Abstract
Recently, cultivated “Qi-Nan” (CQN) agarwood has emerged as a new high-quality agarwood in the agarwood market owing to its similar characteristics, such as high content of resin and richness in two 2-(2-phenylethyl)chromone derivatives, 2-(2-phenylethyl)chromone (59) and 2-[2-(4-methoxyphenyl)ethyl]chromone (60), to the wild harvested “Qi-Nan” (WQN) agarwood. In this study, we compared the chemical constituents and fragrant components of two types of WQN agarwood from A. agallocha Roxb. and A. sinensis, respectively, with CQN agarwood and ordinary agarwood varieties. Additionally, we analyzed different samples of WQN agarwood and CQN agarwood by GC-MS, which revealed several noteworthy differences between WQN and CQN agarwood. The chemical diversity of WQN was greater than that of CQN agarwood. The content of (59) and (60) was higher in CQN agarwood than in WQN agarwood. For the sesquiterpenes, the richness and diversity of sesquiterpenes in WQN agarwood, particularly guaiane and agarofuran sesquiterpenes, were higher than those in CQN. Moreover, guaiane-furans sesquiterpenes were only detected by GC-MS in WQN agarwood of A. sinensis and could be a chemical marker for the WQN agarwood of A. sinensis. In addition, we summarized the odor descriptions of the constituents and established the correlation of scents and chemical constituents in the agarwood.
1. Introduction
Agarwood, also called eaglewood, gaharu, jinko, aloeswood, pokok karas, chen xiang, kalambak, or oud in different countries, is a resinous heartwood from the Aquilaria and Gyrinops species of the Thymelaeceae family and is formed after physical wounding of the stem or infection by pathogens [1,2,3]. As a traditional medicine, agarwood was used for the treatment of various diseases, including rheumatism, arthritis, body pain, asthma, gout, and also acted as a stimulant as well as sedative, analgesic, and carminative agent [4]. Moreover, agarwood is highly valued for its usage in incense and perfume due to its special and pleasant fragrance [5]. “Qi-Nan”, also named Kanankoh, Kyara, or Chi-Nan in different cultures, is commonly considered as the highest quality agarwood in the market, mainly owing to its mysterious and elegant scent, resinoid content, color, and morphological characteristics which are distinct from those of ordinary agarwood [6]. Due to its scarcity and preciousness, “Qi-Nan” is highly appreciated and its price is hundreds or even thousands of times higher than that of ordinary agarwood [5]. The huge economic value and the exhausted wild resource of agarwood have promoted the rapid emergence of the cultivated “Qi-Nan” in recent years. A. sinensis “Reke2”, for example, is a high-quality cultivated “Qi-Nan” propagated by grafting with the ordinary germplasm of A. sinensis, and can produce agarwood with higher content of resin than that of ordinary A. sinensis trees after artificial induction [7]. However, there is little information concerning the difference between the wild and cultivated “Qi-Nan” agarwood varieties in terms of the chemical constituents and aroma components. Li analyzed the incense smoke of cultivated grafting “Qi-Nan” agarwood using thermogravimetric fourier transform infrared spectroscopy (TG-FTIR) and headspace gas chromatography–mass spectrometry (HSGC–MS), and observed a large difference in the main components, including sesquiterpenes and aromatic compounds, between cultivated grafting “Qi-Nan” and wild “Qi-Nan” agarwood. However, this study did not determine the specific constituents, especially the 2-(2-phenylethyl)chromone derivatives, responsible for the differences [8].
The quality of “Qi-Nan” agarwood was often assessed by the traditional grading indexes, such as physical sense, water sinking condition, color, scent/aroma, and morphology [9]. It seems that these indexes were too subjective for agarwood quality grading, and more objective grading parameters—including chemical constituents—are urgently needed. Up to now, the chemical constituents of only two wild “Qi-Nan” agarwood types, originating from A. agallocha Roxb. [10,11,12,13] and A. sinensis [14,15,16,17,18,19,20,21], respectively, have been investigated. Sesquiterpenes and 2-(2-phenylethyl)chromones were reported as their main constituents. Guaianes, eudesmanes, and eremophilanes are the three main types of sesquiterpenes found in the two wild “Qi-Nan” agarwood varieties, together with several agarofurans, agarospiranes, acoranes, cadinanes (Figure 1) [22,23]. In particular, guaianes show great structural diversity and might be used as markers for different kinds of agarwood. Most of the sesquiterpenes contribute to the special odor and to the gorgeous and elegant character of “Qi-Nan” agarwood. As for 2-(2-phenylethyl)chromones, 23 of them, except for 6-methoxy-2-[2-(4-methoxyphenyl)ethyl]chromone (67), have been isolated from the Chinese agarwood “Lv Qi-Nan” of A. sinensis, while only three—2-(2-phenylethyl)chromone (59), 2-[2-(4-methoxyphenyl)ethyl]chromone (60), and (67)—have been isolated from kanankoh; a high-quality agarwood of A. agallocha (Figure 2). Compounds (59) and (60) are two typical 2-(2-phenylethyl)chromones without substitution of the chromone moiety, which are most abundant in high quality “Qi-Nan” agarwood, as well as frequently found in most ordinary agarwood with a low relative content [22,23]. A 2-(2-phenethyl)chromone glycoside (72) was obtained from the high-quality “Lv Qi-Nan” of A. sinensis, which is the only chromone glycoside reported from agarwood up to now [17].
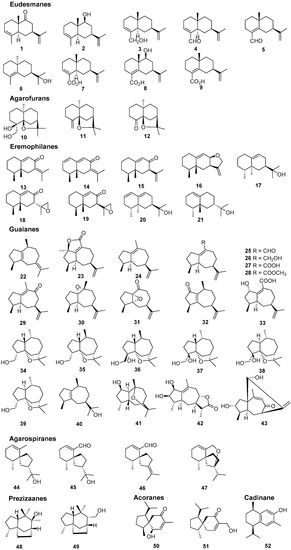
Figure 1.
Structures of sesquiterpenes identified in “Qi-Nan” agarwood from A. agallocha and A. sinensis together with odor sesquiterpenes in agarwood.
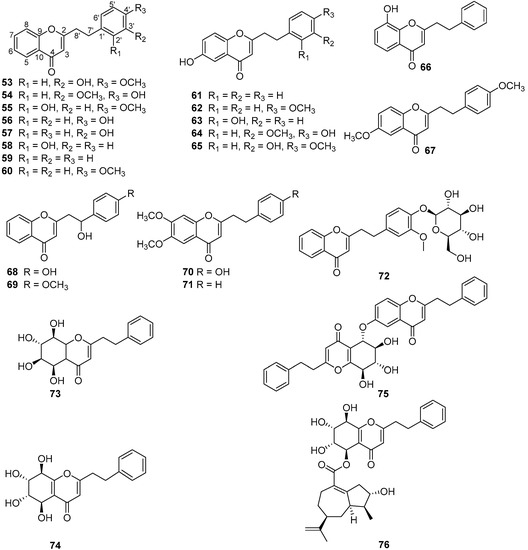
Figure 2.
Structures of 2-(2-phenylethyl)chromones identified in “Qi-Nan” agarwood from A. agallocha and A. sinensis.
In this paper, we analyzed 22 cultivated “Qi-Nan” agarwood and seven wild “Qi-Nan” agarwood varieties by GC-MS, and compared the chemical constituents and fragrant components of cultivated “Qi-Nan” (CQN) agarwood with those of wild harvested “Qi-Nan” (WQN) agarwood and ordinary agarwood, to provide a scientific foundation for the quality evaluation of “Qi-Nan” agarwood.
2. Results and Discussion
2.1. GC-MS Analysis of Wild Harvested “Qi-Nan” Agarwood and Cultivated “Qi-Nan” Agarwood
Seven wild harvested “Qi-Nan” agarwood samples and 22 cultivated “Qi-Nan” agarwood samples were extracted with EtOH and Et2O. The resin content of agarwood is a crucial quantitative criterion for grading the quality of agarwood [24]. The average ethanol extract contents of the CQN agarwood were 46.52 ± 11.25%, which were similar to, or higher than, those of the WQN agarwood (49.85 ± 9.94%), while obviously higher than those of ordinary agarwood [25,26]. Therefore, the quality of CQN agarwood was higher than that of ordinary agarwood and very close to that of WQN agarwood.
The Et2O extracts were analyzed by GC-MS and 56 and 40 compounds were identified in WQN agarwood and CQN agarwood, respectively, which indicated that the chemical diversity of WQN was greater than that of CQN. The main constituents in agarwood are sesquiterpenes and 2-(2-phenylethyl)chromones. As presented in Figure 3A, both of WQN and CQN showed high contents of 2-(2-phenylethyl)chromones. Especially, the relative content of the two main 2-(2-phenylethyl)chromones, 2-(2-phenylethyl)chromone (59) and 2-[2-(4-methoxyphenyl)ethyl]chromone (60), was very high (43.89–73.04% in WQN agarwood and 72.43–95.61% in CQN agarwood in this study), which was consistent with the result of our previous study [7,23] and Ishihara’s discovery [27]. Most of the CQN agarwood possessed higher contents of (59) and (60) than the levels found in WQN agarwood. Therefore, the relative content of (59) and (60) could be a reference not only for identifying and distinguishing both of WQN and CQN agarwood from ordinary agarwood, but also for discriminating the WQN from the CQN agarwood. Furthermore, detailed comparison of the structures of 2-(2-phenylethyl)chromone derivatives identified in the WQN and CQN agarwood varieties revealed that the 2-(2-phenylethyl)chromone derivatives, such as the 2-(2-phenylethyl)chromones numbered (46) and (48–56) in Table S4, were substituted with the hydroxy and/or methoxy on their chromone units in the WQN agarwood samples WQN1–4, while no substituents were observed on the chromone moiety of 2-(2-phenylethyl)chromone derivatives numbered (33–40) in Tables S2 and S3 in the CQN agarwood samples and WQN agarwood samples WQN5–7. This remarkable structural difference in 2-(2-phenylethyl)chromone derivatives could be sufficient evidence to determine the WQN agarwood, but is not a necessary one.
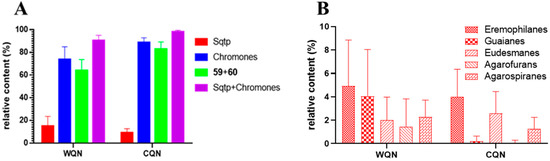
Figure 3.
The chemical distribution in wild harvested “Qi-Nan” (WQN) agarwood and cultivated “Qi-Nan” (CQN) agarwood (Sesquiterpenoids: Sqtp). The relative contents of chromones and sesquiterpenoids in the WQN and CQN agarwood (A). The relative contents of five predominant types of sesquiterpenes in the WQN and CQN agarwood (B).
As for sesquiterpenes, their richness in WQN agarwood was higher in contrast with the CQN agarwood, as illustrated in Figure 3A. Additionally, Figure 3B provided the detailed distribution of five predominant types of sesquiterpenes, including eudesmanes, guaianes, eremophilanes, agarofurans, and agarospiranes, in the WQN and CQN agarwood varieties. Obviously, the contents of guaianes and agarofurans sesquiterpenes were higher in WQN compared with CQN. Ten guaianes and three agarofurans sesquiterpenes were observed in WQN, but only three guaianes and one agarofuran sesquiterpene were detected in CQN, as shown in Tables S2–S4. The results indicate that WQN possess not only a higher concentration, but also a greater chemical diversity of guaiane and agarfuran sesquiterpenes than CQN. To date, the chemical constituents of only two “Qi-Nan” agarwood varieties were reported. In 1991, a series of guaiane sesquiterpenes (23–33) featuring a 7-isopropenyl moiety, except for α-guaiene (22), were isolated and identified from kanankoh, a high-quality agarwood of A. agallocha [12]. Among them, compounds (23), (25–28), and (33) are oxidized at C-14, which is rarely encountered in nature. In this work, GC-MS identified guaiane sesquiterpenes (23), (25), and (27) in WQN, and (26) and (27) in CQN, indicating that guaiane sesquiterpenes with a 7-isopropenyl group are not uncommon in WQN and CQN agarwood. In 2016, six guaiane-furans (35–40) were isolated from a high-quality agarwood of A. sinensis named “Lv Qi-Nan” in Chinese [15]. These guaiane-furans sesquiterpenes possess a 5,11-epoxy ring with multiple stereoisomers, and are functionalized at C-15. None of these sesquiterpenes were found in the 22 samples of CQN agarwood but three of them (35, 37 and 38) were detected in the WQN agarwood. This noteworthy difference indicated that guaiane-furan sesquiterpenes—despite the fact that two of which, sinenofuranol and sinenofuranal, were isolated from ordinary agarwood [28]—may be the identifying components of WQN allowing us to distinguish it from CQN. Both of the two aforementioned agarwood varieties were reported as “Qi-Nan” agarwood and were richer in guaiane sesquiterpenes compared with CQN, while the structural characteristics of guaiane sesquiterpenes in the two agarwood types were totally different, which may be due to their different origin plants. At present, 15 agarofuran sesquiterpenes have been reported from agarwood [23]. Only three (10, 11 and 12) of them were isolated from the two above noted high-quality “Qi-Nan” agarwood varieties and others were identified from ordinary agarwood [23]. Although significant differences in the relative contents of agarofuran sesquiterpenes between WQN and CQN agarwood were observed in this study, it is hard to say that agarfuran sesquiterpenes could be a chemical marker for high quality agarwood.
The compound information of CQN and WQN agarwood was introduced to the orthogonal partial least-squares (OPLS) statistical analysis, except for WQN7 which was formed in the bark, while the other samples were obtained in the xylem. The quality of the model was evaluated by R2X, R2Y and Q2Y. The R2X/R2Y value represents the percentage of predictor/response variance explained. The Q2Y refers to the predictive ability of the model which is calculated by cross-validation, and the RMSE represents the prediction accuracy of the model. As shown in Figure 4, The relatively low R2X value may be attributed to the scattered compound distribution of the WQN agarwood. Meanwhile, the compound distribution of CQN agarwood is centralized. This indicated that the quality uniformity of CQN agarwood is better than the WQN agarwood varieties, which were purchased from a wide range of geographical areas.
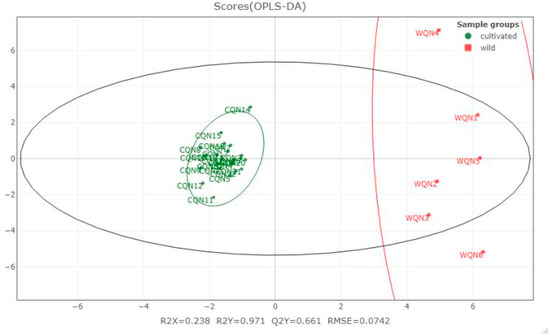
Figure 4.
Orthogonal partial least-squares (OPLS) model of the chemical constituents distribution of cultivated and wild harvested “Qi-Nan” agarwood.
The R2Y, Q2Y and RMSE values proved that the prediction ability of the OPLS model is reliable in a certain degree, which means the WQN and CQN agarwood can be clearly distinguished. The variable importance in projection (VIP) value was then calculated and the VIP > one rule was applied. Table 1 lists the compounds with VIP values which may account for the difference in compound distribution between WQN and CQN agarwood. It can be seen that three 2-(2-phenylethyl)chromones were ranked in the five compounds that VIP > two which indicated these 2-(2-phenylethyl)chromones could be characteristic compounds, able to distinguish WQN agarwood from CQN agarwood.

Table 1.
Variable importance in projection (VIP) values calculated form OPLS model.
2.2. The Fragrant Sesquiterpenes and 2-(2-Phenylethyl)chromones Identified in Agarwood
The scent is one of the most important factors to consider in determining the quality of agarwood. Generally, low quality agarwood possesses a low content of resin and high content of wood. When the low-quality agarwood is burning, it may release woody aromas which will irritate the eyes and nose. However, high-quality agarwood burns evenly and rather slowly while releasing its soft and pleasant scents gradually. Its fragrance lingers at the room for a longer period. Unlike ordinary agarwood, the scent of “Qi-Nan” agarwood can be smelled even at room temperature [29]. The specific odors of agarwood are closely correlated with their chemical compositions. We summarized the odor description of the constitutions in agarwood and have listed them in Table 2. Most of the odor components were sesquiterpenes. For instance, (−)-guaia-1(10),11-dien-15-al (25) has a pleasant β-damascenone-like woody and floral note with a slight cooling side note [10]. Many other sesquiterpenes (1, 4, 5, 18, 19, 34, 35, 36, 37, 38, and 39), in particular those only isolated from the above-mentioned two “Qi-Nan” agarwood, also have fresh floral, sweet, and cool scents, indicating that those sesquiterpenes were the main contributors to the gorgeous and elegant character of “Qi-Nan” agarwood. While the sesquiterpenes (13, 14, 15, 44, 45, 48, 49, etc.) reported only from the ordinary agarwood or from both the “Qi-Nan” agarwood and ordinary agarwood released a woody, or even strong woody scent. 2-(2-Phenylethyl)chromone derivatives were reported be responsible for the warm, sweet, balsamic, long lasting odor when agarwood is burnt or heated [5]. Of particular note here, is that two 2-(2-phenylethyl)chromones, (59) and (60) which account for the highest content in “Qi-Nan” agarwood, were odorless, but they yielded a pleasant scent when heated. They were subjected to pyrolysis into benzaldehyde and 4-methoxy benzaldehyde, respectively, in an air stream at 150 °C for 6 h [30] and these pyrolysis products, together with sesquiterpenes, composed the incense of agarwood [5]. The above information gives insight into the correlation between the scents and the compounds of agarwood, and provides the basis for the scents as an index to grade the quality of agarwood.

Table 2.
The odor sesquiterpenes and 2-(2-phenylethyl)chromones identified in agarwood.
3. Materials and Methods
3.1. GC-MS Analysis
A Hewlett Packard GC 6890 gas chromatography instrument coupled with a Mass Selective Detector (5975C, Agilent Technologies, Wilmington, Delaware, USA) was used for the analysis. Separation of the samples by gas chromatography was carried out using a HP-5MS 5% Phenyl Methyl Siloxane column (30 m × 0.25 mm × 0.25 µm) (Phenomenex, Torrence, CA, USA). The parameters were set: injection volume, 1.0 µL; the front inlet temperature, 250 °C; split ratio, 40:1; the flow rate of the helium (carrier gas), 1.0 mL/min; the interface temperature, 280 °C; ionization of the compounds, electron impact (EI); emission current, 70 eV; the ion source temperature, 230 °C. The oven program commenced at 50 °C and increased to 310 °C at a rate of 5 °C/min, then held for 10 min. The spectra were obtained over the mass range of m/z 29 to 500 and the relative contents of the compounds were determined by normalization. The NIST14 database, WILEY275 database, and the mass spectroscopic data in the literature were used to characterize the compounds.
3.2. Plant Material
In the plantation, the “Qi-Nan” seedlings were obtained by the scions grafting method. The different kinds of excellent wild germplasm of A. sinensis discovered in Dianbai area were selected as parental plants, and the ordinary germplasm of A. sinensis was used as a grafting stock. The scions were usually one year to one and a half years old, 0.5–1.0 cm diameters branches of parental plants, with two or three buds on it. The upper branches of grafting stocks were cut off and only a 5–10 cm high main stem was left, then a 2–3 cm deep cut was made along the longitudinal direction of each main stem. Each scion was cut into a wedge shape and inserted into each rootstock cut, then tied up with a plastic film around the incision site. They were kept under 25 °C, 40–50% shading, and moisturizing conditions for about 30 days, then the graft seedlings began to germinate. When the graft seedlings were 2 or 3 years old, the agarwood formation was induced by holing the trunk. The twenty-two CQN agarwood samples were all harvested about ten months after holing the trunk. All of the 7 WQNs were obtained from two agarwood collectors. It is worth mentioning that, different from all the other samples obtained from the trunk of the plant, WQN7 was obtained from the bark of the trunk, which was named as “tabby cat striation” “Qi-Nan” for the appearance of its outside surface which looks like the skin of a tiger. All the agarwood samples produced a scent under room temperature. The odor descriptions and origin plants of these agarwood varieties were identified by Professor Dai Haofu and have been listed in Table 3. The samples are shown in Figures S1 and S2. The voucher specimens were deposited at the Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences.

Table 3.
The sample information.
3.3. Sample Preparation
All the samples were ground into a powder and dried. The accurately weighed powder (2.00 g) was refluxed in ethanol (3 × 15 mL) and the ethanol extract was filtered and concentrated. The yields of the agarwood samples were listed in Table S1.
The accurately weighed agarwood powder (0.20 g) was extracted under ultrasonic wave (42 KHz, 20 min) with Et2O (3 × 30 mL) at 20 °C for three times. The Et2O extract was filtered and evaporated to obtain a brownish yellow oil. Then, the oil was dissolved in 2.0 mL methanol, filtered through 0.45 µm microporous filtering film and stored at 4 °C.
3.4. Orthogonal Partial Least-Squares (OPLS) Statistical Analysis
The compound information of CQN and WQN agarwood was scaled firstly, then calculated by the ropls R package and plotted by the plotly R package. For the reason of unique forming site of sample WQN7, the process did not contain it.
4. Conclusions
Recently, CQN has emerged as a new high-quality agarwood in the agarwood market, owing to its similar characteristics—such as high content of resin and richness in two 2-(2-phenylethyl)chromonederivatives, 2-(2-phenylethyl)chromone (59) and 2-[2-(4-methoxyphenyl)ethyl]chromone (60)—to the WQN variety. In this study, we compared the chemical constituents and fragrant components of two reported WQN agarwood samples from A. agallocha Roxb, and A. sinensis, respectively—with those of the CQN agarwood variety having been collected in Dianbai, Guangdong province, and ordinary agarwood, as well as the different analyzed samples of WQN agarwood and CQN agarwood having been collected in Dianbai, Guangdong province—by GC-MS, which revealed several noteworthy differences between the WQN and CQN agarwood varieties. The chemical diversity of WQN agarwood was greater than that of CQN agarwood. The content of (59) and (60) was higher in CQN agarwood than in WQN agarwood. For the sesquiterpenes, the richness and diversity of sesquiterpenes, particularly guaiane and agarofuran sesquiterpenes, in WQN agarwood was higher than in CQN agarwood. In addition, guaianefurans sesquiterpenes were only detected by GC-MS in WQN agarwood and could be a chemical marker for WQN agarwood. Moreover, we summarized the odor descriptions of the constituents, most of which were reported from WQN agarwood, and established the correlation of scents and chemical constituents in the agarwood, especially in the high-quality agarwood. In summary, the CQN agarwood possess similar characteristics, including high contents of alcohol extracts and two 2-(2-phenylethyl)chromone derivatives (59 and 60), which were the key criterions for the high quality agarwood, to the WQN agarwood. However, several noteworthy differences in chemical constituents and fragrant components were also found between the two agarwood varieties. These results are helpful for distinguishing the WQN agarwood from CQN agarwood collected in Dianbai, Guangdong province and the advancement of the agarwood industry.
Supplementary Materials
The following are available online; the data contain the samples of cultivated (CQN12-CQN22, Figure S1) and wild harvested (WQN1-WQN7, Figure S2) “Qi-Nan” agarwood. The yields of the agarwood samples were listed in Table S1. The constituents and their structures identified in cultivated (Tables S2, S3 and Figure S3) and wild harvested (Table S4 and Figure S4) “Qi-Nan” agarwood by GC-MS.
Author Contributions
Conceptualization, H.-F.D.; data curation, W.-L.M.; investigation, L.Y., J.-L.Y., W.-H.D., Y.-L.W., J.Z., H.W. and J.-Z.Y.; supervision, H.-F.D.; writing—original draft, L.Y.; writing—review and editing, W.-L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key Research and Development Program of China (2018YFC1706400), National Natural Science Foundation of China (31870668), China Agriculture Research System (CARS-21), and Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (1630052019026).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Material.
Acknowledgments
We are deeply grateful to Xiaowu Zhang, Ben Huang, Boqing Yan, Fengzeng Lu, and Baoyuan Liu for providing the WQN, and CQN samples.
Conflicts of Interest
No potential conflict of interest was reported by the authors.
References
- Rogers, Z.S. A World Checklist of Thymelaeaceae (version 1); Missouri Botanical Garden: St. Louis, MI, USA, 2009. [Google Scholar]
- Li, W.; Cai, C.H.; Dong, W.H.; Guo, Z.K.; Wang, H.; Mei, W.L.; Dai, H.F. 2-(2-Phenylethyl)chromone derivatives from Chinese agarwood induced by artificial holing. Fitoterapia 2014, 98, 117–123. [Google Scholar] [CrossRef]
- Kalita, J.; Bhattacharyya, P.R.; Boruah, H.D.; Unni, B.G.; Lekhak, H.; Nath, S.C. Association of Zeuzera conferta Walker on agarwood formation in Aquilaria malaccensis Lamk. Asian. J. Plant. Sci. Res. 2015, 5, 4–9. [Google Scholar]
- Hashim, Y.Z.H.Y.; Kerr, P.G.; Abbas, P.; Sallehab, H.M. Aquilaria spp. (agarwood) as source of health beneficial compounds: A review of traditional use, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 189, 331–360. [Google Scholar] [CrossRef]
- Naef, R. The volatile and semi-volatile constituents of agarwood, the infected heartwood of Aquilaria species: A review. Flavour Frag. J. 2011, 26, 73–87. [Google Scholar] [CrossRef]
- Ishihara, M. The scent of Kyara, Flavour, Japan Perfumery and Flavouring Association. 2013; Volume 258, pp. 55–67. Available online: http://id.ndl.go.jp/bib/024765960 (accessed on 10 June 2020).
- Wang, Y.G.; Wang, J.; Yang, J.L.; Cai, C.H.; Gai, C.J.; Mei, W.L.; Dai, H.F. The GC-MS analysis of the chemical composition of agarwood from Aquilaria sinensis ‘Reke2’. Chin. J. Trop. Agri. 2020, 40, 79–88. [Google Scholar]
- Chen, Y.; Yan, T.; Zhang, Y.; Wang, Q.; Li, G. Characterization of the incense ingredients of cultivated grafting Kynam by TG-FTIR and HS-GC-MS. Fitoterapia 2020, 142, 104493. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.W. Description of Varieties of Chinese Medicinal Materials; Shanghai Science and Technology Press: Shanghai, China, 1964. [Google Scholar]
- Ishihara, M.; Tsuneya, T.; Shiga, M.; Uneyama, K. Three sesquiterpenes from agarwood. Phytochemistry 1991, 30, 563–566. [Google Scholar] [CrossRef]
- Ishihara, M.; Tsuneya, T.; Uneyama, K. Fragrant sesquiterpenes from agarwood. Phytochemistry 1993, 33, 1147–1155. [Google Scholar] [CrossRef]
- Ishihara, M.; Tsuneya, T.; Uneyama, K. Guaiane sesquiterpenes from agarwood. Phytochemistry 1991, 30, 3343–3347. [Google Scholar] [CrossRef]
- Ishihara, M.; Masatsugu, Y.; Uneyama, K. Preparation of (−)-guaia-1 (10), 11-dien-15, 2-olide and (−)-2α-hydroxyguaia-1 (10), 11-dien-15-oic acid, fragrant sesquiterpenes in agarwood (Aquilaria agallocha). Tetrahedron 1992, 48, 10265–10276. [Google Scholar] [CrossRef]
- Yang, D.L.; Wang, H.; Guo, Z.K.; Li, W.; Mei, W.L.; Dai, H.F. Fragrant agarofuran and eremophilane sesquiterpenes in agarwood ‘Qi-Nan’ from Aquilaria sinensis. Phytochem. Lett. 2014, 8, 121–125. [Google Scholar] [CrossRef]
- Yang, D.L.; Li, W.; Dong, W.H.; Wang, J.; Mei, W.L.; Dai, H.F. Five new 5,11-epoxyguaiane sesquiterpenes in agarwood “Qi-Nan” from Aquilaria sinensis. Fitoterapia 2016, 112, 191–196. [Google Scholar] [CrossRef]
- Shao, H.; Mei, W.L.; Kong, F.D.; Dong, W.H.; Li, W.; Zhu, G.P.; Dai, H.F. A new 2-(2-phenylethyl) chromone glycoside in Chinese agarwood “Qi-Nan” from Aquilaria sinensis. J. Asian Nat. Prod. Res. 2017, 19, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Kong, F.D.; Wang, H.; Mei, W.L.; Dai, H.F. Qinanmer, a new compound from Chinese agarwood ‘Qi-Nan’ originating from Aquilaria sinensis. J. Asian Nat. Prod. Res. 2017, 19, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.L.; Wang, H.; Guo, Z.K.; Dong, W.H.; Mei, W.L.; Dai, H.F. A new 2-(2-phenylethyl) chromone derivative in Chinese agarwood ‘Qi-Nan’from Aquilaria sinensis. J. Asian Nat. Prod. Res. 2014, 16, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.L.; Wang, J.; Li, W.; Dong, W.H.; Mei, W.L.; Dai, H.F. New guaiane and acorane sesquiterpenes in high quality agarwood Qi-Nan from Aquilaria sinensis. Phytochem. Lett. 2016, 17, 94–99. [Google Scholar] [CrossRef]
- Yang, D.L.; Mei, W.L.; Zeng, Y.B.; Guo, Z.K.; Zhao, Y.X.; Wang, H.; Zuo, W.J.; Dong, W.H.; Wang, Q.H.; Dai, H.F. 2-(2-Phenylethyl) chromone derivatives in Chinese agarwood “Qi-Nan” from Aquilaria sinensis. Planta Med. 2013, 79, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mei, W.L.; Dong, W.H.; Cai, C.H.; Gai, C.J.; Dai, H.F. Study on chemical constituents of Chinese agarwood ‘Qi-Nan’. J. Trop. Subtrop. Bot. 2019, 27, 196–202. [Google Scholar]
- Yang, D.L.; Mei, W.L.; Yang, J.L.; Zeng, Y.B.; Dai, H.F. GC-MS Analysis of the fragrant sesquiterpenes and 2-(2-phenylethyl)chromone derivatives in four types of agarwood “Qi-Nan”. Chin. J. Trop. Crops 2014, 35, 1235–1243. [Google Scholar]
- Li, W.; Chen, H.Q.; Wang, H.; Mei, W.L.; Dai, H.F. Natural products in agarwood and Aquilaria plants: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2020. [CrossRef]
- Azah, M.N.; Husni, S.S.; Mailina, J.; Sahrim, L.; Majid, J.A.; Faridz, Z.M. Classification of agarwood (gaharu) by resin content. J. Trop. For. Sci. 2013, 25, 213–219. [Google Scholar]
- Liu, Y.; Chen, H.; Yang, Y.; Zhang, Z.; Wei, J.; Meng, H.; Chen, W.; Feng, J.; Gan, B.; Chen, X.; et al. Whole-tree agarwood-inducing technique: An efficient novel technique for producing high-quality agarwood in cultivated Aquilaria sinensis trees. Molecules 2013, 18, 3086–3106. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Wei, J.; Zhang, Z.; Chen, B. Analysis on the quality of agarwood produced via the whole-tree agarwood·inducing technique in different area. Mod. Chin. Med. 2014, 16, 183–186. [Google Scholar]
- Ishihara, M.; Tsuneya, T.; Uneyama, K. Components of the volatile concentrate of agarwood. J. Essent. Oil Res. 1993, 5, 283–289. [Google Scholar] [CrossRef]
- Xu, J.F.; Zhu, L.F.; Lu, B.Y.; Liu, Z.J. Study on the chemical constituents of essential oil of Aquilaria sinensis (Lour.) Gilg. Acta Bot. Sin. 1988, 30, 635–638. [Google Scholar]
- Liu, Y.Y.; Wei, J.H.; Gao, Z.H.; Zhang, Z.; Lyu, J.C. A review of quality assessment and grading for agarwood. Chin. Herb. Med. 2017, 9, 22–30. [Google Scholar] [CrossRef]
- Hashimoto, K.; Nakahara, S.; Inoue, T.; Sumida, Y.; Takahashi, M.; Masada, Y. A new chromone from agarwood and pyrolysis products of chromone derivatives. Chem. Pharm. Bull. 1985, 33, 5088–5091. [Google Scholar] [CrossRef]
- Ishihara, M. (Okayama University, Okayama, Japan). Personal communication, 28 March 1993.
- Tissandié, L.; Viciana, S.; Brevard, H.; Meierhenrich, U.J.; Filippi, J.J. Towards a complete characterisation of guaiacwood oil. Phytochemistry 2018, 149, 64–81. [Google Scholar] [CrossRef]
- Nagashima, T.; Kawasaki, I.; Yoshida, T.; Nakanishi, T.; Yoneda, K.; Miura, I. New Sesquiterpenoids from agarwood. In Proceedings of the IXth International Essential Oil Congress, Singapore, 15 August 1983; p. 12. [Google Scholar]
- Ishihara, M.; Kitaura., T. Method for producing optically active dihydrokaranone. JP 2004189643, 8 July 2004. [Google Scholar]
- Ishihara, M.; Kitaura., T. Method for preparing optically active karanone. JP 2004231519, 19 August 2004. [Google Scholar]
- Naf, R.; Velluz, A.; Brauchli, R.; Thommen, W. Agarwood oil (Aquilaria agallocha Roxb.). Its composition and eight new valencane-, eremophilane- and vetispirane- derivatives. Flavour Frag. J. 1995, 10, 147. [Google Scholar] [CrossRef]
- Sakurai, K.; Kitahara, T.; Mori, K. Stereocontrolled synthesis of (−)-prezizanol,(−)-prezizaene, their epimers and (−)-allokhusiol. Tetrahedron 1990, 46, 761. [Google Scholar] [CrossRef]
- Nagashima, T.; Yoshida, T. (+)- Or (-)-7-hydroxymethyl-2,6,6-trimethyltricyclo[6,2,1,01,5]undecane. US Patent 4,444,982, 24 April 1984. [Google Scholar]
- Yang, D.L. Study on Fragrant Constituents in Agarwood ‘Qi-Nan’ and Quality Evaluation of Agarwood. Ph.D. Thesis, Hainan University, Haikou, China, 1 May 2014. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
