New Anti-Leukemic Effect of Carvacrol and Thymol Combination through Synergistic Induction of Different Cell Death Pathways
Abstract
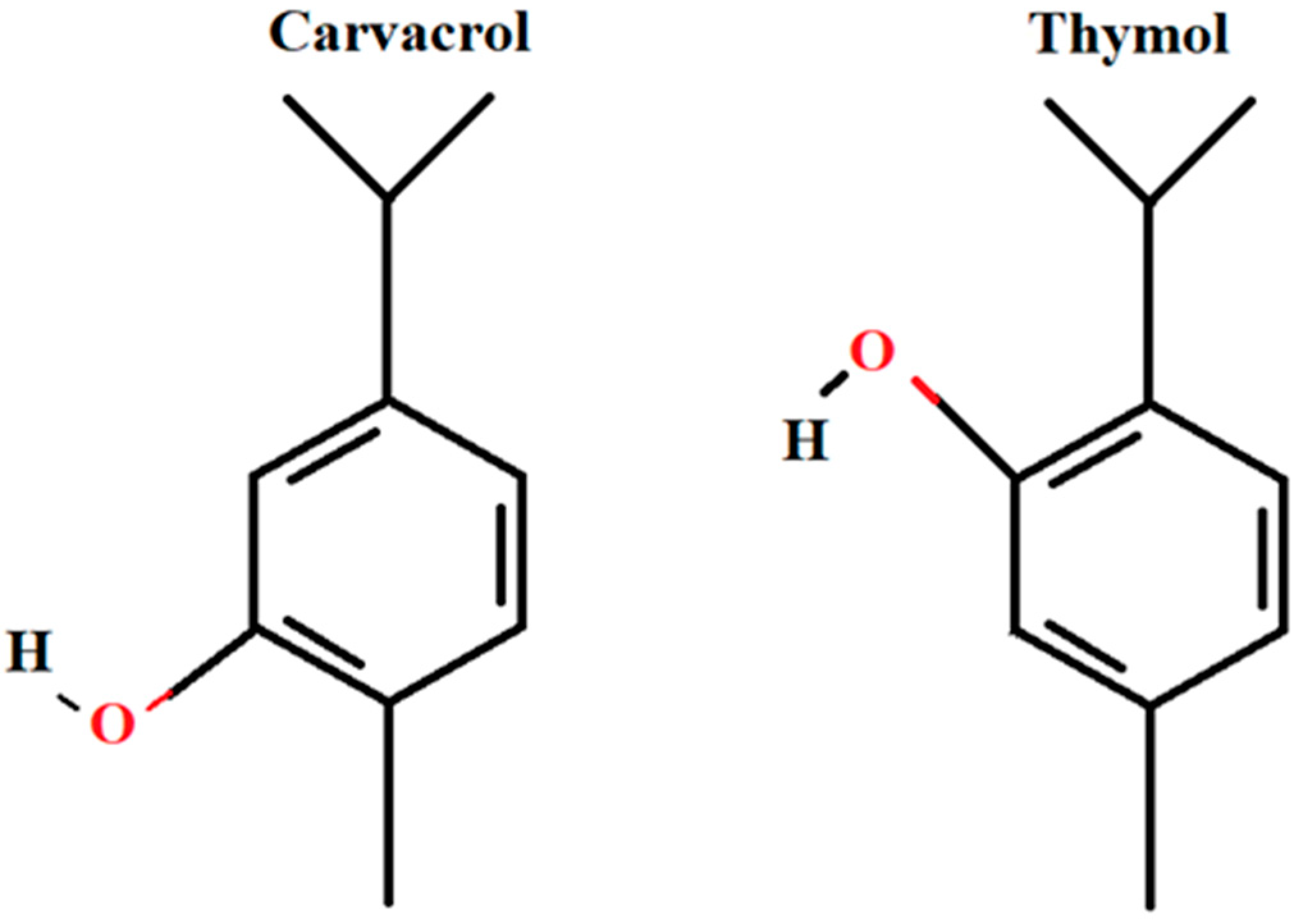
:1. Introduction
2. Results
2.1. Cytotoxic Effects of Ptychotis Verticillata (PV) Extract Oil on Myeloid Leukemic Cell Lines
2.2. Potential Synergistic Cell Death Induction by Thymol and Carvacrol on Myeloïd Leukemic Cell Lines
2.3. Carvacrol- and Thymol Combination-Induced Cell Death Is Associated with Apoptotic, Oxidative, and Cell Stress Pathways (Gene and Protein Levels)
2.3.1. Upregulation of Proapoptotic Gene Expression
2.3.2. Differential Modulation of the Oxidative and Stress Gene Pathways
2.3.3. Protein Confirmation of Apoptotic and Reticular Stress Signaling Pathway Implication
2.4. Carvacrol- and Thymol Combination-Induced Potential Synergistic Cell Death Is Associated with Nonapoptotic Pathways
2.4.1. Dependence on Caspases Activation and ROS Generation Pathways
2.4.2. Implication of Autophagy and Necrosis Pathways
3. Discussion
4. Materials and Methods
4.1. Plant Material and Essential Oil Isolation
4.2. Chemicals Reagents
4.3. Cell Lines, Culture Conditions, and Drug Treatment
4.4. Trypan Blue Assay
4.5. Cell Viability Assay
4.6. Cell Death Assay
4.7. RNA Isolation and Q-PCR Analysis
4.8. Protein Extraction and Immunoblotting
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| Allo-SCT | Allogeneic stem cell transplantation |
| AML | Acute myeloid leukemia |
| APC-annexin | Allophycocyanin annexin V |
| BCL-2 | B-cell lymphoma 2 |
| Car | Carvacrol |
| Casp-3 | Caspase3 |
| Casp-9 | Caspase 9 |
| Chop | C/EBP-homologous protein |
| DCFH-DA | Dichlorofluorescin diacetate |
| DMSO | Dimethylsulfoxide |
| DNA | Deoxyribonucleic acid |
| EO | Essential oil |
| ER | Endoplasmic reticulum |
| FBS | Fetal bovine serum |
| FLT3 | FMS-like tyrosine kinase 3 |
| HBSS | Hank’s Buffered Saline Solution |
| IGFR1 | Insulin growth factor 1 receptor |
| MSC | Mesenchymal stromal cells |
| mToR | mammalian target of rapamycin |
| NAC | N-acetyl-l-cysteine |
| PBMC | Peripheral blood mononuclear cells |
| PBS | Phosphate-buffered saline |
| PI | Propidium iodide |
| PI3k | Phosphatidylinositol 3-kinase |
| PS | Phosphatidylserine |
| PV | Ptychotis verticillata |
| ROS | Reactive oxygen species |
| Thy | Thymol |
| UPR | Unfolded protein response |
| 7-AAD | 7-aminoactinomycine D |
References
- Ortiz, L.M.G.; Orellana, M.I.R.; Ratovitski, E.A. Natural Compounds as Modulators of Non-apoptotic Cell Death in Cancer Cells. Curr. Genom. 2017, 18, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Estey, E. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am. J. Hematol. 2018, 93, 1267–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellakhdar, J. La Pharmacopée Marocaine Traditionnelle. Médecine Arabe Ancienne et Savoirs Populaires; Ibis Press: Paris, France, 1997. [Google Scholar]
- Bnouham, M.; Merhfour, F.Z.; Ziyyat, A.; Aziz, M.; Legssyer, A.; Mekhfi, H. Antidiabetic effect of some medicinal plants of Oriental Morocco in neonatal non-insulin-dependent diabetes mellitus rats. Hum. Exp. Toxicol. 2010, 29, 865–871. [Google Scholar] [CrossRef] [PubMed]
- El Ouariachi, E.M.; Tomi, P.; Bouyanzer, A.; Hammouti, B.; Desjobert, J.-M.; Costa, J.; Paolini, J. Chemical composition and antioxidant activity of essential oils and solvent extracts of Ptychotis verticillata from Morocco. Food Chem. Toxicol. 2011, 49, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Bouhtit, F.; Najar, M.; Agha, D.M.; Melki, R.; Najimi, M.; Sadki, K.; Lewalle, P.; Hamal, A.; Lagneaux, L.; Merimi, M. The biological response of mesenchymal stromal cells to thymol and carvacrol in comparison to their essential oil: An innovative new study. Food Chem. Toxicol. 2019, 134, 110844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Greenwald, D.R.; Ruoslahti, E. Coadministration of a Tumor-Penetrating Peptide Enhances the Efficacy of Cancer Drugs. Science 2010, 328, 1031–1035. [Google Scholar] [CrossRef] [Green Version]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Crystal, A.S.; Shaw, A.T.; Sequist, L.V.; Friboulet, L.; Niederst, M.J.; Lockerman, E.L.; Frias, R.L.; Gainor, J.F.; Amzallag, A.; Greninger, P.; et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science 2014, 346, 1480–1486. [Google Scholar] [CrossRef] [Green Version]
- Meeran, M.F.N.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Maria, D.M.C.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Kang, D.; Joo, Y.; Lee, J.; Oh, G.-H.; Choi, S.; Ko, S.; Je, S.; Choi, H.J.; Song, J.J. TGF-β downregulation-induced cancer cell death is finely regulated by the SAPK signaling cascade. Exp. Mol. Med. 2018, 50, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorea, J.; Prasad, M.; Cohen, L.; Li, N.; Schefzik, R.; Ghosh, S.; Rotblat, B.; Brors, B.; Elkabets, M. IGF1R upregulation confers resistance to isoform-specific inhibitors of PI3K in PIK3CA-driven ovarian cancer. Cell Death Dis. 2018, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Saif, A.; Kazmi, S.F.A.; Naseem, R.; Shah, H.; Butt, M.O. Acute Myeloid Leukemia: Is That All There Is? Cureus 2018, 10, 3198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bnouham, M.; Benalla, W.; Asehraou, A.; Berrabah, M. Antibacterial activity of essential oil from Ptychotis verticillata. Spatula DD 2012, 2, 69–73. [Google Scholar] [CrossRef]
- Najar, M.; Bouhtit, F.; Melki, R.; Afif, H.; Hamal, A.; Fahmi, H.; Merimi, M.; Lagneaux, L. Mesenchymal Stromal Cell-Based Therapy: New Perspectives and Challenges. J. Clin. Med. 2019, 8, 626. [Google Scholar] [CrossRef] [Green Version]
- Bhakkiyalakshmi, E.; Suganya, N.; Sireesh, D.; Krishnamurthi, K.; Devi, S.S.; Rajaguru, P.; Ramkumar, K.M. Carvacrol induces mitochondria-mediated apoptosis in HL-60 promyelocytic and Jurkat T lymphoma cells. Eur. J. Pharmacol. 2016, 772, 92–98. [Google Scholar] [CrossRef]
- Pathania, A.S.; Guru, S.K.; Verma, M.; Sharma, C.; Abdullah, S.T.; Malik, F.; Chandra, S.; Katoch, M.; Bhushan, S. Disruption of the PI3K/AKT/mTOR signaling cascade and induction of apoptosis in HL-60 cells by an essential oil from Monarda citriodora. Food Chem. Toxicol. 2013, 62, 246–254. [Google Scholar] [CrossRef]
- Handschuh, L. Not Only Mutations Matter: Molecular Picture of Acute Myeloid Leukemia Emerging from Transcriptome Studies. J. Oncol. 2019, 2019, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Kleinsimon, S.; Longmuss, E.; Rolff, J.; Jäger, S.; Eggert, A.; Delebinski, C.; Seifert, G. GADD45A and CDKN1A are involved in apoptosis and cell cycle modulatory effects of viscumTT with further inactivation of the STAT3 pathway. Sci. Rep. 2018, 8, 5750. [Google Scholar] [CrossRef] [Green Version]
- Snezhkina, A.V.; Kudryavtseva, A.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxidative Med. Cell. Longev. 2019, 2019, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, H.; Shi, H.; Du, Y.; Yu, J.; Gu, S.; Chen, X.; Liu, K.J.; Hu, C.-A.A. PUMA Overexpression Induces Reactive Oxygen Species Generation and Proteasome-Mediated Stathmin Degradation in Colorectal Cancer Cells. Cancer Res. 2005, 65, 1647–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhang, S.; Zhang, M.; Sun, B. Novel approach for extraction of grape skin antioxidants by accelerated solvent extraction: Box–Behnken design optimization. J. Food Sci. Technol. 2019, 56, 4879–4890. [Google Scholar] [CrossRef] [PubMed]
- Krstić, J.; Trivanović, D.; Mojsilović, S.; Santibanez, J.F. Transforming Growth Factor-Beta and Oxidative Stress Interplay: Implications in Tumorigenesis and Cancer Progression. Oxidative Med. Cell. Longev. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wang, F.; Zhao, Z.; Zhao, X.; Qiu, J.; Nie, C.; Wei, Y. BIM-Mediated AKT Phosphorylation Is a Key Modulator of Arsenic Trioxide-Induced Apoptosis in Cisplatin-Sensitive and -Resistant Ovarian Cancer Cells. PLoS ONE 2011, 6, e20586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeeshan, H.M.A.; Lee, G.H.; Kim, H.-R.; Chae, H.-J. Endoplasmic Reticulum Stress and Associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.; Wang, S.; Ren, B.; Wang, J.; Chen, J.; Lu, J.; Zhan, S.; Fu, Y.; Huang, L.; Tan, J. CHOP favors endoplasmic reticulum stress-induced apoptosis in hepatocellular carcinoma cells via inhibition of autophagy. PLoS ONE 2017, 12, e0183680. [Google Scholar] [CrossRef] [Green Version]
- Iurlaro, R.; Muñoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Nakano, H.; Shinohara, K. Role of wild-type p53 in apoptotic and non-apoptotic cell death induced by X-irradiation and heat treatment in p53-mutated mouse M10 cells. J. Radiat. Res. 2010, 51, 665–673. [Google Scholar] [CrossRef] [Green Version]
- Ranjan, A.; Iwakuma, T. Non-Canonical Cell Death Induced by p53. Int. J. Mol. Sci. 2016, 17, 2068. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, H.; Miyazaki, S.; Matsuyama, S.; Takeda, M.; Kawano, M.; Nakagawa, H.; Nishimura, K.; Matsuo, S. Selection of autophagy or apoptosis in cells exposed to ER-stress depends on ATF4 expression pattern with or without CHOP expression. Biol. Open 2013, 2, 1084–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgopoulos, N.T.; Steele, L.P.; Thomson, M.J.; Selby, P.J.; Southgate, J.; Trejdosiewicz, L.K. A novel mechanism of CD40-induced apoptosis of carcinoma cells involving TRAF3 and JNK/AP-1 activation. Cell Death Differ. 2006, 13, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Klausen, C.; Cheng, J.-C.; Leung, P.C.K. CD40 ligand induces RIP1-dependent, necroptosis-like cell death in low-grade serous but not serous borderline ovarian tumor cells. Cell Death Dis. 2015, 6, e1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tait, S.W.; Ichim, G.; Green, D.R. Die another way—Non-apoptotic mechanisms of cell death. J. Cell Sci. 2014, 127, 2135–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boufker, H.I.; Lagneaux, L.; Fayyad-Kazan, H.; Badran, B.; Najar, M.; Wiedig, M.; Ghanem, G.E.; Laurent, G.; Body, J.-J.; Journé, F. Role of farnesoid X receptor (FXR) in the process of differentiation of bone marrow stromal cells into osteoblasts. Bone 2011, 49, 1219–1231. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Boskabady, M.H. The effect of carvacrol on inflammatory mediators and respiratory symptoms in veterans exposed to sulfur mustard, a randomized, placebo-controlled trial. Respir. Med. 2019, 150, 21–29. [Google Scholar] [CrossRef]
- Alavinezhad, A.; Khazdair, M.R.; Boskabady, M.H. Possible therapeutic effect of carvacrol on asthmatic patients: A randomized, double blind, placebo-controlled, Phase II clinical trial. Phytotherapy Res. 2018, 32, 151–159. [Google Scholar] [CrossRef]
- Wei, H.-K.; Xue, H.-X.; Zhou, Z.X.; Peng, J. A carvacrol–thymol blend decreased intestinal oxidative stress and influenced selected microbes without changing the messenger RNA levels of tight junction proteins in jejunal mucosa of weaning piglets. Animal 2017, 11, 193–201. [Google Scholar] [CrossRef] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouhtit, F.; Najar, M.; Moussa Agha, D.; Melki, R.; Najimi, M.; Sadki, K.; Boukhatem, N.; Bron, D.; Meuleman, N.; Hamal, A.; et al. New Anti-Leukemic Effect of Carvacrol and Thymol Combination through Synergistic Induction of Different Cell Death Pathways. Molecules 2021, 26, 410. https://doi.org/10.3390/molecules26020410
Bouhtit F, Najar M, Moussa Agha D, Melki R, Najimi M, Sadki K, Boukhatem N, Bron D, Meuleman N, Hamal A, et al. New Anti-Leukemic Effect of Carvacrol and Thymol Combination through Synergistic Induction of Different Cell Death Pathways. Molecules. 2021; 26(2):410. https://doi.org/10.3390/molecules26020410
Chicago/Turabian StyleBouhtit, Fatima, Mehdi Najar, Douâa Moussa Agha, Rahma Melki, Mustapha Najimi, Khalid Sadki, Noureddine Boukhatem, Dominique Bron, Nathalie Meuleman, Abdellah Hamal, and et al. 2021. "New Anti-Leukemic Effect of Carvacrol and Thymol Combination through Synergistic Induction of Different Cell Death Pathways" Molecules 26, no. 2: 410. https://doi.org/10.3390/molecules26020410
APA StyleBouhtit, F., Najar, M., Moussa Agha, D., Melki, R., Najimi, M., Sadki, K., Boukhatem, N., Bron, D., Meuleman, N., Hamal, A., Lagneaux, L., Lewalle, P., & Merimi, M. (2021). New Anti-Leukemic Effect of Carvacrol and Thymol Combination through Synergistic Induction of Different Cell Death Pathways. Molecules, 26(2), 410. https://doi.org/10.3390/molecules26020410







