Screening of Wood/Forest and Vine By-Products as Sources of New Drugs for Sustainable Strategies to Control Fusarium graminearum and the Production of Mycotoxins
Abstract
1. Introduction
2. Results
2.1. Characterization of Wood/Forest and Vine By-Product Extracts
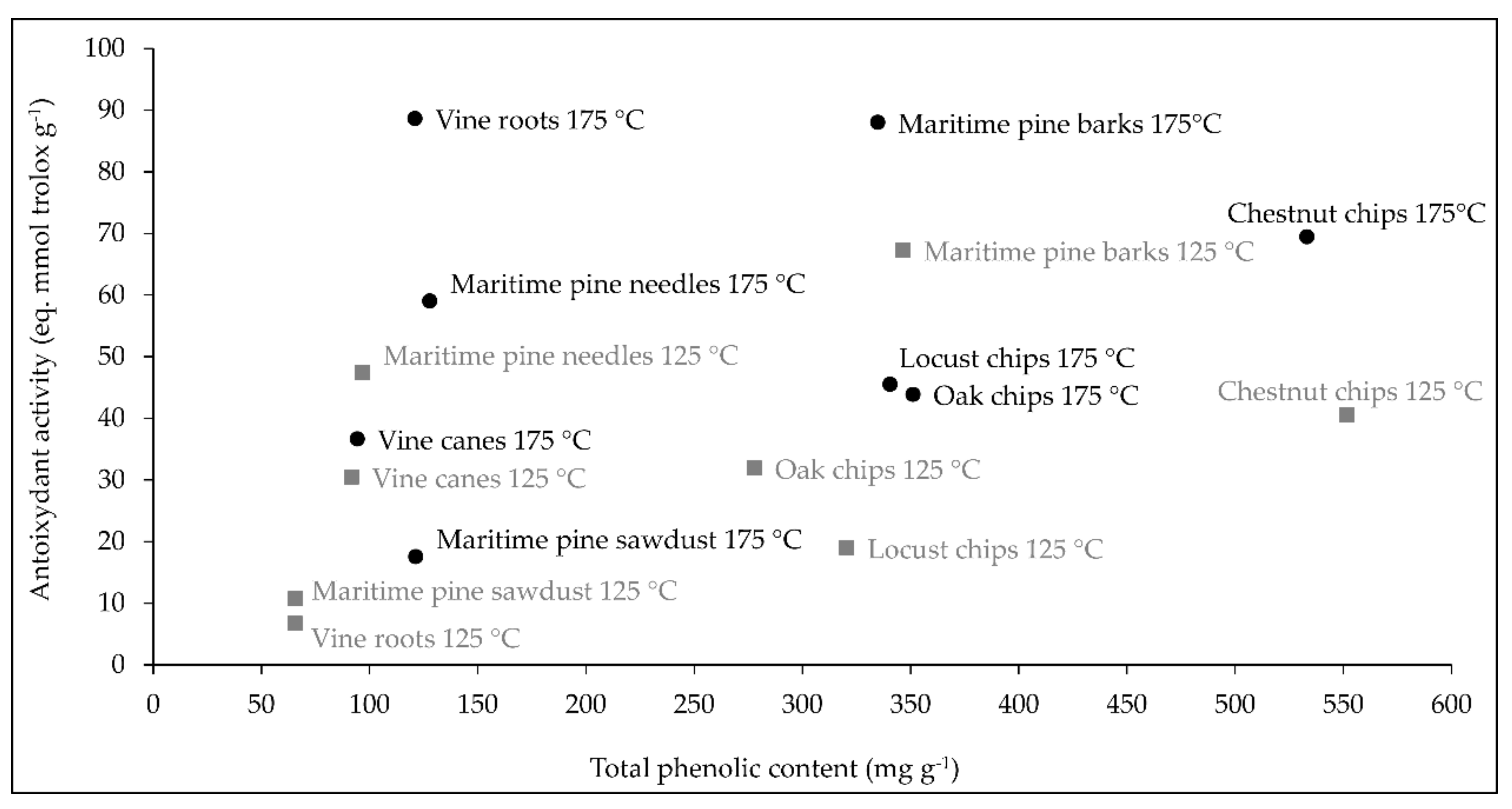
2.1.1. Plant Extraction Yield
2.1.2. Total Phenolic Content and Antioxidant Activity of Wood/Forest and Vine By-Product Extracts
2.2. Screening of the Wood/Forest and Vine By-Product Extracts for Their Antifungal Activity Against F. graminearum CBS 185.32
2.3. Impact of Selected Wood/Forest and Vine By-Product Extracts on the Mycelial Biomass of F. graminearum and the Production of TCTB in Liquid Cultures
2.3.1. Comparative Efficiencies of Maritime Pine Sawdust 175 °C, Maritime Pine Sawdust 125 °C, Vine Cane 125 °C and Maritime Pine Bark 125 °C Extracts to Affect the Fungal Growth and the Production of TCTB by F. graminearum CBS 185.32
2.3.2. Effect of the Maritime Pine Sawdust 175 °C Extract on the Fungal Biomass and TCTB Yield by a Panel of F. graminearum Strains
2.4. Characterization of the Phenolic Composition of the Maritime Pine Sawdust 175 °C Extract
3. Discussion
3.1. Variations in Antioxidant Activity and Total Phenolic Composition of Wood/Forest and Vine By-Product Extracts
3.2. The Maritime Pine Sawdust 175 °C Extract Is a Strong Inhibitor of Fungal Growth and TCTB Production by F. graminearum CBS 185.32
4. Materials and Methods
4.1. Chemicals and Standards
4.2. Natural Sources and Plant Material Preparation Prior to Extraction
4.3. Preparation of Natural Extracts and Determination of Extraction Yield and Extract Concentration
4.4. Fusarium Strains, Media, and Culture Conditions
4.5. Extraction and TCTB Analysis
4.6. Determination of Total Phenolic Content
4.7. Determination of Free Radical Scavenging Potential by the Oxygen Radical Absorbance Capacity (ORAC)
4.8. LC/MS Analysis
4.9. Expression of Results and Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Mahdjoubi, C.K.; Arroyo-Manzanares, N.; Hamini-Kadar, N.; García-Campaña, A.M.; Mebrouk, K.; Gámiz-Gracia, L. Multi-mycotoxin occurrence and exposure assessment approach in foodstuffs from Algeria. Toxins 2020, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, M.-G. Characterization of Fusarium graminearum isolates resistant to both carbendazim and a new fungicide. Phytopathology 2009, 99, 441–446. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 19. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Ponts, N.; Pinson-Gadais, L.; Boutigny, A.L.; Barreau, C.; Richard-Forget, F. Cinnamic-derived acids significantly affect Fusarium graminearum growth and in vitro synthesis of type B trichothecenes. Phytopathology 2011, 101, 929–934. [Google Scholar] [CrossRef]
- Gauthier, L.; Bonnin-Verdal, M.-N.; Marchegay, G.; Pinson-Gadais, L.; Ducos, C.; Richard-Forget, F.; Atanasova-Penichon, V. Fungal biotransformation of chlorogenic and caffeic acids by Fusarium graminearum: New insights in the contribution of phenolic acids to resistance to deoxynivalenol accumulation in cereals. Int. J. Food Microbiol. 2016, 221, 61–68. [Google Scholar] [CrossRef]
- Ferruz, E.; Atanasova-Pénichon, V.; Bonnin-Verdal, M.-N.; Marchegay, G.; Pinson-Gadais, L.; Ducos, C.; Lorán, S.; Ariño, A.; Barreau, C.; Richard-Forget, F. Effects of phenolic acids on the growth and production of T-2 and HT-2 Toxins by Fusarium langsethiae and F. sporotrichioides. Molecules 2016, 21, 449. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Legoahec, L.; Bernillon, S.; Deborde, C.; Maucourt, M.; Verdal-Bonnin, M.-N.; Pinson-Gadais, L.; Ponts, N.; Moing, A.; Richard-Forget, F. Mycotoxin biosynthesis and central metabolism are two interlinked pathways in Fusarium graminearum, as demonstrated by the extensive metabolic changes induced by caffeic acid exposure. Appl. Environ. Microbiol. 2018, 84, e01705-17. [Google Scholar] [CrossRef]
- Boutigny, A.-L.; Barreau, C.; Atanasova-Penichon, V.; Verdal-Bonnin, M.-N.; Pinson-Gadais, L.; Richard-Forget, F. Ferulic acid, an efficient inhibitor of type B trichothecene biosynthesis and Tri gene expression in Fusarium liquid cultures. Mycol. Res. 2009, 113, 746–753. [Google Scholar] [CrossRef]
- Coma, V.; Portes, E.; Gardrat, C.; Richard-Forget, F.; Castellan, A. In vitro inhibitory effect of tetrahydrocurcuminoids on Fusarium proliferatum growth and fumonisin B-1 biosynthesis. Food Addit. Contam. A 2011, 28, 218–225. [Google Scholar] [CrossRef]
- Valette, N.; Perrot, T.; Sormani, R.; Gelhaye, E.; Morel-Rouhier, M. Antifungal activities of wood extractives. Fungal Biol. Rev. 2017, 31, 113–123. [Google Scholar] [CrossRef]
- Rodrigues, A.M.S.; Stien, D.; Eparvier, V.; Espindola, L.S.; Beauchêne, J.; Amusant, N.; Leménager, N.; Baudassé, C.; Raguin, L. The wood preservative potential of long-lasting Amazonian wood extracts. Int. Biodeter. Biodegr. 2012, 75, 146–149. [Google Scholar] [CrossRef]
- Rosdiana, N.A.; Dumarçay, S.; Gérardin, C.; Chapuis, H.; Santiago-Medina, F.J.; Sari, R.K.; Syafii, W.; Gelhaye, E.; Raharivelomanana, P.; Mohammed, R.; et al. Characterization of bark extractives of different industrial Indonesian wood species for potential valorization. Ind. Crops Prod. 2017, 108, 121–127. [Google Scholar] [CrossRef]
- Gabaston, J.; Richard, T.; Biais, B.; Waffo-Teguo, P.; Pedrot, E.; Jourdes, M.; Corio-Costet, M.-F.; Mérillon, J.-M. Stilbenes from common spruce ( Picea abies ) bark as natural antifungal agent against downy mildew (Plasmopara viticola). Ind. Crops Prod. 2017, 103, 267–273. [Google Scholar] [CrossRef]
- Burčová, Z.; Kreps, F.; Greifová, M.; Jablonský, M.; Ház, A.; Schmidt, Š.; Šurina, I. Antibacterial and antifungal activity of phytosterols and methyl dehydroabietate of Norway spruce bark extracts. J. Biotechnol. 2018, 282, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Zacharof, M.-P. Grape winery waste as feedstock for bioconversions: Applying the biorefinery concept. Waste Biomass Valor. 2017, 8, 1011–1025. [Google Scholar] [CrossRef]
- Bourgignon, D.; European Parliament. European Parliamentary Research Service. Understanding Waste Streams: Treatment of Specific Waste. Briefing. 2015. Available online: https://www.europarl.europa.eu/thinktank/en/document.html?reference=EPRS_BRI(2015)564398 (accessed on 12 January 2021).
- Chupin, L.; Motillon, C.; Charrier-El Bouhtoury, F.; Pizzi, A.; Charrier, B. Characterisation of maritime pine (Pinus pinaster) bark tannins extracted under different conditions by spectroscopic methods, FTIR and HPLC. Ind. Crops Prod. 2013, 49, 897–903. [Google Scholar] [CrossRef]
- Vázquez, G.; Fontenla, E.; Santos, J.; Freire, M.S.; González-Álvarez, J.; Antorrena, G. Antioxidant activity and phenolic content of chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. I Ind. Crops Prod. 2008, 28, 279–285. [Google Scholar] [CrossRef]
- Aires, A.; Carvalho, R.; Saavedra, M.J. Valorization of solid wastes from chestnut industry processing: Extraction and optimization of polyphenols, tannins and ellagitannins and its potential for adhesives, cosmetic and pharmaceutical industry. J. Waste Manag. 2016, 48, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Legault, J.; Girard-Lalancette, K.; Dufour, D.; Pichette, A. Antioxidant potential of bark extracts from boreal forest conifers. Antioxidants 2013, 2, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Aspé, E.; Fernández, K. Comparison of phenolic extracts obtained of Pinus radiata bark from pulp and paper industry and sawmill industry. Maderas Cienc. Technol. 2011, 13, 243–252. [Google Scholar] [CrossRef]
- Jerez, M.; Selga, A.; Sineiro, J.; Torres, J.L.; Núñez, M.J. A comparison between bark extracts from Pinus pinaster and Pinus radiata: Antioxidant activity and procyanidin composition. Food Chem. 2007, 100, 439–444. [Google Scholar] [CrossRef]
- De Vasconcelos, M.C.; Bennett, R.N.; Rosa, E.A.; Ferreira-Cardoso, J.V. Composition of European chestnut (Castanea sativa Mill.) and association with health effects: Fresh and processed products. J. Sci. Food Agric. 2010, 90, 1578–1589. [Google Scholar] [CrossRef]
- Yokozawa, T.; Chen, C.P.; Dong, E.; Tanaka, T.; Nonaka, G.-I.; Nishioka, I. Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2-picrylhydrazyl radical. Biochem. Pharmacol. 1998, 56, 213–222. [Google Scholar] [CrossRef]
- Conde, E.; Díaz-Reinoso, B.; Moure, A.; Hemming, J.; Willför, S.M.; Domínguez, H.; Parajó, J.C. Extraction of phenolic and lipophilic compounds from pinus pinaster knots and stemwood by supercritical CO2. In Proceedings of the III Iberoamerican Conference on Supercritical Fluids—PROSCIBA, Cartagena de Indias, Colombia, 1–5 April 2013. [Google Scholar]
- Willför, S.; Hemming, J.; Reunanen, M.; Holmbom, B. Phenolic and lipophilic extractives in scots pine knots and stemwood. Holzforschung 2003, 57, 359–372. [Google Scholar] [CrossRef]
- Maimoona, A.; Naeem, I.; Saddiqe, Z.; Jameel, K. A review on biological, nutraceutical and clinical aspects of French maritime pine bark extract. J. Ethnopharmacol. 2011, 133, 261–277. [Google Scholar] [CrossRef]
- Jung, K.-H.; Yoo, S.-K.; Moon, S.-K.; Lee, U.-S. Furfural from pine needle extract inhibits the growth of a plant pathogenic fungus, Alternaria mali. Mycobiology 2007, 35, 39–43. [Google Scholar] [CrossRef]
- Salim, H.; Rimawi, W.H.; Shaheen, S.; Mjahed, A. Phytochemical analysis and antibacterial activity of extracts from Palestinian Aleppo pine seeds, bark and cones. Asian. J. Chem. 2019, 31, 143–147. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Genisheva, Z.; Botelho, C.; Santos, J.; Ramos, C.; Teixeira, J.A.; Rocha, C.M.R. Unravelling the biological potential of Pinus pinaster bark extracts. Antioxidants 2020, 9, 334. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, B.; Yun, K.W. Evaluation of antimicrobial activity and total phenolic content of three Pinus species. J. Ecol. Environ. 2013, 36, 57–63. [Google Scholar] [CrossRef]
- Montibus, M.; Pinson-Gadais, L.; Richard-Forget, F.; Barreau, C.; Ponts, N. Coupling of transcriptional response to oxidative stress and secondary metabolism regulation in filamentous fungi. Crit. Rev. Microbiol. 2015, 41, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, M.; Ricelli, A.; Zjalic, S.; Fabbri, A.A.; Fanelli, C. Natural functions of mycotoxins and control of their biosynthesis in fungi. Appl. Microbiol. Biotechnol. 2010, 87, 899–911. [Google Scholar] [CrossRef]
- Huang, Z.; Hashida, K.; Makino, R.; Kawamura, F.; Shimizu, K.; Kondo, R.; Ohara, S. Evaluation of biological activities of extracts from 22 African tropical wood species. J. Wood Sci. 2009, 55, 225–229. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant secondary metabolites in cereals: Potential involvement in resistance to Fusarium and mycotoxin accumulation. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Fitzgerald, D.J.; Stratford, M.; Gasson, M.J.; Narbad, A. Structure−function analysis of the vanillin molecule and its antifungal properties. J. Agric. Food Chem. 2005, 53, 1769–1775. [Google Scholar] [CrossRef]
- Bastos, M.; Lima, M.; Conserva, L.M.; Andrade, V.S.; Rocha, E.M.; Lemos, R.P. Studies on the antimicrobial activity and brine shrimp toxicity of Zeyheria tuberculosa (Vell.) Bur. (Bignoniaceae) extracts and their main constituents. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 16. [Google Scholar] [CrossRef]
- Koh, J.C.O.; Barbulescu, D.M.; Salisbury, P.A.; Slater, A.T. Pterostilbene is a potential candidate for control of blackleg in Canola. PLoS ONE 2016, 11, e0156186. [Google Scholar] [CrossRef]
- Gautier, C.; Pinson-Gadais, L.; Verdal-Bonnin, M.-N.; Ducos, C.; Tremblay, J.; Chéreau, S.; Atanasova, V.; Richard-Forget, F. Investigating the efficiency of hydroxycinnamic acids to inhibit the production of enniatins by Fusarium avenaceum and modulate the expression of enniatins biosynthetic genes. Toxins 2020, 12, 735. [Google Scholar] [CrossRef]
- Boonmee, S.; Atanasova, V.; Chéreau, S.; Marchegay, G.; Hyde, K.D.; Richard-Forget, F. Efficiency of hydroxycinnamic phenolic acids to inhibit the production of ochratoxin A by Aspergillus westerdijkiae and Penicillium verrucosum. Int. J. Mol. Sci. 2020, 21, 8548. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Yang, S.; Cheng, Y.J.; Chen, F.; Pan, S.; Fan, G. Antifungal activity and action mode of pinocembrin from propolis against Penicillium italicum. Food Sci. Biotechnol. 2012, 21, 1533–1539. [Google Scholar] [CrossRef]
- Lee, H.; Woo, E.-R.; Lee, D.G. (-)-Nortrachelogenin from Partrinia scabiosaefolia elicits apoptotic response in Candida albicans. FEMS Yeast Res. 2016, fow013. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, S.; Larkov, O.; Lamdan, N.-L.; Goldshmidt-Tran, O.; Horwitz, B.A. Plant phenolic acids induce programmed cell death of a fungal pathogen: MAPK signaling and survival of Cochliobolus heterostrophus. Environ. Microbiol. 2016, 18, 4188–4199. [Google Scholar] [CrossRef] [PubMed]
- Gabaston, J.; Richard, T.; Cluzet, S.; Palos Pinto, A.; Dufour, M.-C.; Corio-Costet, M.-F.; Mérillon, J.-M. Pinus pinaster knot: A source of polyphenols against Plasmopara viticola. J. Agric. Food Chem. 2017, 65, 8884–8891. [Google Scholar] [CrossRef]
- Cooney, J.M.; Lauren, D.R.; di Menna, M.E. Impact of competitive fungi on trichothecene production by Fusarium graminearum. J. Agric. Food Chem. 2001, 49, 522–526. [Google Scholar] [CrossRef]
- Yammine, S.; Delsart, C.; Ghidossi, R.; Vitrac, X.; Mietton Peuchot, M.; Ghidossi, R. Characterisation of polyphenols and antioxidant potential of red and white pomace by-product extracts using subcritical water extraction. OENO One 2020, 54. [Google Scholar] [CrossRef]
- Gabaston, J.; Leborgne, C.; Waffo-Téguo, P.; Pedrot, E.; Richard, T.; Mérillon, J.-M.; Valls Fonayet, J. Separation and isolation of major polyphenols from maritime pine (Pinus pinaster) knots by two-step centrifugal partition chromatography monitored by LC-MS and NMR spectroscopy. J. Sep. Sci. 2020, 43, 1080–1088. [Google Scholar] [CrossRef]




| Plant Species | Natural Extract | Extraction Yield (%) 1 | Concentration (g L−1) 2 | Total Phenolic Content (mg g−1) |
|---|---|---|---|---|
| Forest and Wood By-Products | ||||
| Pinus pinaster | Maritime pine barks 175 °C | 5.0 | 10.0 | 334.8 ± 4.7 |
| Maritime pine barks 125 °C | 4.7 | 9.5 | 346.6 ± 6.2 | |
| Maritime pine sawdust 175 °C | 2.3 | 4.6 | 121.3 ± 17.5 | |
| Maritime pine sawdust 125 °C | 1.8 | 3.7 | 65.8 ± 0.3 | |
| Maritime pine needles 175 °C | 7.9 | 15.9 | 127.8 ± 3.2 | |
| Maritime pine needles 125 °C | 7.3 | 14.6 | 96.7 ± 2.4 | |
| Quercus robur | Oak chips 175 °C | 2.5 | 5.1 | 351.1 ± 11.1 |
| Oak chips 125 °C | 2.5 | 5.1 | 277.9 ± 5.7 | |
| Castanea sativa | Chestnut chips 175 °C | 2.6 | 5.2 | 533.1 ± 7.4 |
| Chestnut chips 125 °C | 2.1 | 4.2 | 551.6 ± 27.4 | |
| Robinia pseudoacacia | Locust chips 175 °C | 1.6 | 3.1 | 340.5 ± 4.2 |
| Locust chips 125 °C | 1.1 | 2.1 | 320.6 ± 4.8 | |
| Vine By-Products | ||||
| Vitis vinifera | Vine canes 175 °C | 5.3 | 10.5 | 94.2 ± 1.7 |
| Vine canes 125 °C | 5.7 | 11.3 | 91.7 ± 3.7 | |
| Vine roots 175 °C | 7.1 | 14.1 | 120.9 ± 2.7 | |
| Vine roots 125 °C | 1.9 | 3.8 | 65.6 ± 1.7 | |
| Compounds | Retention Time (min) | λmax (nm) | (M − H)− | Concentration (mg g−1 of Extract) |
|---|---|---|---|---|
| Phenolic Acids/Aldehydes/Alcohols | ||||
| Protocatechuic acid | 1.8 | 260 | 153 | 0.25 (± 0.01) |
| Vanillic acid | 3.7 | 262 | 167 | 0.53 (± 0.06) |
| Caffeic acid | 3.8 | 325 | 179 | 0.15 (± 0.01) |
| Coniferyl alcohol | 4.6 | 264 | 179 | 4.89 (± 0.22) |
| Vanillin | 4.7 | 280 | 151 | 0.64 (± 0.01) |
| Ferulic acid | 5.1 | 325 | 193 | 0.35 (± 0.01) |
| Coniferyl aldehyde | 5.9 | 340 | 177 | 0.83 (± 0.01) |
| Unknown 1 | 10.6 | 253 | 329 | 1.90 (± 0.06) |
| Lignans | ||||
| Nortrachelogenin | 6.9 | 281 | 373 | 7.30 (± 0.25) |
| Pinoresinol 2 | 7.7 | 281 | 357 | 2.84 (± 0.29) |
| Flavonoids | ||||
| Pinobanksin 3 | 9.2 | 289 | 271 | 1.01 (± 0.01) |
| Pinocembrin | 11.8 | 289 | 255 | 0.41 (± 0.02) |
| Total | 21.09 (± 0.40) | |||
| Strain | Source | Host | Country | Chemotype |
|---|---|---|---|---|
| Fg 605 | INRAE MycSA collection, France 1 | Unknown | Unknown | DON/15ADON |
| Fg 156 | INRAE MycSA collection, France 1 | Wheat | France | DON/15ADON |
| Fg 164 | INRAE MycSA collection, France 1 | Wheat | France | DON/15ADON |
| Fg 91 | INRAE MycSA collection, France 1 | Corn | France | NIV/FX |
| 34W23.4F9 | AgResearch, Hamilton, New Zealand [48] | Unknown | New Zealand | DON/15ADON |
| PH-1 | Fungal Genetic Stock Center, USA | Corn | USA | DON/15ADON |
| CBS 185.32 | Westerdijk Fungal Biodiversity Institute, The Netherlands 2 | Corn | Unknown | DON/15ADON |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montibus, M.; Vitrac, X.; Coma, V.; Loron, A.; Pinson-Gadais, L.; Ferrer, N.; Verdal-Bonnin, M.-N.; Gabaston, J.; Waffo-Téguo, P.; Richard-Forget, F.; et al. Screening of Wood/Forest and Vine By-Products as Sources of New Drugs for Sustainable Strategies to Control Fusarium graminearum and the Production of Mycotoxins. Molecules 2021, 26, 405. https://doi.org/10.3390/molecules26020405
Montibus M, Vitrac X, Coma V, Loron A, Pinson-Gadais L, Ferrer N, Verdal-Bonnin M-N, Gabaston J, Waffo-Téguo P, Richard-Forget F, et al. Screening of Wood/Forest and Vine By-Products as Sources of New Drugs for Sustainable Strategies to Control Fusarium graminearum and the Production of Mycotoxins. Molecules. 2021; 26(2):405. https://doi.org/10.3390/molecules26020405
Chicago/Turabian StyleMontibus, Mathilde, Xavier Vitrac, Véronique Coma, Anne Loron, Laetitia Pinson-Gadais, Nathalie Ferrer, Marie-Noëlle Verdal-Bonnin, Julien Gabaston, Pierre Waffo-Téguo, Florence Richard-Forget, and et al. 2021. "Screening of Wood/Forest and Vine By-Products as Sources of New Drugs for Sustainable Strategies to Control Fusarium graminearum and the Production of Mycotoxins" Molecules 26, no. 2: 405. https://doi.org/10.3390/molecules26020405
APA StyleMontibus, M., Vitrac, X., Coma, V., Loron, A., Pinson-Gadais, L., Ferrer, N., Verdal-Bonnin, M.-N., Gabaston, J., Waffo-Téguo, P., Richard-Forget, F., & Atanasova, V. (2021). Screening of Wood/Forest and Vine By-Products as Sources of New Drugs for Sustainable Strategies to Control Fusarium graminearum and the Production of Mycotoxins. Molecules, 26(2), 405. https://doi.org/10.3390/molecules26020405





