Coumarin Derivatives in Inflammatory Bowel Disease
Abstract
:1. Introduction
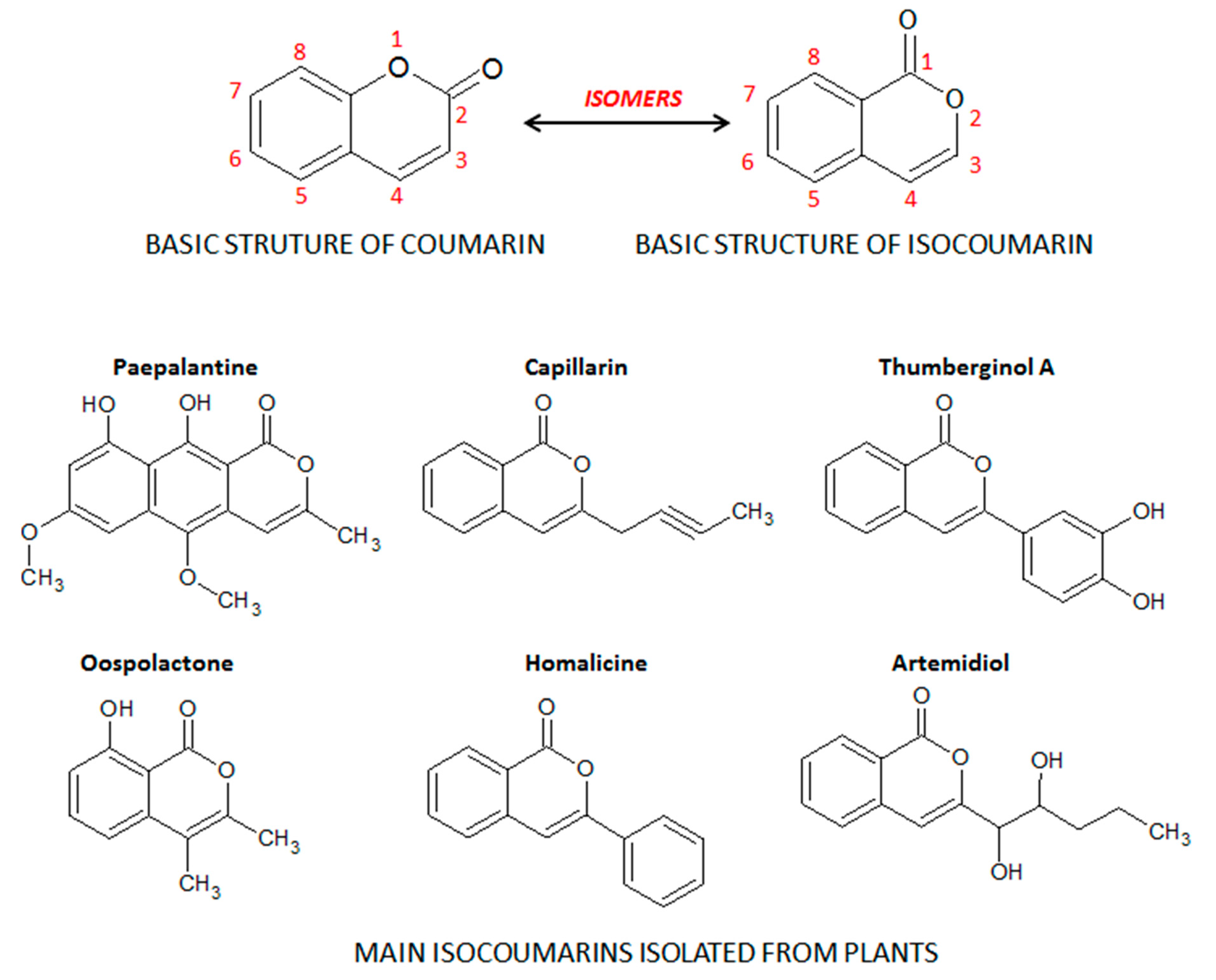
2. Coumarin and the Main Coumarin Derivatives
3. Inflammatory Bowel Diseases: General Aspects
4. Intestinal Anti-Inflammatory Activity of Coumarin Derivatives
4.1. Effects of Coumarin Derivatives on Oxidative Stress
4.1.1. The Isocoumarin Paepalantine
4.1.2. Coumarin and 4-hydroxycoumarin
4.1.3. Esculetin (6,7-dihydroxycoumarin) and 4-methyl Esculetin
4.1.4. Daphnetin (7,8-dihydroxycoumarin)
4.1.5. Esculin (7-hydroxy-6-O-glucosylcoumarin)
4.1.6. Other Simple Antioxidant Coumarin Derivatives
4.2. Effects of Coumarin Derivatives on Aarachidonic Acid Metabolism
4.3. Effects of Coumarin Derivatives on the Immune Response
4.4. Effects of Coumarin Derivatives on the Nuclear Signaling Pathways
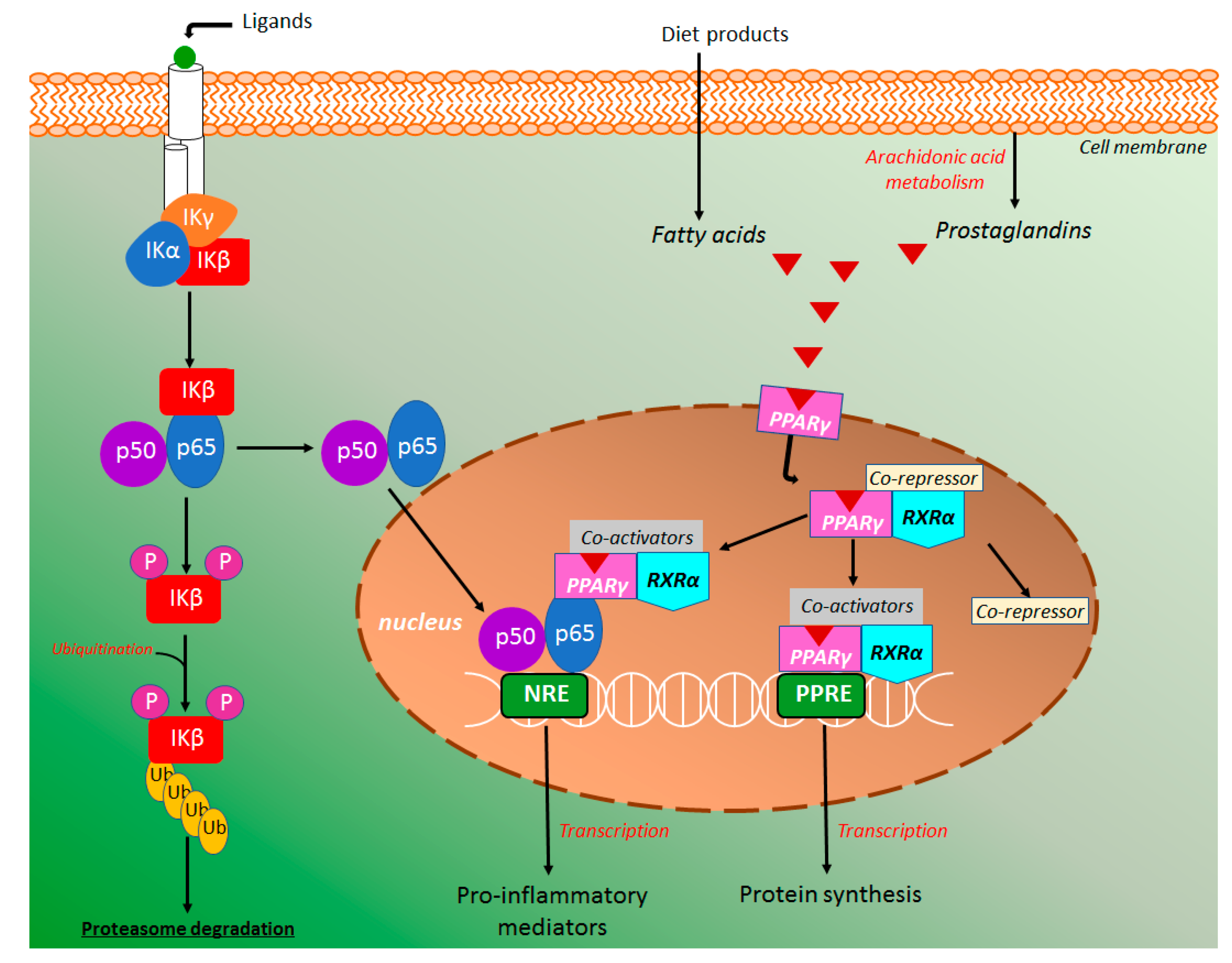
4.4.1. NF-κB and PPAR-γ Signaling Pathways
4.4.2. MAPK Signaling Pathway
4.4.3. HIF-1α Signaling Pathway
4.4.4. The Pregnane X Signaling Pathway
4.5. Effects of Coumarin Derivatives Intestinal Microbiota
5. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- GDB 2017 Burden Disease Collaborators. Global, regional, and national age-sex specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [Green Version]
- Jarmakiewicz-Czaja, S.; Pia, D.; Filip, R. The influence of nutrients on Inflammatory Bowel Diseases. J. Nutr. Metab. 2020, 2020, 2894169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vezza, T.; Rodríguez-Nogales, A.; Algieri, F.; Utrilla, M.P.; Rodriguez-Cabezaz, M.H.; Gávez, J. Flavonoids in Inflammatory Bowel Disease: A review. Nutrients 2016, 8, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoensch, H.P.; Weigmann, B. Regulation of the intestinal immune system by flavonoids and its utility in chronic inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 877–881. [Google Scholar] [CrossRef] [PubMed]
- González-Quilen, C.; Rodríguez-Gallego, E.; Beltrán-Debón, R.; Pinent, M.; Ardévol, A.; Blay, M.T.; Terra, X. Health-promoting properties of proanthocyanidins for intestinal dysfunction. Nutrients 2020, 12, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Wu, B.; Fu, W.; Reddivari, L. The anti-inflammatory effects of dietary anthocyanins against ulcerative colitis. Int. J. Mol. Sci. 2019, 20, 2588. [Google Scholar] [CrossRef] [Green Version]
- Fan, F.; Sang, L.; Jiang, M. Catechins and their therapeutic benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [Green Version]
- Hoult, J.R.S.; Paya, M. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmacol. Vasc. Syst. 1996, 27, 713–722. [Google Scholar] [CrossRef]
- Lake, B.G. Coumarin metabolism, toxicity and carcinogenicity: Relevance for human risk assessment. Food Chem. Toxicol. 1999, 37, 423–453. [Google Scholar] [CrossRef]
- Jain, P.K.; Joshi, H. Coumarin: Chemical and pharmacological profile. J. Appl. Pharm. Sci. 2012, 2, 236–240. [Google Scholar]
- Akkol, E.K.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and coumarin-related compounds in pharmacotherapy of Cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef] [PubMed]
- Wardrop, D.; Keeling, D. The story of the discovery of heparin and warfarin. Br. J. Haematol. 2008, 141, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.B. Warfarin: From rat poison to clinical use. Nat. Rev. Cardiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Pariente, B.; Mould, D.R.; Schreiber, S.; Petersson, J.; Hommes, D. New tools and approaches for improved management of inflammatory bowel diseases. J. Crohn’s Colitis 2014, 8, 1246–1253. [Google Scholar] [CrossRef] [Green Version]
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Ghouri, Y.A.; Tahan, V.; Shen, B. Secondary causes of inflammatory bowel diseases. World J. Gastroenterol. 2020, 26, 3998–4017. [Google Scholar] [CrossRef]
- Di Stasi, L.C.; Costa, C.A.R.A.; Witaicenis, A. Products for the treatment of inflammatory bowel disease: A patent review (2013–2014). Expert Opin. Ther. Pat. 2015, 25, 629–642. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Annese, V. Genetics and epigenetics of IBD. Pharmacol. Res. 2020, 159, 104892. [Google Scholar] [CrossRef]
- Ellinghaus, D.; Bethune, J.; Petersen, B.; Frankle, A. The genetics of Crohn’s disease and ulcerative colitis—Status quo and beyond. Scand. J. Gastroenterol. 2015, 50, 13–23. [Google Scholar] [CrossRef] [PubMed]
- de Souza, S.P.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.C.; Madsen, K.; Doyle, J.; Meddings, J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut 2009, 58, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasano, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, G.P.; Dughera, F.; Ribaldone, D.G.; Rosso, C.; Abate, M.L.; Pellicano, R.; Bresso, F.; Smedile, A.; Saracco, G.M.; Astegiano, M. Serum zonulin in patients with infammatory bowel disease: A pilot study. Min. Med. 2019, 110, 95–100. [Google Scholar]
- Rezaie, A.; Parker, R.D.; Abdollahi, M. Oxidative stress and pathogenesis of inflammatory bowel disease: An epiphenomenon or the cause? Dig. Dis. Sci. 2007, 52, 2015–2021. [Google Scholar] [CrossRef]
- Moura, F.A.; Andrade, K.Q.; Santos, J.C.F.; Araújo, O.R.P.; Goulart, M.O.F. Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biol. 2015, 6, 617–639. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E. Study of antioxidant properties of agents from perspective of their action mechanism. Molecules 2020, 25, 4251. [Google Scholar] [CrossRef]
- Opara, E.C. Oxidative stress. Dis. Mon. 2006, 52, 183–198. [Google Scholar] [CrossRef]
- Chami, B.; Martin, N.J.J.; Dennis, J.M.; Witting, P.K. Myeloperoxidase in the inflamed colon: A novel target for treating inflammatory bowel disease. Arch. Biochem. Biophys. 2018, 645, 61–71. [Google Scholar] [CrossRef]
- Dedon, P.C.; Tannenbaum, S.R. Reactive nitrogen species in the chemical biology of inflammation. Arch. Biochem. Biophys. 2004, 423, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, A.; Witaicenis, A.; Caruso, I.P.; Piva, H.M.R.; Araujo, G.C.; Moraes, F.R.; Fossey, M.C.; Cornélio, M.L.; Souza, F.P.; Di Stasi, L.C. 4-methylesculetin, a natural coumarin with intestinal anti-inflammatory activity, elicits a glutathione antioxidant response by different mechanisms. Chem. Biol. Int. 2020, 315, 108876. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Leong, P.K.; Ko, K.M. Induction of the glutathione antioxidant response/glutathione redox cycling by nutraceuticals: Mechanism of protection against oxidant-induced cell death. J. Nutrac. Food Sci. 2016, 1, 1–8. [Google Scholar]
- Nandi, A.; Yan, L.; Jana, C.K.; Das, N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [Green Version]
- Arthur, J.R. The glutathione peroxidases. Cell. Mol. Life Sci. 2000, 57, 1825–1835. [Google Scholar] [CrossRef]
- Chen, B.; Deen, W.M. Analysis of the effects of cell spacing and liquid depth on nitric oxide and its oxidation products in cell cultures. Chem. Res. Toxicol. 2001, 14, 135–147. [Google Scholar] [CrossRef]
- Loria, V.; Dato, I.; Graziani, F.; Biasucci, L.M. Myeloperoxidase: A new biomarker in ischemic heart disease and acute coronary syndromes. Mediat. Inflamm. 2008, 2008, 135625. [Google Scholar] [CrossRef] [Green Version]
- Karakas, M.; Koening, W. Myeloperoxidase production by macrophage and risk of atherosclerosis. Curr. Atheroscler. Rep. 2012, 14, 277–283. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Su, Z.; Kong, A.T. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef]
- Iranshahy, M.; Iranshahi, M.; Abtahi, S.R.; Karimi, G. The role of nuclear factor erythroid 2-related factor 2 in hepatoprotective activity of natural products: A review. Food Chem. Toxicol. 2018, 120, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Suzuki, T.; Kobayashi, A.; Wakabayashi, J.; Mahler, J.; Motohashi, H.; Yamamoto, M. Physiological significance of reactive cysteine residues of keap1 in determing Nrf2 activity. Mol. Cell. Biol. 2008, 28, 2758–2770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Stasi, L.C.; Camuesco, D.; Nieto, A.; Vilegas, W.; Zarzuelo, A.; Gálvez, J. Intestinal anti-inflammatory activity of paepalantine, an isocumarin isolated from the capitula of Paepalanthus bromelioides, in the trinitrobenzene sulphonic acid model of rat colitis. Planta Med. 2004, 70, 315–320. [Google Scholar]
- Vilegas, W.; Roque, N.F.; Salatino, A.; Giesbrecht, A.M.; Davino, S. Isocoumarin from Paepalanthus bromelioides. Phytochemistry 1990, 29, 2299–2301. [Google Scholar] [CrossRef]
- Kitagawa, R.R.; Raddi, M.S.G.; Khalil, N.M.; Vilegas, W.; Fonseca, L.M. Effects of the isocoumarin paepalantine on the luminol and lucigenin amplified chemiluminescence of rat neutrophils. Biol. Pharm. Bull. 2003, 26, 905–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cálgaro-Helena, A.F.; Devienne, K.F.; Rodrigues, T.; Dorta, D.J.; Raddi, M.S.G.; Vilegas, W.; Uyemura, S.A.; Santos, A.C.; Curti, C. Effects of isocoumarins isolated from Paepalanthus bromelioides on mitochondria: Uncoupling, and induction/inhibition of mitochondrial permeability transition. Chem. Biol. Interact. 2006, 161, 155–164. [Google Scholar] [CrossRef]
- Devienne, K.F.; Cálgaro-Helena, A.F.; Dorta, D.J.; Prado, I.M.R.; Raddi, M.S.G.; Vilegas, W.; Uyemura, S.A.; Santos, A.C.; Curti, C. Antioxidant activity of isocoumarins isolated from Paepalanthus bromelioides on mitochondria. Phytochemistry 2007, 68, 1075–1080. [Google Scholar] [CrossRef]
- Ardisson, J.S.; Gonçalves, R.C.R.; Rodrigues, R.P.; Kitagawa, R.R. Antitumour, immunomodulatory activity and in silico studies of naphthopyranones targeting iNOS, a relevant target for the treatment of helicobacter pylori infection. Biomed. Pharmacother. 2018, 107, 1160–1165. [Google Scholar] [CrossRef]
- Luchini, A.C.; Orsi, P.R.; Cestari, S.H.; Seito, L.N.; Witaicenis, A.; Pellizzon, C.H.; Di Stasi, L.C. Intestinal anti-inflammatory activity of coumarin and 4-hydroxycoumarin in the trinitrobenzene sulphonic acid model of rat colitis. Biol. Pharm. Bull. 2008, 31, 1343–1350. [Google Scholar] [CrossRef] [Green Version]
- Witaicenis, A.; Seito, L.N.; Di Stasi, L.C. Intestinal anti-inflammatory activity of esculetin and 4-methylesculetin in the trinitrobenzene sulphonic acid model of rat colitis. Chem. Biol. Interact. 2010, 186, 211–218. [Google Scholar] [CrossRef]
- Yum, S.; Jeong, S.; Lee, S.; Kim, W.; Nam, J.; Jung, Y. HIF-prolyl hydroxylase is a potential molecular target for esculetin-mediated anti-colitic effects. Fitoterapia 2015, 103, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Rubio, V.; García-Pérez, A.I.; Herráez, A.; Diez, J.C. Different roles of Nrf2 and NF-κB in the antioxidant imbalance produced by esculetin or quercetin on NB4 leukemia cells. Chem. Biol. Interact. 2018, 294, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Witaicenis, A.; Luchini, A.C.; Hiruma-Lima, C.A.; Felisbino, S.L.; Garrido-Mesa, N.; Utrilla, P.; Gávez, J.; Di Stasi, L.C. Supression of TNBS-induced colitis in rats by 4-methylesculetin, a natural coumarin: Cmparison with prednisolone and sulphasalazine. Chem. Biol. Interact. 2012, 195, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witaicenis, A.; Oliveira, E.C.S.; Tanimoto, A.; Zorzella-Pezavento, S.F.G.; Oliveira, S.L.; Sartori, A.; Di Stasi, L.C. 4-metylesculetin, a coumarin derivative, ameliorates dextran sulfate sodium-induced intestinal inflammation. Chem. Biol. Interact. 2018, 280, 59–63. [Google Scholar] [CrossRef]
- Witaicenis, A.; Seito, L.N.; Chagas, A.S.; Almeida-Junior, L.D.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Di Stasi, L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine 2014, 21, 240–246. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Sayed, A.M.; Hussein, O.E.; Mahmoud, A.M. Coumarins as modulators of the keap1/Nrf2/ARE signaling pathway. Oxidative Med. Cell. Longev. 2020, 2000, 1675957. [Google Scholar] [CrossRef] [Green Version]
- Lv, H.; Zhu, C.; Wei, W.; Lv, X.; Yu, Q.; Deng, X.; Ci, X. Enhanced keap1-Nrf2/Trx-1 axis by daphnetin protects against oxidative stress-driven hepatotoxicity via inhibiting ASK1/JNK and Txnip/NLRP3 inflammasome activation. Phytomedicine 2020, 71, 153241. [Google Scholar] [CrossRef]
- Tian, X.; Peng, Z.; Luo, S.; Zhang, S.; Li, B.; Zhou, C.; Fan, H. Aesculin protects against DSS-induced colitis through activating PPARγ and inhbiting NF-κB pathway. Eur. J. Pharmacol. 2019, 857, 172453. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Wang, X.; He, Z.; Liu, F.; Zhi, W.; Zhanf, H.; Niu, X. Esculin attenuates endotoxin shock induced by lipopolysaccharide in mouse and NO production in vitro through inhibition of NF-κB activation. Eur. J. Pharmacol. 2016, 791, 726–734. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Shen, Y.; Du, Y.; Chen, J.; Pei, F.; Fu, W.; Qiao, J. Esculin prevents lipolysaccharide/D-galactosamine-induced acute liver injury in mice. Microb. Pathog. 2018, 125, 418–422. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, H.; Park, H.J.; Choi, K.; Sadikot, R.T.; Cha, J.; Joo, M. Glycosilation enables aesculin to activate Nrf2. Sci. Rep. 2016, 6, 29956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khairy, H.; Saleh, H.; Badr, A.M.; Marie, M.S. Therapeutic efficacy of osthole against dinitrobenzene sulphonic acid induced-colitis in rats. Biomed. Pharmacother. 2018, 100, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Gao, Z.; Ji, K.; Li, X.; Wu, J.; Liu, Y.; Wang, X.; Liang, H.; Liu, Y.; Li, X.; et al. The in vitro and in vivo anti-inflammatory effect of osthole, the major natural coumarin from Cnidium monnieri (L.) Cuss, via the blocking of the activation of the NF-κB and MAPK/p38 pathways. Phytomedicine 2019, 58, 152864. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Feng, L.; Song, P.; Xu, F.; Li, A.; Wang, Y.; Shen, Y.; Wu, X.; Luo, Q.; Wu, X.; et al. Isomeranzin suppress inflammation by inhibiting M1 macrophage polarization through the NF-κB and ERK pathway. Int. Immunopharmacol. 2016, 38, 175–185. [Google Scholar] [CrossRef]
- Kohno, H.; Suzuki, R.; Curini, M.; Epifano, F.; Maltese, F.; Gonzales, S.P.; Tanaka, T. Dietary administrations with prenyloxycoumarins, auraptene and collinin, inhibits colitis-related colon carcinogenesis in mice. Int. J. Cancer 2006, 118, 2936–2942. [Google Scholar] [CrossRef]
- Kawabata, K.; Murakami, A.; Ohigashi, H. Auraptene decreases the activity of matrix metalloproteinases in dextran sulfate sodium-induced ulcerative colitis in ICR mice. Biosci. Biotech. Biochem. 2006, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenson, W.F. The universe of arachidonic acid metabolites in inflammatory bowel disease: Can we tell the good from the bad? Curr. Opin. Gastroenterol. 2014, 30, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.; Ronne, A.; Elmgreen, J. Abnormal metabolism of arachidonic acid in chronic inflammatory bowel disease: Enhanced release of leukotriene B4 from activated neutrophils. Gut 1987, 28, 181–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Nicolaides, D.N. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef]
- Sekiya, K.; Okuda, H.; Arichi, S. Selective inhibtion of platelet lipoxygenase by esculetin. Biochim. Biophys. Acta (BBA) Lipids Lip. Metab. 1982, 713, 68–72. [Google Scholar]
- Zhu, H.; Huang, J. Anti-inflammatory effects of scopoletin. Zhongcaoyao 1989, 15, 462–465. [Google Scholar]
- Huang, G.J.; Deng, J.S.; Liao, J.C.; Hou, W.C.; Wang, S.Y.; Sung, P.J.; KUo, Y.H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory activity of imperatorin from Glehnia littoralis. J. Agric. Food Chem. 2012, 60, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Q.; Liu, H.; Lu, C.; Liang, C.L.; Qiu, F.; Han, L.; Dai, Z. Esculetin ameliorates psoriasis-like skin disease in mice by inducing CD4+Foxp3+ regulatory T cells. Front. Immunol. 2018, 9, 2092. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Ge, X.; Chen, Y.; Zhu, B.; Wu, Q.; Zhang, J.; Shan, J.; Cheng, H.; Shi, L. Daphnetin ameliorates experimental colitis by modulating microbiota compositions and Treg/Th17 balance. FASEB J. 2019, 33, 9308–9322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Cai, Y.; Zhang, X.; Chen, H.; Lin, Y.; Li, H. Osthole pretreatment alleviates TNBS-induced colitis in mice via both cAMP/PKA-dependent and independent pathways. Acta Pharmacol. Sin. 2017, 38, 1120–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Shah, Y.M.; Gonzalez, F.J. Pregnane X receptor as a target for treatment of inflammatory bowel disorders. Trends Pharmacol. Sci. 2012, 33, 323–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDaniel, D.K.; Eden, K.; Ringel, V.M.; Allen, I.C. Emerging roles for non-canonical NF-κB signaling in the modulation of Inflammatory Bowel Disease pathobiology. Inflamm. Bowel Dis. 2016, 22, 2265–2279. [Google Scholar] [CrossRef] [Green Version]
- Rogler, G.; Brand, K.; Vogl, D.; Page, S.; Hofmeister, R.; Andus, T.; Knuechel, R.; Baeuerle, P.A.; Scholmerich, J.; Gross, V. NuclearFactor κB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 1988, 115, 357–369. [Google Scholar] [CrossRef]
- Spehlman, M.E.; Eckmann, L. Nuclear factor-kappa B in intestinal protection and destruction. Curr. Opin. Gastroenterol. 2009, 25, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Karrash, T.; Jobin, C. NF-κB and the intestine: Friend or foe? Inflamm. Bowel Dis. 2008, 14, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ma, J.; Shi, X.; Song, X.; Yang, Y.; Xiao, S.; Li, J.; Gu, W.; Huang, Z.; Zhang, J.; et al. A novel pyrazole-containing indolizine derivative suppresses NF-κB activation and protects against TNBS-induced colitis via a PPAR-γ-dependent pathway. Biochem. Pharmacol. 2017, 135, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Shah, Y.M.; Morimura, K.; Gonzalez, F.J. Expression of peroxisome proliferator-activated receptor-γ in macrophage. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G657–G666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croasdell, A.; Duffney, P.F.; Kim, N.; Lacy, S.H.; Sime, P.J.; Phipps, R.P. PPARγ and the innate immune system mediate the resolution of inflammation. PPAR Res. 2015, 2015, 549691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Hidalgo, M.; Villegas, M.I.; Alarcón de la Lastra, C. Rosiglitazone, an agonist of perosisome proliferator-activated receptor gamma, reduces chronic colonic inflammation is rats. Biochem. Pharmacol. 2005, 69, 1733–1744. [Google Scholar] [CrossRef]
- Hou, Y.; Moreau, F.; Chadee, K. PPARγ is an E3 ligase that induces the degradation of NF-κB/p65. Nat. Commun. 2012, 3, 1300. [Google Scholar] [CrossRef] [Green Version]
- Korbecki, J.; Bobinski, R.; Dutka, M. Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm. Res. 2019, 68, 443–458. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.; Rani, K.; Datt, C. Molecular link between dietary fibre, gut microbiota and health. Mol. Biol. Rep. 2020, 47, 6229–6237. [Google Scholar] [CrossRef]
- Arora, R.; Sawney, S.; Saini, V.; Steffi, C.; Tiwari, M.; Saluja, D. Esculetin induces antiproliferative and apoptotic response in pancreatic cancer cells by directly binding to keap1. Mol. Cancer 2016, 15, 64. [Google Scholar] [CrossRef] [Green Version]
- Coskun, M.; Olsen, J.; Seidelin, J.B.; Nielsen, O.H. MAP kinases in inflammatory bowel disease. Clin. Chim. Acta 2011, 412, 513–520. [Google Scholar] [CrossRef]
- Broom, O.J.; Widjaya, B.; Troelsen, J.; Olsen, J.; Nielsen, O.H. Mitogen activated protein kinases: A role in inflammatory bowel disease? Clin. Exp. Immunol. 2009, 158, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK signal transduction pathways activated stress and inflammation: A 10-year update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quaglio, A.E.V.; Castilho, A.C.S.; Di Stasi, L.C. Experimental evidence of MAP kinase gene expression on the response of intestinal anti-inflammatory drugs. Life Sci. 2015, 136, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Kim, H.; Choi, D.; Kim, D.; Park, S.; Kim, Y.; Kim, Y.M.; Jung, Y. Quercetin activates an angiogenic pathway, hypoxia inducible factor (HIF)-1-vascular endothelial growth factor, by inhibiting HIF-prolyl hydroxylase: A structural analysis of quercetin for inhibiting HIF-prolyl hydroxylases. Mol. Pharmacol. 2007, 71, 1676–1684. [Google Scholar] [CrossRef]
- Choi, D.; Han, J.; Lee, Y.; Choi, J.; Han, S.; Hong, S.; Jeon, H.; Kim, Y.M.; Jung, Y. Caffeic acid phenethyl ester is a potent inhibitor of HIF prolyl hydroxylase: Structural analysis and pharmacological implication. J. Nutr. Biochem. 2010, 21, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; Keely, S.; Karhausen, J.; Gerich, M.E.; Furuta, G.T.; Colgan, S.P. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 2008, 134, 145–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tambuwala, M.M.; Cummins, E.P.; Lenihan, C.R.; Kiss, J.; Stauch, M.; Scholz, C.C.; Fraisl, P.; Lasitschka, F.; Mollenhauer, M.; Saunders, S.P.; et al. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology 2010, 139, 2093–2101. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; KO, J.; Ju, C.; Eltzschig, H.K. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 2019, 51, 68. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, L.; Yang, J.; Wang, Z.; Du, G. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia. Mol. Med. Rep. 2018, 18, 3547–3554. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zhang, G.; Zheng, C.; Song, M.; Liu, F.; Huang, X.; Bai, S.; Huang, X.; Lin, C.; Zhu, C.; et al. Activating the pregnane X receptor by imperatorin attenuates dextran sulphate sodium-induced colitis in mice. Br. J. Pharmacol. 2018, 175, 3563–3580. [Google Scholar] [CrossRef]
- Gao, J.; Xie, W. Pregnane X receptor and constitutive androstane receptor at the crossroads of drug metabolism and energy metabolism. Drug Metab. Dispos. 2010, 38, 2091–2095. [Google Scholar] [CrossRef] [Green Version]
- Shah, Y.M.; Ma, X.; Morimura, K.; Kim, I.; Gonzalez, F.J. Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-κB target gene expression. Am. J. Physiol. Gastrointes. Liver Physiol. 2007, 292, G1114–G1122. [Google Scholar] [CrossRef] [PubMed]
- DeMeo, M.T.; Mutlu, E.A.; Keshavarzian, A.; Tobin, M.C. Intestinal permeation and gastrointestinal disease. J. Clin. Gastroenterol. 2002, 34, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Deuring, J.J.; Li, M.; Cao, W.; Chen, S.; Wang, W.; de Haar, C.; van der Woude, C.J.; Peppelenbosch, M. Pregnane X receptor activation constrains mucosal NF-κB activity in active inflammatory bowel disease. PLoS ONE 2019, 14, e0221924. [Google Scholar]
- Wag, Y.; Chen, J.; Chen, J.; Dong, C.; Yan, X.; Zhu, Z.; Lu, P.; Song, Z.; Liu, H.; Chen, S. Daphnetin ameliorates glucocorticoid-induced osteoporosis via activation of Wnt/GSK-3β/β-catenin signaling. Toxicol. Appl. Pharmacol. 2020, 409, 115333. [Google Scholar]
- Lee, J.-H.; Kim, Y.-G.; Cho, S.S.; Ryu, S.Y.; Cho, M.H.; Lee, J. Coumarins reduce biofilm formation and virulence of Escherichia coli O157:H7. Phytomedicine 2014, 21, 1037–1042. [Google Scholar] [CrossRef]
- Martínez Aguilar, Y.; Rodriguez, F.S.; Saavedra, M.A.; Hermosilla Espinosa, R.; Yero, O.M. Secondary metabolites and in vitro antibacterial activity of extracts from Anacardium occidentale L. (cashew tree) leaves. Rev. Cuba. Plantas Med. 2012, 17, 320–329. [Google Scholar]
- Widelski, J.; Luca, S.V.; Skiba, A.; Chinou, I.; Marcout, L.; Wolfender, J.-L.; Skalicka-Wozniak, K. Isolation and antimicrobial activity of coumarin derivatives from fruits of Peucedanum luxurians Tamamsch. Molecules 2018, 23, 1222. [Google Scholar] [CrossRef] [Green Version]
- Saho, J.; Kumar Mekap, S.; Sudhir, K.P. Synthesis, spectral characterization of some new 3-heteroaryl-azo-4-hydroxy coumarin derivatives and their antimicrobial evaluation. J. Taibah Univ. Sci. 2015, 9, 187–195. [Google Scholar] [CrossRef] [Green Version]
- López-Rojas, P.; Janeczko, M.; Kubinski, K.; Amestt, Á.; Maslyk, M.; Estévez-Braun, A. Synthesis and antimicrobial activity of 4-substituted 1, 2, 3-triazole-coumarin derivatives. Molecules 2018, 23, 199. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Xu, H.; Zhao, H.; Geng, Y.; Ren, Y.; Guo, L.; Shi, J.; Xu, Z. Edgeworthia gardneri (Wall.) Meisn. Water extract improves diabetes and modulates gut microbiota. J. Ethnopharmacol. 2019, 239, 111854. [Google Scholar] [CrossRef]
- Zhao, X.-Q.; Guo, S.; Lu, Y.-Y.; Hua, Y.; Zhang, F.; Yan, H.; Shang, E.-X.; Wang, H.-Q.; Zhang, W.-H.; Duan, J.-A. Lyceum barbarum L. leaves ameliorate type 2 diabetes in rats by modulating metabolic profiles and gut microbiota composition. Biomed. Pharmacother. 2020, 121, 109559. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stasi, L.C. Coumarin Derivatives in Inflammatory Bowel Disease. Molecules 2021, 26, 422. https://doi.org/10.3390/molecules26020422
Di Stasi LC. Coumarin Derivatives in Inflammatory Bowel Disease. Molecules. 2021; 26(2):422. https://doi.org/10.3390/molecules26020422
Chicago/Turabian StyleDi Stasi, Luiz C. 2021. "Coumarin Derivatives in Inflammatory Bowel Disease" Molecules 26, no. 2: 422. https://doi.org/10.3390/molecules26020422
APA StyleDi Stasi, L. C. (2021). Coumarin Derivatives in Inflammatory Bowel Disease. Molecules, 26(2), 422. https://doi.org/10.3390/molecules26020422




