Synthesis and Biological Evaluation of 99mTc(I) Tricarbonyl Complexes Dual-Targeted at Tumoral Mitochondria
Abstract
1. Introduction
2. Results
2.1. Chemistry
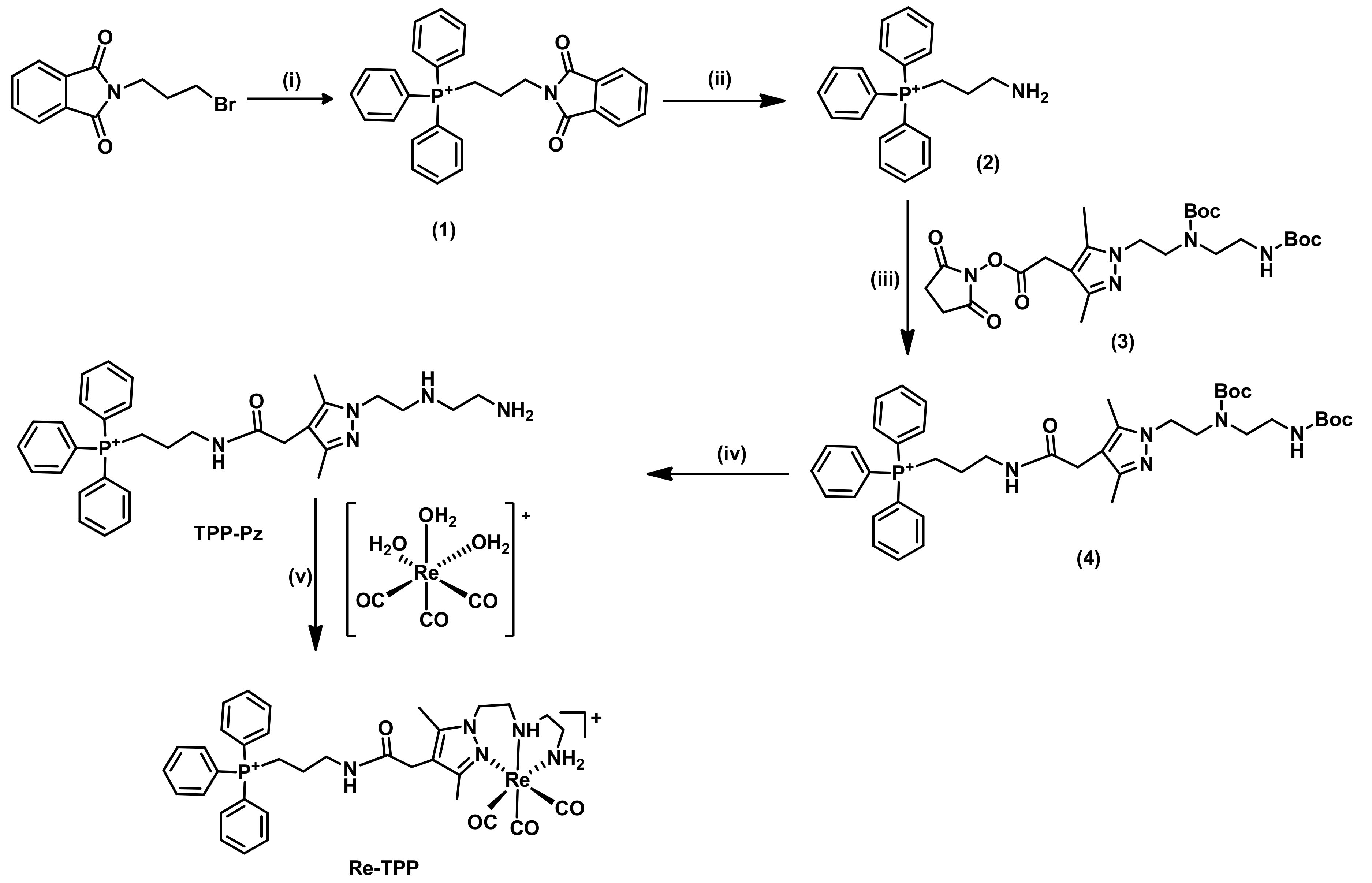
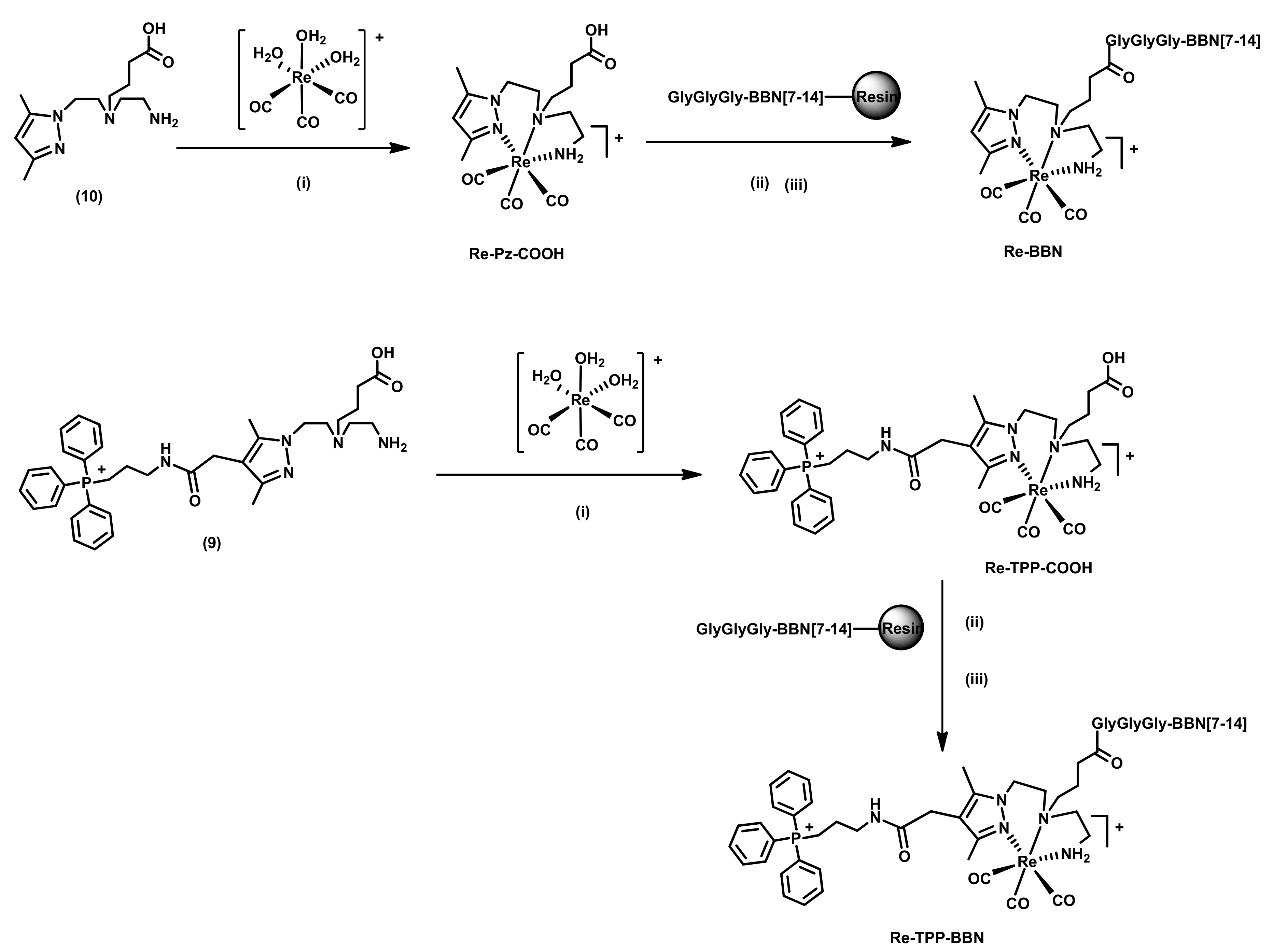
2.1.1. Synthesis and Characterization of Single-Targeted TPP Derivatives: Chelators and Re Complexes
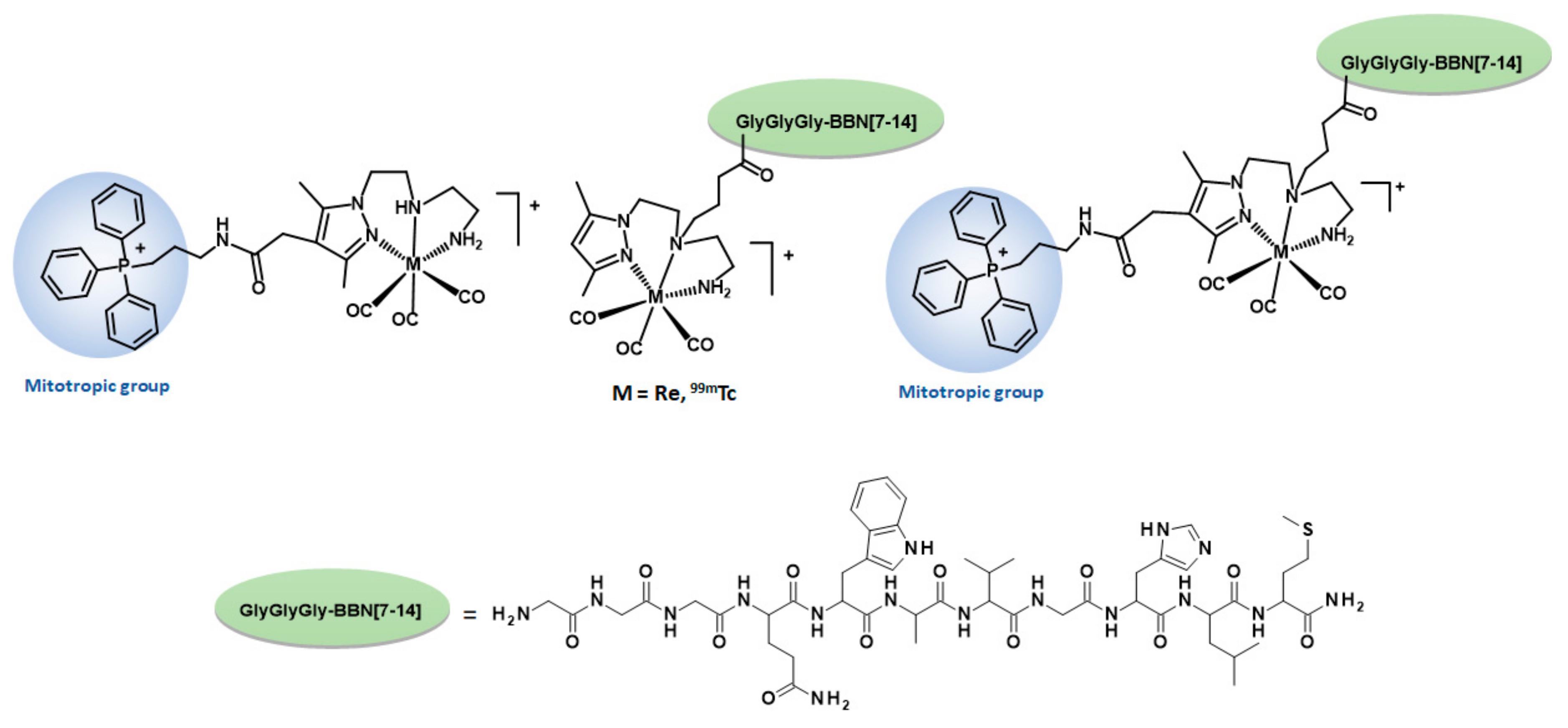
2.1.2. Synthesis and Characterization of BBN Derivatives: Peptides, Chelators, and Re Complexes
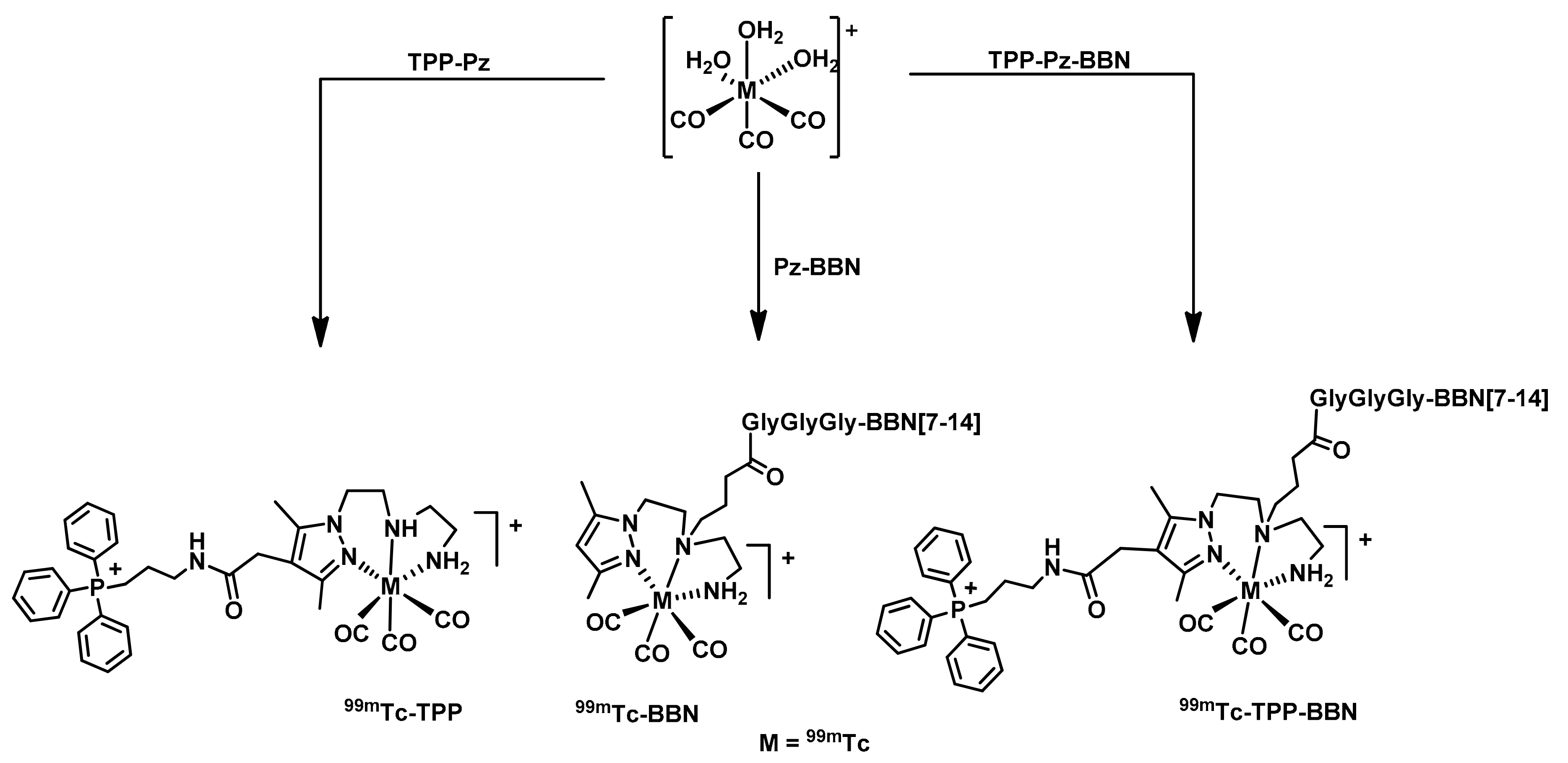
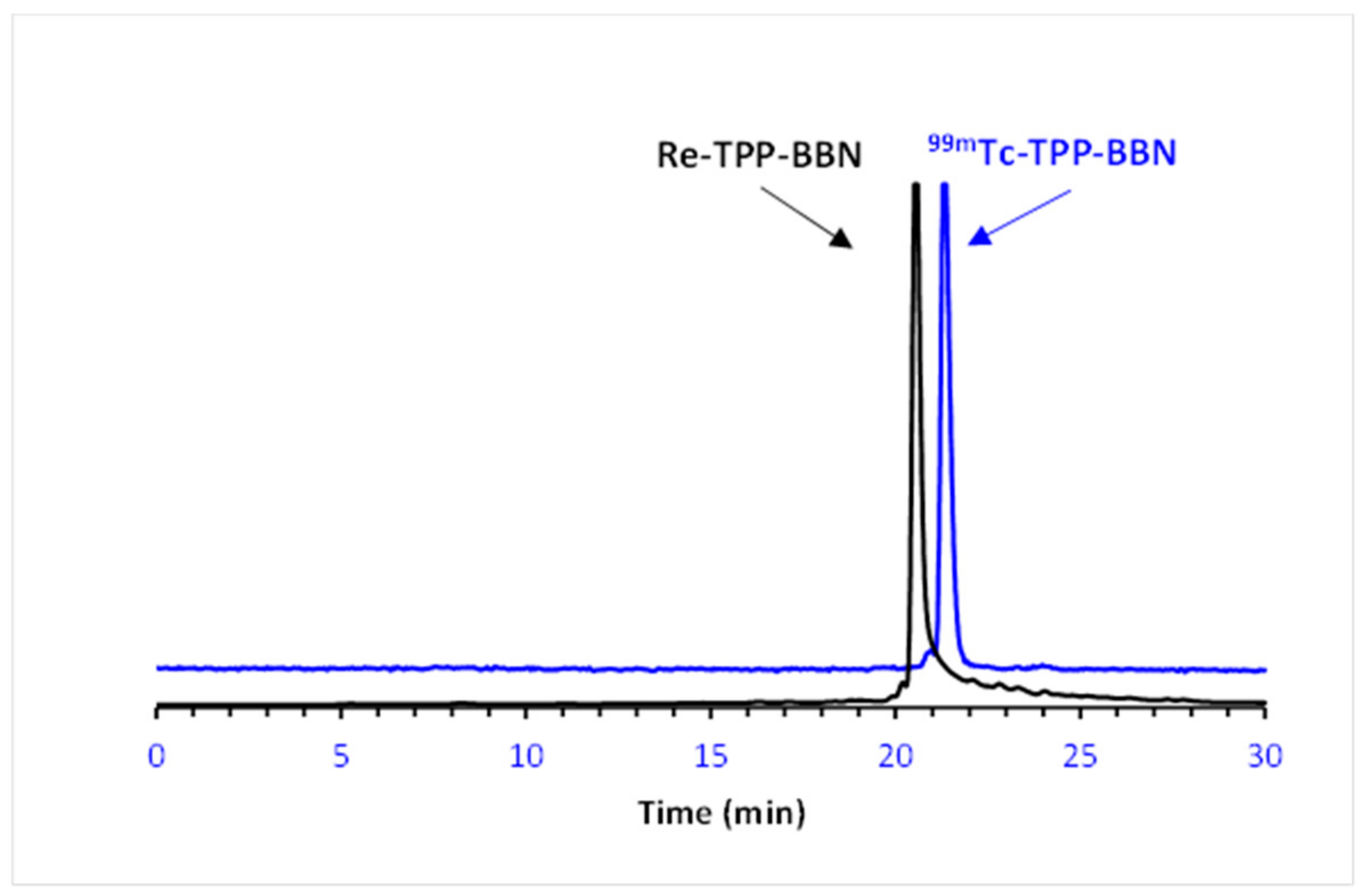
2.2. Radiochemistry
2.2.1. Synthesis of 99mTc(I) Complexes
2.2.2. In Vitro Stability and Lipophilicity of the 99mTc(I) Complexes
2.3. Biological Studies
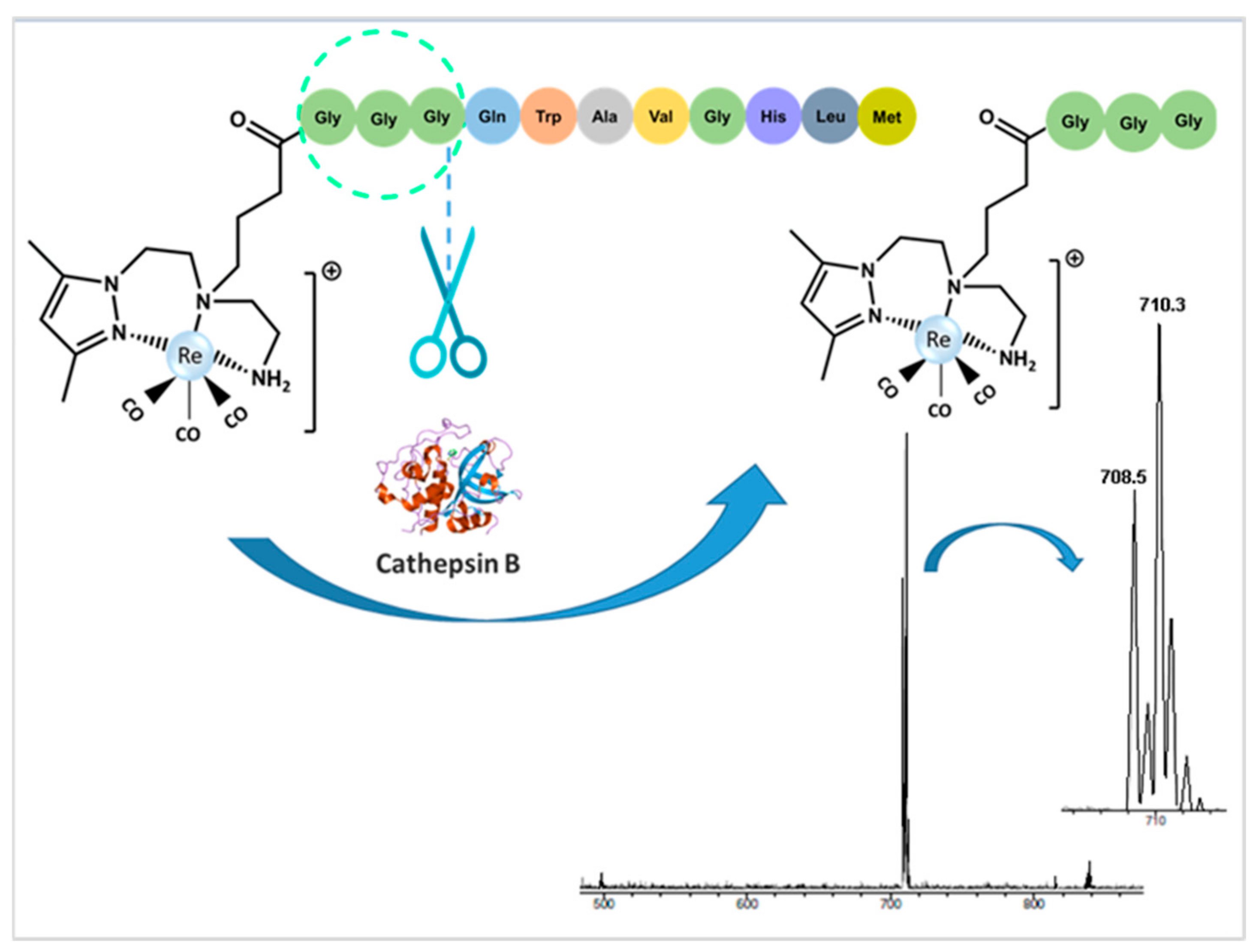
2.3.1. In Vitro Enzymatic Assays
2.3.2. Cellular Uptake and Internalization Studies
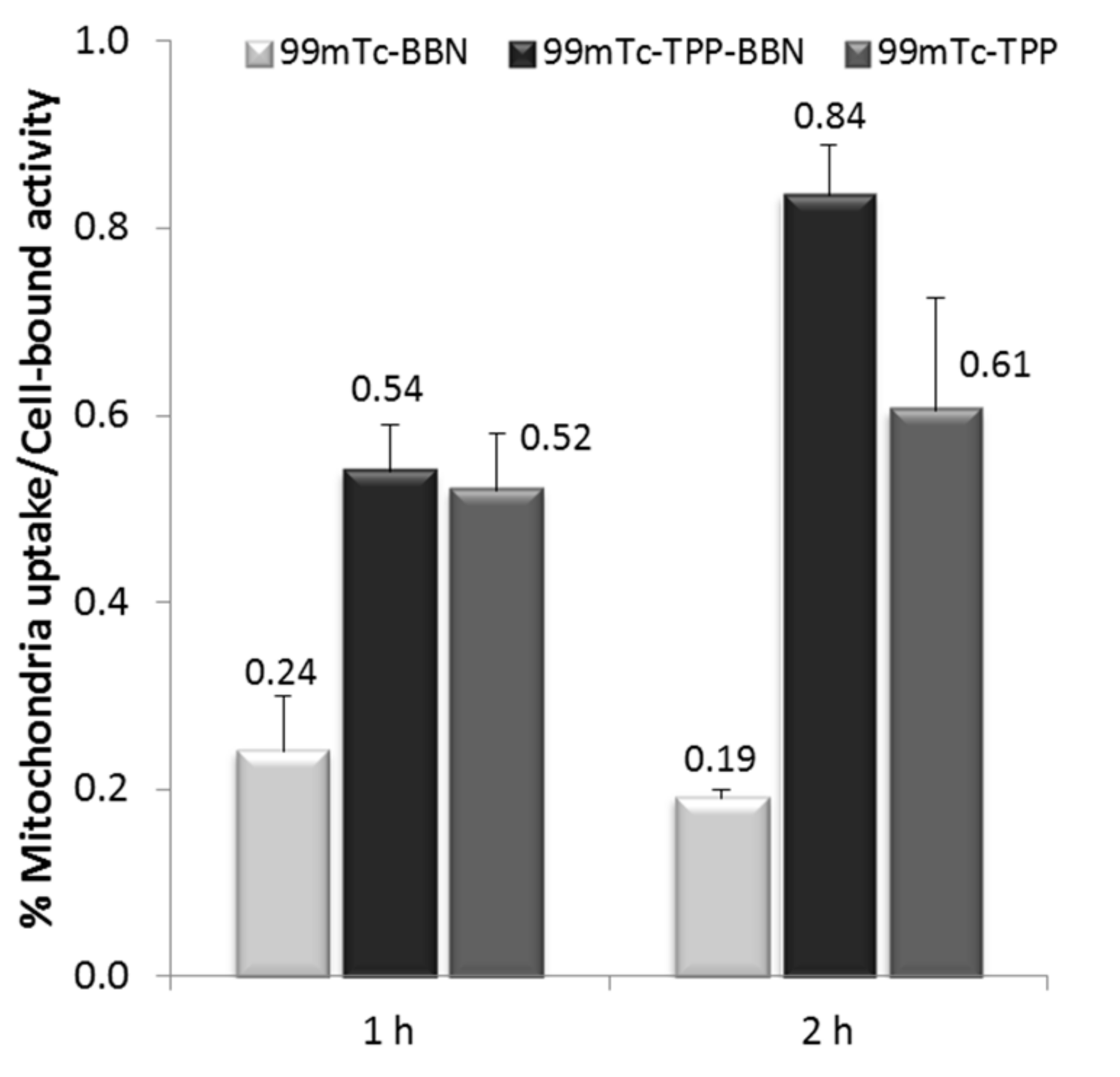
2.3.3. Mitochondria Uptake
2.3.4. Preliminary Radiobiological Studies: Clonogenic Assay
3. Discussion
4. Materials and Methods
4.1. Synthesis of Triphenylphosphonium Derivatives
4.1.1. (3-Phtalimidylpropyl) triphenylphosphonium (1)
4.1.2. (3-Aminopropyl)triphenylphosphonium (2)
4.1.3. tert-Butyl(2-((tert-butoxycarbonyl)amino)ethyl)(2-(3,5-dimethyl-4-(2-oxo-2-((3-(triphenylphosphoranyl)propyl)amino)ethyl)-1H-pyrazol-1-yl)ethyl)carbamate (4)
4.1.4. 2-(1-(2-((2-Aminoethyl)amino)ethyl)-3,5-dimethyl-1H-pyrazol-4-yl)-N-(3-(triphenylphosphoranyl)propyl)acetamide (TPP-Pz)
4.1.5. Ethyl 4-((2-((tert-butoxycarbonyl)amino)ethyl)(2-(3,5-dimethyl-4-(2-oxo-2-((3-(triphenylphospho ranyl) propyl)amino)ethyl)-1H-pyrazol-1-yl)ethyl)amino)butanoate (7)
4.1.6. 4-((2-((tert-Butoxycarbonyl)amino)ethyl)(2-(3,5-dimethyl-4-(2-oxo-2-((3-(triphenylphosphoranyl) propyl)amino)ethyl)-1H-pyrazol-1-yl)ethyl)amino)butanoic acid (8)
4.1.7. 4-((2-Aminoethyl)(2-(3,5-dimethyl-4-(2-oxo-2-((3-(triphenylphosphoranyl)propyl)amino)-ethyl)-1H-pyrazol-1-yl)ethyl)amino)butanoic acid (9)
4.2. Synthesis of Re(I) Complexes
4.3. Synthesis of Peptide Derivatives
4.4. Synthesis and In Vitro Evaluation of 99mTc(I) Complexes
4.4.1. Radiolabeling Procedure
4.4.2. In Vitro Stability Studies
4.4.3. Lipophilicity Determination
4.5. Enzymatic Assays
4.6. Cell Studies
4.6.1. Cell Culture
4.6.2. Internalization and Cellular Uptake
4.6.3. Mitochondrial Uptake
4.6.4. Clonogenic Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability: Not available. |
References
- Goldsmith, S.J. Targeted Radionuclide Therapy: A Historical and Personal Review. Semin. Nucl. Med. 2020, 50, 87–97. [Google Scholar] [CrossRef]
- Kratochwil, C.; Haberkorn, U.; Giesel, F.L. Radionuclide Therapy of Metastatic Prostate Cancer. Semin. Nucl. Med. 2019, 49, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger electrons for cancer therapy—A review. EJNMMI Radiopharm. Chem. 2019, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Hindié, E.; Zanotti-Fregonara, P.; Quinto, M.A.; Morgat, C.; Champion, C. Dose Deposits from 90Y, 177Lu, 111In, and 161Tb in Micrometastases of Various Sizes: Implications for Radiopharmaceutical Therapy. J. Nucl. Med. 2016, 57, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Esteves, T.; Marques, F.; Paulo, A.; Rino, J.; Nanda, P.; Smith, C.J.; Santos, I. Nuclear targeting with cell-specific multifunctional tricarbonyl M(I) (M is Re, 99mTc) complexes: Synthesis, characterization, and cell studies. J. Biol. Inorg. Chem. 2011, 16, 1141–1153. [Google Scholar] [CrossRef]
- Pereira, E.; do Quental, L.; Palma, E.; Oliveira, M.C.; Mendes, F.; Raposinho, P.; Correia, I.; Lavrado, J.; Di Maria, S.; Belchior, A.; et al. Evaluation of Acridine Orange Derivatives as DNA-Targeted Radiopharmaceuticals for Auger Therapy: Influence of the Radionuclide and Distance to DNA. Sci. Rep. 2017, 7, 42544. [Google Scholar] [CrossRef] [PubMed]
- Belchior, A.; Di Maria, S.; Fernandes, C.; Vaz, P.; Paulo, A.; Raposinho, P. Radiobiological and dosimetric assessment of DNA-intercalated 99mTc-complexes bearing acridine orange derivatives. EJNMMI Res. 2020, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Bavelaar, B.M.; Lee, B.Q.; Gill, M.R.; Falzone, N.; Vallis, K.A. Subcellular Targeting of Theranostic Radionuclides. Front. Pharmacol. 2018, 9, 996. [Google Scholar] [CrossRef]
- Galley, H.F. Bench-to-Bedside review: Targeting antioxidants to mitochondria in sepsis. Crit. Care 2010, 14, 230. [Google Scholar] [CrossRef]
- Taylor, R.W.; Turnbull, D.M. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 2005, 6, 389–402. [Google Scholar] [CrossRef]
- Battogtokh, G.; Choi, Y.S.; Kang, D.S.; Park, S.J.; Shim, M.S.; Huh, K.M.; Cho, Y.Y.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondria-targeting drug conjugates for cytotoxic, anti-oxidizing and sensing purposes: Current strategies and future perspectives. Acta Pharmaceutica Sinica B 2018, 8, 862–880. [Google Scholar] [CrossRef] [PubMed]
- Kam, W.W.; Banati, R.B. Effects of ionizing radiation on mitochondria. Free Radic. Biol. Med. 2013, 65, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wei, H.; He, Q.; Ferreira, C.A.; Kutyreff, C.J.; Ni, D.; Rosenkrans, Z.T.; Cheng, L.; Yu, F.; Engle, J.W.; et al. Efficient Uptake of 177Lu-Porphyrin-PEG Nanocomplexes by Tumor Mitochondria for Multimodal-Imaging-Guided Combination Therapy. Angewandte Chemie Int. Ed. Engl. 2018, 57, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, J.; Zhou, Y. Mitochondrial-Targeted Molecular Imaging in Cardiac Disease. BioMed Res. Int. 2017, 2017, 5246853. [Google Scholar] [CrossRef]
- Sharma, V.; Piwnica-Worms, D. Monitoring Multidrug Resistance P-Glycoprotein Drug Transport Activity with Single-Photon Emission Computed Tomography and Positron Emission Tomography Radiopharmaceuticals. In Contrast Agents III: Radiopharmaceuticals—From Diagnostics to Therapeutics; Krause, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 155–178. [Google Scholar]
- Crane, P.; Laliberté, R.; Heminway, S.; Thoolen, M.; Orlandi, C. Effect of mitochondrial viability and metabolism on technetium-99m-sestamibi myocardial retention. Eur. J. Nucl. Med. 1993, 20, 20–25. [Google Scholar] [CrossRef]
- Mendes, F.; Gano, L.; Fernandes, C.; Paulo, A.; Santos, I. Studies of the myocardial uptake and excretion mechanisms of a novel 99mTc heart perfusion agent. Nucl. Med. Biol. 2012, 39, 207–213. [Google Scholar] [CrossRef]
- Mendes, F.; Gano, L.; Grilo, J.; Cunha, S.; Fernandes, C.; Paulo, A. Imaging probes for non-invasive tumoral detection and functional monitoring of cancer multidrug resistance. Cancer Drug Resist. 2020, 3, 209–224. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Yang, C.-T.; Wang, J.; Wang, L.; Li, Z.-B.; Chen, X.; Liu, S. Effects of targeting moiety, linker, bifunctional chelator, and molecular charge on biological properties of 64Cu-labeled triphenylphosphonium cations. J. Med. Chem. 2008, 51, 2971–2984. [Google Scholar] [CrossRef]
- Wang, J.; Yang, C.-T.; Kim, Y.-S.; Sreerama, S.G.; Cao, Q.; Li, Z.-B.; He, Z.; Chen, X.; Liu, S. 64Cu-Labeled Triphenylphosphonium and Triphenylarsonium Cations as Highly Tumor-Selective Imaging Agents. J. Med. Chem. 2007, 50, 5057–5069. [Google Scholar] [CrossRef]
- Yang, C.-T.; Kim, Y.-S.; Wang, J.; Wang, L.; Shi, J.; Li, Z.-B.; Chen, X.; Fan, M.; Li, J.-J.; Liu, S. 64Cu-Labeled 2-(Diphenylphosphoryl)ethyldiphenylphosphonium Cations as Highly Selective Tumor Imaging Agents: Effects of Linkers and Chelates on Radiotracer Biodistribution Characteristics. Bioconjug. Chem. 2008, 19, 2008–2022. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S. 64Cu-Labeled Phosphonium Cations as PET Radiotracers for Tumor Imaging. Bioconjug. Chem. 2011, 22, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Cho, H.; Chen, H.H.; Panagia, M.; Sosnovik, D.E.; Josephson, L. Fluorescent and radiolabeled triphenylphosphonium probes for imaging mitochondria. Chem. Commun. 2013, 49, 10361–10363. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kim, H.S.; Reder, S.; Zheng, J.H.; Herz, M.; Higuchi, T.; Pyo, A.Y.; Bom, H.S.; Schwaiger, M.; Min, J.J. Comparison of 18F-Labeled Fluoroalkylphosphonium Cations with 13N-NH3 for PET Myocardial Perfusion Imaging. J. Nucl. Med. 2015, 56, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Momcilovic, M.; Jones, A.; Bailey, S.T.; Waldmann, C.M.; Li, R.; Lee, J.T.; Abdelhady, G.; Gomez, A.; Holloway, T.; Schmid, E.; et al. In vivo imaging of mitochondrial membrane potential in non-small-cell lung cancer. Nature 2019, 575, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lopez, M.; Hardy, M.; McAllister, D.M.; Kalyanaraman, B.; Zhao, M. A 99mTc-Labeled triphenylphosphonium derivative for the early detection of breast tumors. Cancer Biother. Radiopharm. 2009, 24, 579–587. [Google Scholar] [CrossRef]
- Moura, C.; Mendes, F.; Gano, L.; Santos, I.; Paulo, A. Mono- and dicationic Re(I)/99mTc(I) tricarbonyl complexes for the targeting of energized mitochondria. J. Inorg. Biochem. 2013, 123, 34–45. [Google Scholar] [CrossRef]
- Paparidis, G.; Akrivou, M.; Tsachouridou, V.; Shegani, A.; Vizirianakis, I.S.; Pirmettis, I.; Papadopoulos, M.S.; Papagiannopoulou, D. Synthesis and evaluation of 99mTc/Re-tricarbonyl complexes of the triphenylphosphonium cation for mitochondrial targeting. Nucl. Med. Biol. 2018, 57, 34–41. [Google Scholar] [CrossRef]
- Kyriazopoulos, A.; Alexiou, A.-L.; Miliotou, A.; Papadopoulou, L.; Hatzidimitriou, A.; Papagiannopoulou, D. Effect of the triphenylphosphonium cation on the biological properties of new rhenium and technetium-99m fac-[M(CO)3(NSN)]±-type complexes: Synthesis, structural characterization, in vitro and in vivo studies. Inorganica Chimica Acta 2020, 511, 119807. [Google Scholar] [CrossRef]
- Singh, R.; Setiady, Y.Y.; Ponte, J.; Kovtun, Y.V.; Lai, K.C.; Hong, E.E.; Fishkin, N.; Dong, L.; Jones, G.E.; Coccia, J.A.; et al. A New Triglycyl Peptide Linker for Antibody-Drug Conjugates (ADCs) with Improved Targeted Killing of Cancer Cells. Mol. Cancer Ther. 2016, 15, 1311–1320. [Google Scholar] [CrossRef]
- Bargh, J.D.; Isidro-Llobet, A.; Parker, J.S.; Spring, D.R. Cleavable linkers in antibody—Drug conjugates. Chem. Soc. Rev. 2019, 48, 4361–4374. [Google Scholar] [CrossRef]
- Morais, G.R.; Paulo, A.; Santos, I. Organometallic Complexes for SPECT Imaging and/or Radionuclide Therapy. Organometallics 2012, 31, 5693–5714. [Google Scholar] [CrossRef]
- Morais, M.; Paulo, A.; Gano, L.; Santos, I.; Correia, J.D.G. Target-Specific Tc(CO)3-complexes for in vivo imaging. J. Organomet. Chem. 2013, 744, 125–139. [Google Scholar] [CrossRef]
- Silva, F.; Fernandes, C.; Campello, M.P.C.; Paulo, A. Metal complexes of tridentate tripod ligands in medical imaging and therapy. Polyhedron 2017, 125, 186–205. [Google Scholar] [CrossRef]
- Moura, C.; Esteves, T.; Gano, L.; Raposinho, P.D.; Paulo, A.; Santos, I. Synthesis, characterization and biological evaluation of tricarbonyl M(i) (M = Re, 99mTc) complexes functionalized with melanin-binding pharmacophores. N. J. Chem. 2010, 34, 2564–2578. [Google Scholar] [CrossRef]
- Alves, S.; Paulo, A.; Correia, J.D.G.; Domingos, Â.; Santos, I. Coordination capabilities of pyrazolyl containing ligands towards the fac-[Re(CO)3]+ moiety. J. Chem. Soc. Dalton Trans. 2002, 4714–4719. [Google Scholar] [CrossRef]
- Alves, S.; Correia, J.D.G.; Santos, I.; Veerendra, B.; Sieckman, G.L.; Hoffman, T.J.; Rold, T.L.; Figueroa, S.D.; Retzloff, L.; McCrate, J.; et al. Pyrazolyl conjugates of bombesin: A new tridentate ligand framework for the stabilization of fac-[M(CO)3]+ moiety. Nucl. Med. Biol. 2006, 33, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rentero, S.; Grijalvo, S.; Peñuelas, G.; Fàbrega, C.; Eritja, R. Thioctic acid derivatives as building blocks to incorporate DNA oligonucleotides onto gold nanoparticles. Molecules 2014, 19, 10495–10523. [Google Scholar] [CrossRef]
- Carvalho, P.A.; Chiu, M.L.; Kronauge, J.F.; Kawamura, M.; Jones, A.G.; Holman, B.L.; Piwnica-Worms, D. Subcellular distribution and analysis of technetium-99m-MIBI in isolated perfused rat hearts. J. Nucl. Med. 1992, 33, 1516–1522. [Google Scholar]
- Matsui, T.; Nuryadi, E.; Komatsu, S.; Hirota, Y.; Shibata, A.; Oike, T.; Nakano, T. Robustness of Clonogenic Assays as a Biomarker for Cancer Cell Radiosensitivity. Int. J. Mol. Sci. 2019, 20, 4148. [Google Scholar] [CrossRef]
- Agorastos, N.; Borsig, L.; Renard, A.; Antoni, P.; Viola, G.; Spingler, B.; Kurz, P.; Alberto, R. Cell-Specific and Nuclear Targeting with [M(CO)3]+ (M=99mTc, Re)-Based Complexes Conjugated to Acridine Orange and Bombesin. Chem. Eur. J. 2007, 13, 3842–3852. [Google Scholar] [CrossRef]
- Zelenka, K.; Borsig, L.; Alberto, R. Metal complex mediated conjugation of peptides to nucleus targeting acridine orange: A modular concept for dual-modality imaging agents. Bioconjug. Chem. 2011, 22, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Ginj, M.; Hinni, K.; Tschumi, S.; Schulz, S.; Maecke, H.R. Trifunctional somatostatin-based derivatives designed for targeted radiotherapy using auger electron emitters. J. Nucl. Med. 2005, 46, 2097–2103. [Google Scholar] [PubMed]
- Kluba, C.A.; Bauman, A.; Valverde, I.E.; Vomstein, S.; Mindt, T.L. Dual-Targeting conjugates designed to improve the efficacy of radiolabeled peptides. Org. Biomol. Chem. 2012, 10, 7594–7602. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.F.; Filipovska, A.; Smith, R.A.; Gait, M.J.; Murphy, M.P. Cell-Penetrating peptides do not cross mitochondrial membranes even when conjugated to a lipophilic cation: Evidence against direct passage through phospholipid bilayers. Biochem. J. 2004, 383, 457–468. [Google Scholar] [CrossRef]
- Lazarova, N.; James, S.; Babich, J.; Zubieta, J. A convenient synthesis, chemical characterization and reactivity of [Re(CO)3(H2O)3]Br: The crystal and molecular structure of [Re(CO)3(CH3CN)2Br]. Inorg. Chem. Commun. 2004, 7, 1023–1026. [Google Scholar] [CrossRef]
- Alves, S.; Paulo, A.; Correia, J.D.G.; Gano, L.; Smith, C.J.; Hoffman, T.J.; Santos, I. Pyrazolyl Derivatives as Bifunctional Chelators for Labeling Tumor-Seeking Peptides with the fac-[M(CO)3]+ Moiety (M = 99mTc, Re): Synthesis, Characterization, and Biological Behavior. Bioconjug. Chem. 2005, 16, 438–449. [Google Scholar] [CrossRef]
- Troutner, D.E.; Volkert, W.A.; Hoffman, T.J.; Holmes, R.A. A neutral lipophilic complex of 99mTc with a multidentate amine oxime. Int. J. Appl. Radiat. Isotopes 1984, 35, 467–470. [Google Scholar] [CrossRef]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]



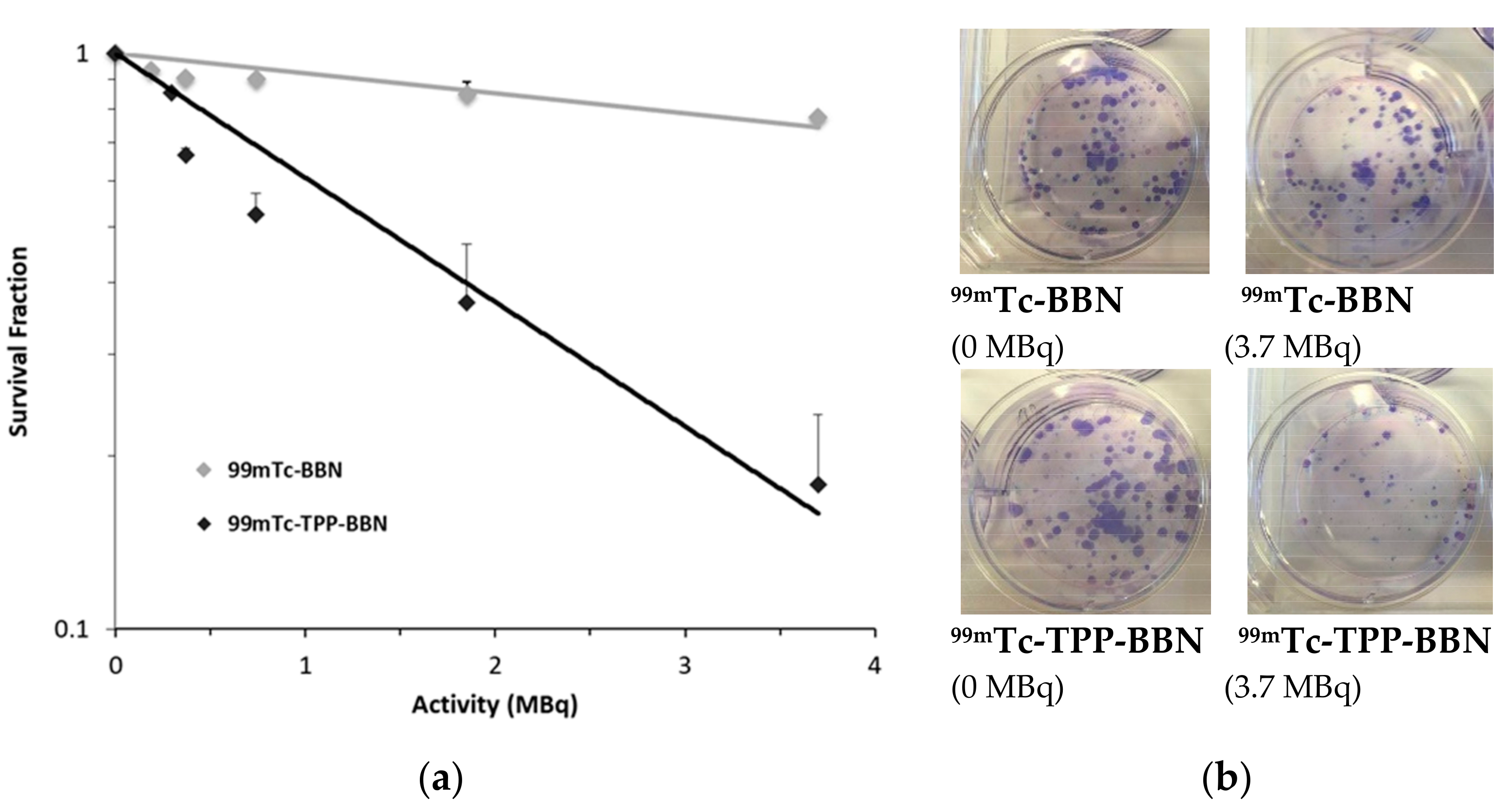
| Radiocomplex | LD50 | LD20 | LD10 |
|---|---|---|---|
| (MBq) | (MBq) | (MBq) | |
| 99mTc-TPP-BBN | 1.39 | 0.45 | 0.21 |
| 99mTc-BBN | >5 | 2.79 | 1.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo, D.; Fernandes, C.; Silva, F.; Palma, E.; Raposinho, P.; Belchior, A.; Vaz, P.; Paulo, A. Synthesis and Biological Evaluation of 99mTc(I) Tricarbonyl Complexes Dual-Targeted at Tumoral Mitochondria. Molecules 2021, 26, 441. https://doi.org/10.3390/molecules26020441
Figueiredo D, Fernandes C, Silva F, Palma E, Raposinho P, Belchior A, Vaz P, Paulo A. Synthesis and Biological Evaluation of 99mTc(I) Tricarbonyl Complexes Dual-Targeted at Tumoral Mitochondria. Molecules. 2021; 26(2):441. https://doi.org/10.3390/molecules26020441
Chicago/Turabian StyleFigueiredo, Diogo, Célia Fernandes, Francisco Silva, Elisa Palma, Paula Raposinho, Ana Belchior, Pedro Vaz, and António Paulo. 2021. "Synthesis and Biological Evaluation of 99mTc(I) Tricarbonyl Complexes Dual-Targeted at Tumoral Mitochondria" Molecules 26, no. 2: 441. https://doi.org/10.3390/molecules26020441
APA StyleFigueiredo, D., Fernandes, C., Silva, F., Palma, E., Raposinho, P., Belchior, A., Vaz, P., & Paulo, A. (2021). Synthesis and Biological Evaluation of 99mTc(I) Tricarbonyl Complexes Dual-Targeted at Tumoral Mitochondria. Molecules, 26(2), 441. https://doi.org/10.3390/molecules26020441









