 (s, 2H, C4NH2), 6.90 (d, 2H, N-Ph-(H)ortho), 6.98 (s, 1H, HN-Ph), 7.60–7.97 (m, 7H, N-Ph-(H) (meta) and pyrimidine-4-Ph, pyrimidine C2H), 8.23 (s, 1H, NHCO), 8.68 (s,1H, pteridine-C7H), 11.39 (s, 1H, CH2CH2COOH), 12.28 (s,1H, CH2COOH). Calcd. for C30H24N10O6 (620.57): C, 58.06; H, 3.90; N, 22.57; found: C, 57.83; H, 3.76; N, 22.50.