Synthetic Routes to Coumarin(Benzopyrone)-Fused Five-Membered Aromatic Heterocycles Built on the α-Pyrone Moiety. Part 1: Five-Membered Aromatic Rings with One Heteroatom
Abstract
1. Introduction

2. Synthesis of Benzopyrone-Fused, Five-Membered Aromatic Heterocycles
2.1. Five-Membered Aromatic Rings with One Heteroatom
2.1.1. Furans
4H-Furo[2,3-c]benzopyran-4-one
- Furan Construction
- Pyrone Construction
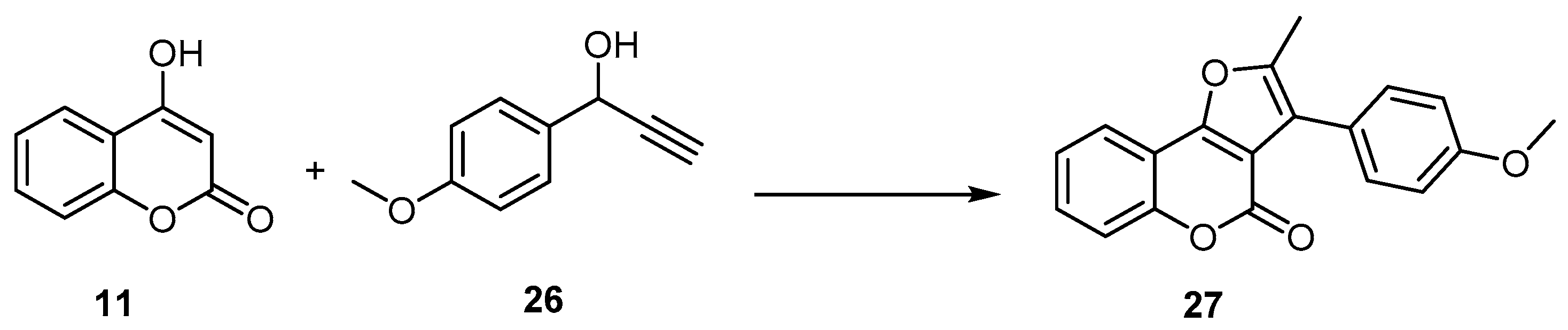
4H-Furo[3,4-c]benzopyran-4-one
- Furan and Pyrone Construction
4H-Furo[3,2-c]benzopyran-4-one
- Furan Construction
- Pyrone Construction
2.1.2. Pyrroles
3H,4H[1]Benzopyrano[3,4-b]pyrrol-4-one
- Pyrrole Construction
3H,4H[1]Benzopyrano[3,4-e]pyrrol-4-one
- Pyrrole Construction
[1]Benzopyrano[4,3-b]pyrrol-4-one
- Pyrrole Construction
2.1.3. Thiophenes
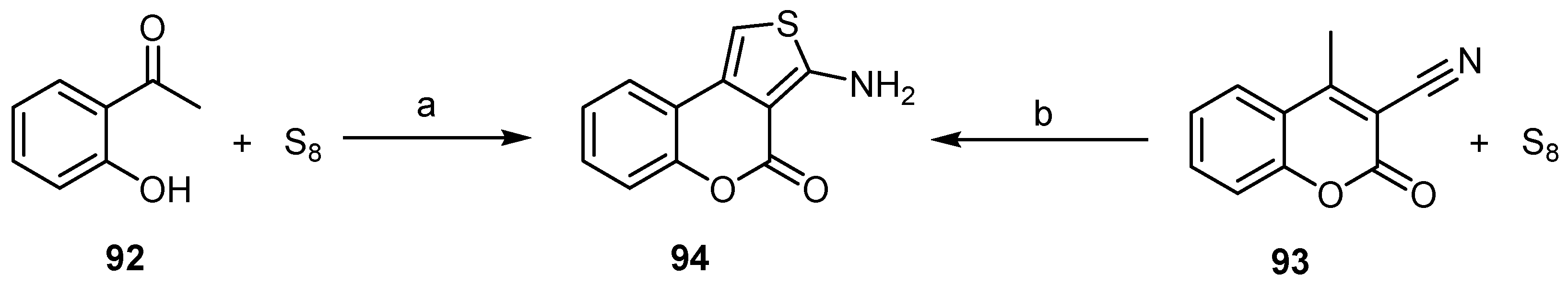
4H-Thieno[2,3-c]benzopyran-4-one
- Thiophene Construction
- Pyrone Construction
4H-Thieno[3,4-c]benzopyran-4-one
- Thiophene Construction
4H-Thieno[3,2-c][1]benzopyran-4(4H)-one
- Thiophene Construction
- Pyrone Construction
2.1.4. Selenophene
4H-Selenophen[2,3-c] and [3,2-c]benzopyran-4-ones
Author Contributions
Funding
Conflicts of Interest
References
- Molnar, M.; Lončarić, M.; Kovač, M. Green Chemistry Approaches to the Synthesis of Coumarin Derivatives. Curr. Org. Chem. 2020, 24, 4–43. [Google Scholar] [CrossRef]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An Important Class of Phytochemicals. Phytochem. Isol. Characterisation Role Hum. Health 2015. [Google Scholar] [CrossRef]
- Lončarić, M.; Gašo-Sokač, D.; Jokić, S.; Molnar, M. Recent Advances in the Synthesis of Coumarin Derivatives from Different Starting Materials. Biomolecules 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; Helal, M.H.; Gouda, M.A.; Ammar, Y.A.; El-Gaby, M.S.A.; Abbas, S.Y. An Overview on Synthetic Strategies to Coumarins. Synth. Commun. 2018, 48, 1534–1550. [Google Scholar] [CrossRef]
- Irfan, A.; Rubab, L.; Rehman, M.U.; Anjum, R.; Ullah, S.; Marjana, M.; Qadeer, S.; Sana, S. Coumarin Sulfonamide Derivatives: An Emerging Class of Therapeutic Agents. Heterocycl. Commun. 2020, 26, 46–59. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on Natural Coumarin Lead Compounds for Their Pharmacological Activity. BioMed. Res. Int. 2013, 2013, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Pandey, A.; Manvati, S. Coumarin: An Emerging Antiviral Agent. Heliyon 2020, 6, e03217. [Google Scholar] [CrossRef]
- Chattopadhyay, D.S.K. Pharmacological Potentiality of Coumarins as Anti-Viral Agent. Int. J. Res. Anal. Rev. 2018, 5, 11. [Google Scholar]
- Bizzarri, B.M.; Botta, L.; Capecchi, E.; Celestino, I.; Checconi, P.; Palamara, A.T.; Nencioni, L.; Saladino, R. Regioselective IBX-Mediated Synthesis of Coumarin Derivatives with Antioxidant and Anti-Influenza Activities. J. Nat. Prod. 2017, 80, 3247–3254. [Google Scholar] [CrossRef]
- Hassan, M.Z.; Osman, H.; Ali, M.A.; Ahsan, M.J. Therapeutic Potential of Coumarins as Antiviral Agents. Eur. J. Med. Chem. 2016, 123, 236–255. [Google Scholar] [CrossRef]
- Menezes, J.C.; Diederich, M. Translational Role of Natural Coumarins and Their Derivatives as Anticancer Agents. Future Med. Chem. 2019, 11, 1057–1082. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kohli, S.; Sandhu, S.; Bansal, Y.; Bansal, G. Coumarin: A Promising Scaffold for Anticancer Agents. Anticancer Agents Med. Chem. 2015, 15, 1032–1048. [Google Scholar] [CrossRef] [PubMed]
- Majnooni, M.B.; Fakhri, S.; Smeriglio, A.; Trombetta, D.; Croley, C.R.; Bhattacharyya, P.; Sobarzo-Sánchez, E.; Farzaei, M.H.; Bishayee, A. Antiangiogenic Effects of Coumarins against Cancer: From Chemistry to Medicine. Molecules 2019, 24, 4278. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, K.; Bhagat, J.; Gupta, M.K.; Singh, J.V.; Gulati, H.K.; Singh, A.; Kaur, K.; Kaur, G.; Sharma, S.; Rana, A.; et al. Design, Synthesis, Antimicrobial Evaluation, and Molecular Modeling Studies of Novel Indolinedione–Coumarin Molecular Hybrids. ACS Omega 2019, 4, 8720–8730. [Google Scholar] [CrossRef]
- Al-Majed, Y.K.; Kadhum, A.H.; Al-Amier, A.A.; Mohama, A. Coumarins: The Antimicrobial Agents. Syst. Rev. Pharm. 2017, 8, 62–70. [Google Scholar] [CrossRef]
- Arsenyan, P.; Vasiljeva, J.; Shestakova, I.; Domracheva, I.; Jaschenko, E.; Romanchikova, N.; Leonchiks, A.; Rudevica, Z.; Belyakov, S. Selenopheno[3,2-c]- and [2,3-c]Coumarins: Synthesis, Cytotoxicity, Angiogenesis Inhibition, and Antioxidant Properties. C. R. Chim. 2015, 18, 399–409. [Google Scholar] [CrossRef]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef]
- Boisde, P.M.; Meuly, W.C. Coumarin. In Kirk-Othmer Encyclopedia of Chemical Technology; American Cancer Society: Atlanta, GA, USA, 2000; ISBN 978-0-471-23896-6. [Google Scholar]
- Api, A.M.; Belmonte, F.; Belsito, D.; Biserta, S.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; et al. RIFM Fragrance Ingredient Safety Assessment, Coumarin, CAS Registry Number 91-64-5. Food Chem. Toxicol. 2019, 130, 110522. [Google Scholar] [CrossRef]
- Coumarin|Cosmetics Info. Available online: https://cosmeticsinfo.org/ingredient/coumarin (accessed on 18 September 2020).
- Lončar, M.; Jakovljević, M.; Šubarić, D.; Pavlić, M.; Buzjak Služek, V.; Cindrić, I.; Molnar, M. Coumarins in Food and Methods of Their Determination. Foods 2020, 9, 645. [Google Scholar] [CrossRef]
- Krüger, S.; Winheim, L.; Morlock, G.E. Planar Chromatographic Screening and Quantification of Coumarin in Food, Confirmed by Mass Spectrometry. Food Chem. 2018, 239, 1182–1191. [Google Scholar] [CrossRef]
- Sun, X.; Liu, T.; Sun, J.; Wang, X. Synthesis and Application of Coumarin Fluorescence Probes. RSC Adv. 2020, 10, 10826–10847. [Google Scholar] [CrossRef]
- Sarih, N.M.; Ciupa, A.; Moss, S.; Myers, P.; Slater, A.G.; Abdullah, Z.; Tajuddin, H.A.; Maher, S. Furo[3,2-c]Coumarin-Derived Fe3+ Selective Fluorescence Sensor: Synthesis, Fluorescence Study and Application to Water Analysis. Sci. Rep. 2020, 10, 7421. [Google Scholar] [CrossRef] [PubMed]
- Akchurin, I.O.; Yakhutina, A.I.; Bochkov, A.Y.; Solovjova, N.P.; Traven, V.F. Synthesis of Novel Push-Pull Fluorescent Dyes—7-(Diethylamino)Furo[3,2-c]Coumarin and 7-(Diethylamino)Thieno[3,2-c]Coumarin Derivatives. Heterocycl. Commun. 2018, 24, 85–91. [Google Scholar] [CrossRef]
- Shi, L.; Yu, H.; Zeng, X.; Yang, S.; Gong, S.; Xiang, H.; Zhang, K.; Shao, G. A Novel Ratiometric Fluorescent Probe Based on Thienocoumarin and Its Application for the Selective Detection of Hypochlorite in Real Water Samples and in Vivo. New J. Chem. 2020, 44, 6232–6237. [Google Scholar] [CrossRef]
- Cadierno, V. Chapter 4—Metal-Catalyzed Routes for the Synthesis of Furocoumarins and Coumestans. In Green Synthetic Approaches for Biologically Relevant Heterocycles; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 77–100. ISBN 978-0-12-800070-0. [Google Scholar]
- Ram, R.; Krupadanam, S.; Srimannarayana, G. Synthesis of 2-Methyl-9-Oxo-9H-Furo[2,3-c]Benzopyrans and 2-Methyl-3H,4H[1]Benzopyrano[3,4-b]Pyrrol-4-Ones. Synth. Commun. 1998, 28, 2421–2428. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Khan, A.T.; Das, D.P. Facile Regioselective Synthesis of 2-Methyl Furo [3,2-c][1] Benzopyran-4-One 2-Methyl Furo[2,3-c] [1]Benzopyran-4-One. Synth. Commun. 1989, 19, 917–930. [Google Scholar] [CrossRef]
- Pandya, M.K.; Chhasatia, M.R.; Vala, N.D.; Parekh, T.H. Synthesis of Furano[2,3-c] /Pyrrolo[2,3-c]Coumarins and Synthesis of 1(H)-[1]Benzopyrano[3,4-b][1]Benzopyrano[3′,4′-d] Furan-7(H)-Ones /1(H)-[1]Benzopyrano[3,4-b][1]Benzopyrano [3′,4′-d]Pyrrole-7(H)-Ones. J. Drug Deliv. Ther. 2019, 9, 32–42. [Google Scholar] [CrossRef]
- Dong, Y.; Yu, J.-T.; Sun, S.; Cheng, J. Rh(III)-Catalyzed Sequential Ortho -C–H Oxidative Arylation/Cyclization of Sulfoxonium Ylides with Quinones toward 2-Hydroxy-Dibenzo[b,d]Pyran-6-Ones. Chem. Commun. 2020, 56, 6688–6691. [Google Scholar] [CrossRef]
- Brahmbhatt, D.I.; Gajera, J.M.; Patel, C.N.; Pandya, V.P.; Pandya, U.R. First Synthesis of Furo[3,4-c]Coumarins. J. Heterocycl. Chem. 2006, 43, 1699–1702. [Google Scholar] [CrossRef]
- Wang, X.; Nakagawa-Goto, K.; Kozuka, M.; Tokuda, H.; Nishino, H.; Lee, K.-H. Cancer Preventive Agents. Part 6: Chemopreventive Potential of Furanocoumarins and Related Compounds. Pharm. Biol. 2006, 44, 116–120. [Google Scholar] [CrossRef]
- Rajitha, B.; Geetanjali, Y.; Somayajulu, V. Synthesis of Coumestrol Analogs as Possible Antifertility Agents. Indian J. Chem 1986, 25B, 872–873. [Google Scholar]
- Al-Sehemi, A.G.; El-Gogary, S.R. Synthesis and Photooxygenation of Furo[3,2-c]Coumarin Derivatives as Antibacterial and DNA Intercalating Agent. Chin. J. Chem. 2012, 30, 316–320. [Google Scholar] [CrossRef]
- Gorbunov, Y.O.; Mityanov, V.S.; Melekhina, V.G.; Krayushkin, M.M. Synthesis of Novel 4H-Furo[3,2-c]Pyran-4-Ones and 4H-Furo[3,2-c]Chromen-4-Ones. Russ. Chem. Bull. 2018, 67, 304–307. [Google Scholar] [CrossRef]
- Panja, S.K.; Ghosh, J.; Maiti, S.; Bandyopadhyay, C. Synthesis of Furocoumarins and Biscoumarins by an Isocyanide-Induced Multicomponent Reaction: Isocyanide as a Masked Amine. J. Chem. Res. 2012, 36, 222–225. [Google Scholar] [CrossRef]
- Reisch, J. Die Synthese von Hydroxy- und Furo-cumarinderivaten. Archiv. Pharm. 1966, 299, 798–805. [Google Scholar] [CrossRef]
- Rahman, M.-U.; Khan, K.-Z.; Siddiqi, Z.S.; Zaman, A. A Convenient Route for 3-Formyl-4-Hydroxycoumarin and Reactions of 3- Bromo-4-Hydroxycoumarin. J. Chem. Sect. Org. Chem. Incl. Med. Chem. Indian 1990, 29, 941–943. [Google Scholar]
- Kadam, A.; Bellinger, S.; Zhang, W. Atom- and Step-Economic Synthesis of Biaryl-Substituted Furocoumarins, Furoquinolones and Furopyrimidines by Multicomponent Reactions and One-Pot Synthesis. Green Process. Synth. 2013, 2, 491–497. [Google Scholar] [CrossRef]
- Lee, Y.R.; Kim, B.S.; Wang, H.C. Silver(I)/Celite Promoted Oxidative Cycloaddition of 4-Hydroxycoumarin to Olefins. A Facile Synthesis of Dihydrofurocoumarins and Furocoumarins. Tetrahedron 1998, 54, 12215–12222. [Google Scholar] [CrossRef]
- Majumdar, N.; Paul, N.D.; Mandal, S.; de Bruin, B.; Wulff, W.D. Catalytic Synthesis of 2H-Chromenes. ACS Catal. 2015, 5, 2329–2366. [Google Scholar] [CrossRef]
- Nebra, N.; Díaz-Álvarez, A.E.; Díez, J.; Cadierno, V. Expeditious Entry to Novel 2-Methylene-2,3-Dihydrofuro[3,2-c] Chromen-2-Ones from 6-Chloro-4-Hydroxychromen-2-One and Propargylic Alcohols. Molecules 2011, 16, 6470–6480. [Google Scholar] [CrossRef]
- Raffa, G.; Rusch, M.; Balme, G.; Monteiro, N. A Pd-Catalyzed Heteroannulation Approach to 2,3-Disubstituted Furo[3,2-c]Coumarins. Org. Lett. 2009, 11, 5254–5257. [Google Scholar] [CrossRef] [PubMed]
- Medina, F.G.; Marrero, J.G.; Macías-Alonso, M.; González, M.C.; Córdova-Guerrero, I.; Teissier García, A.G.; Osegueda-Robles, S. Coumarin Heterocyclic Derivatives: Chemical Synthesis and Biological Activity. Nat. Prod. Rep. 2015, 32, 1472–1507. [Google Scholar] [CrossRef] [PubMed]
- Nealmongkol, P.; Tangdenpaisal, K.; Sitthimonchai, S.; Ruchirawat, S.; Thasana, N. Cu(I)-Mediated Lactone Formation in Subcritical Water: A Benign Synthesis of Benzopyranones and Urolithins A–C. Tetrahedron 2013, 69, 9277–9283. [Google Scholar] [CrossRef]
- Cheng, G.; Hu, Y. One-Pot Synthesis of Furocoumarins through Cascade Addition–Cyclization–Oxidation. Chem. Commun. 2007, 3285–3287. [Google Scholar] [CrossRef]
- Cheng, G.; Hu, Y. Two Efficient Cascade Reactions to Synthesize Substituted Furocoumarins. J. Org. Chem. 2008, 73, 4732–4735. [Google Scholar] [CrossRef] [PubMed]
- Sanz, R.; Miguel, D.; Martínez, A.; Álvarez-Gutiérrez, J.M.; Rodríguez, F. Brønsted Acid Catalyzed Propargylation of 1,3-Dicarbonyl Derivatives. Synthesis of Tetrasubstituted Furans. Org. Lett. 2007, 9, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, J.; Shen, Q.; Zhou, X. Yb(OTf)3-Catalyzed Propargylation and Allenylation of 1,3-Dicarbonyl Derivatives with Propargylic Alcohols: One-Pot Synthesis of Multi-Substituted Furocoumarin. Tetrahedron 2007, 63, 11636–11643. [Google Scholar] [CrossRef]
- Cadierno, V. A Novel Propargylation/Cycloisomerization Tandem Process Catalyzed by a Ruthenium(II)/Trifluoroacetic Acid System: One-Pot Entry to Fully Substituted Furans from Readily Available Secondary Propargylic Alcohols and 1,3-Dicarbonyl Compounds. Adv. Synth. Catal. 2007, 349, 382–394. [Google Scholar] [CrossRef]
- Cadierno, V.; García-Garrido, S.E.; Gimeno, J.; Nebra, N. Atom-Economic Transformations of Propargylic Alcohols Catalyzed by the 16-Electron Allyl-Ruthenium(II) Complex [Ru(H3-2-C3H4Me)(CO)(Dppf)][SbF6] (Dppf=1,1′-Bis(Diphenylphosphino)Ferrocene). Inorg. Chim. Acta 2010, 363, 1912–1934. [Google Scholar] [CrossRef]
- Conreaux, D.; Belot, S.; Desbordes, P.; Monteiro, N.; Balme, G. Et3N-Induced Demethylation−Annulation of 3-Alkynyl-4-Methoxy-2-Pyridones and Structurally Related Compounds in the Synthesis of Furan-Fused Heterocycles. J. Org. Chem. 2008, 73, 8619–8622. [Google Scholar] [CrossRef]
- Lei, C.; Li, Y.; Mh, X. One-Pot Synthesis of Furocoumarins via Sequential Pd/Cu-Catalyzed Alkynylation and Intramolecular Hydroalkoxylation. Org. Biomol. Chem. 2010, 8, 3073–3077. [Google Scholar] [CrossRef]
- Pham, P.H.; Nguyen, Q.T.D.; Tran, N.K.Q.; Nguyen, V.H.H.; Doan, S.H.; Ha, H.Q.; Truong, T.; Phan, N.T.S. Metal-Free Synthesis of Furocoumarins: An Approach via Iodine-Promoted One-Pot Cyclization between 4-Hydroxycoumarins and Acetophenones: Metal-Free Synthesis of Furocoumarins: An Approach via Iodine-Promoted One-Pot Cyclization between 4-Hydroxycoumarins and Acetophenones. Eur. J. Org. Chem. 2018, 2018, 4431–4435. [Google Scholar] [CrossRef]
- Traven, V.F.; Kravtchenko, D.V.; Chibisova, T.A.; Shorshnev, S.V.; Eliason, R.; Wakefield, D.H. A New Short Way to Furocoumarins. Heterocycl. Commun. 1996, 2. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Bhattacharyya, T. Regioselective Synthesis of Furo[3,2-c][1]Benzopyran-4-One and Furo[3,2-c]Quinolin-4-One. J. Chem. Res. 1997, 244–245. [Google Scholar] [CrossRef]
- Lee, T.-H.; Jayakumar, J.; Cheng, C.-H.; Chuang, S.-C. One Pot Synthesis of Bioactive Benzopyranones through Palladium-Catalyzed C–H Activation and CO Insertion into 2-Arylphenols. Chem. Commun. 2013, 49, 11797. [Google Scholar] [CrossRef]
- Xiao, B.; Gong, T.-J.; Liu, Z.-J.; Liu, J.-H.; Luo, D.-F.; Xu, J.; Liu, L. Synthesis of Dibenzofurans via Palladium-Catalyzed Phenol-Directed C-H Activation/C-O Cyclization. J. Am. Chem. Soc. 2011, 133, 9250–9253. [Google Scholar] [CrossRef]
- Moon, Y.; Kim, Y.; Hong, H.; Hong, S. Synthesis of Heterocyclic-Fused Benzofurans via C–H Functionalization of Flavones and Coumarins. Chem. Commun. 2013, 49, 8323. [Google Scholar] [CrossRef]
- Fu, L.; Li, S.; Cai, Z.; Ding, Y.; Guo, X.-Q.; Zhou, L.-P.; Yuan, D.; Sun, Q.-F.; Li, G. Ligand-Enabled Site-Selectivity in a Versatile Rhodium(Ii)-Catalysed Aryl C–H Carboxylation with CO2. Nat. Catal. 2018, 1, 469–478. [Google Scholar] [CrossRef]
- Khan, M.A.; De Brito Morley, M.L. Condensed Benzopyrans III. 3H,4H [1] Benzopyrano [3,4- b] Pyrrol-4-Ones. J. Heterocycl. Chem. 1978, 15, 1399–1401. [Google Scholar] [CrossRef]
- Soman, S.S.; Thaker, T.H.; Rajput, R.A. Novel Synthesis and Cytotoxic Activity of Some Chromeno[3,4-b]Pyrrol-4(3H)-Ones. Chem. Heterocycl. Comp. 2011, 46, 1514. [Google Scholar] [CrossRef]
- Majumdar, K.; Chattopadhyay, B. Amino-Claisen versus Oxy-Claisen Rearrangement: Regioselective Synthesis of Pyrrolocoumarin Derivatives. Synthesis 2008, 2008, 921–924. [Google Scholar] [CrossRef]
- Gtigga, R.; Vipond, D. 4-phenylsulphinyl-and 4-Phenylsulphonylcoumarins as components in cycloaddition reactions. Temahedron 1989, 45, 7587–7592. [Google Scholar]
- Xue, S.; Yao, J.; Liu, J.; Wang, L.; Liu, X.; Wang, C. Three-Component Reaction between Substituted 2-(2-Nitrovinyl)-Phenols, Acetylenedicarboxylate and Amines: Diversity-Oriented Synthesis of Novel Pyrrolo[3,4-c]Coumarins. RSC Adv. 2016, 6, 1700–1704. [Google Scholar] [CrossRef]
- Alizadeh, A.; Ghanbaripour, R.; Zhu, L.-G. An Approach to the Synthesis of 2-Acylchromeno[3,4-c]Pyrrol-4(2H)-One Derivatives via a Sequential Three-Component Reaction. Synlett 2013, 24, 2124–2126. [Google Scholar] [CrossRef]
- Shaabani, A.; Sepahvand, H.; Bazgir, A.; Khavasi, H.R. Tosylmethylisocyanide (TosMIC) [3+2] Cycloaddition Reactions: A Facile Van Leusen Protocol for the Synthesis of the New Class of Spirooxazolines, Spiropyrrolines and Chromeno[3,4-c]Pyrrols. Tetrahedron 2018, 74, 7058–7067. [Google Scholar] [CrossRef]
- Padilha, G.; Iglesias, B.A.; Back, D.F.; Kaufman, T.S.; Silveira, C.C. Synthesis of Chromeno[4,3-b]Pyrrol-4(1 H)-Ones, from β-Nitroalkenes and 4-Phenylaminocoumarins, under Solvent-Free Conditions. ChemistrySelect 2017, 2, 1297–1304. [Google Scholar] [CrossRef]
- Saha, M.; Pradhan, K.; Das, A.R. Facile and Eco-Friendly Synthesis of Chromeno[4,3-b]Pyrrol-4(1H)-One Derivatives Applying Magnetically Recoverable Nano Crystalline CuFe2O 4 Involving a Domino Three-Component Reaction in Aqueous Media. RSC Adv. 2016, 6, 55033–55038. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, X.; Su, W. An Efficient Protocol for Multicomponent Synthesis of Functionalized Chromeno[4,3-b]Pyrrol-4(1H)-One Derivatives. Tetrahedron Lett. 2015, 56, 2476–2479. [Google Scholar] [CrossRef]
- Alberola, A.; Calvo, L.; González-Ortega, A.; Encabo, A.P.; Sañudo, M.C. Synthesis of [1]Benzopyrano[4,3-b]Pyrrol-4(1H)-Ones from 4-Chloro-3-Formylcoumarin. Synthesis 2001, 2001, 1941–1948. [Google Scholar] [CrossRef]
- Navarro, R.A.; Bleye, L.C.; González-Ortega, A.; Ruíz, C.S. Synthesis of 1H-[1]Benzopyrano[4,3-b]Pyrrole and 4hthieno[3,2-c][1]Benzopyran Derivatives. Functionalisation by Aromatic Electrophilic Substitution. Heterocycles 2001, 55, 2369–2386. [Google Scholar]
- Alberola, A.; Álvaro, R.; Ortega, A.G.; Sádaba, M.L.; Carmen Sañudo, M. Synthesis of [1]Benzopyrano[4,3-b]Pyrrol-4(1H)-Ones from N(α)-(2-Oxo-2H-1-Benzopyran-4-Yl)Weinreb α-Aminoamides. Tetrahedron 1999, 55, 13211–13224. [Google Scholar] [CrossRef]
- Alberola, A.; Alvaro, R.; Andres, J.M.; Calvo, B.; González, A. Synthesis of [1]Benzopyrano[4,3-b]Pyrrol-4(1H)-Ones from 4-Chlorocoumarin. Synthesis 1994, 279–281. [Google Scholar] [CrossRef]
- Peng, S.; Wang, L.; Huang, J.; Sun, S.; Guo, H.; Wang, J. Palladium-Catalyzed Oxidative Annulation C-H/N-H Functionalization: Access to Substituted Pyrroles. Adv. Synth. Catal. 2013, 355, 2550–2557. [Google Scholar] [CrossRef]
- Lin, C.-H.; Yang, D.-Y. Synthesis of Coumarin/Pyrrole-Fused Heterocycles and Their Photochemical and Redox-Switching Properties. Org. Lett. 2013, 15, 2802–2805. [Google Scholar] [CrossRef]
- Yang, X.; Jing, L.; Chen, Z. An Efficient Method for One-Pot Synthesis of 3-Alkoxy-Substituted Chromeno[4,3-b]Pyrrol-4(1H)-One Derivatives. Chem. Heterocycl. Comp. 2018, 54, 1065–1069. [Google Scholar] [CrossRef]
- Yahyavi, H.; Heravi, M.M.; Mahdavi, M.; Foroumadi, A. Iodine-Catalyzed Tandem Oxidative Coupling Reaction: A One-Pot Strategy for the Synthesis of New Coumarin-Fused Pyrroles. Tetrahedron Lett. 2018, 59, 94–98. [Google Scholar] [CrossRef]
- Yang, Y.; Qi, X.; Liu, R.; He, Q.; Yang, C. One-Pot Transition-Metal-Free Cascade Synthesis of Thieno[2,3-c]Coumarins from Chromones. RSC Adv. 2016, 6, 103895–103898. [Google Scholar] [CrossRef]
- Vishnumurthy, K.; Makriyannis, A. Novel and Efficient One-Step Parallel Synthesis of Dibenzopyranones via Suzuki−Miyaura Cross Coupling. J. Comb. Chem. 2010, 12, 664–669. [Google Scholar] [CrossRef][Green Version]
- Janosik, T.; Bergman, J. Five-Membered Ring Systems: Thiophenes and Se/Te Analogues in Progress in Heterocyclic Chemistry; Gordon, W.G., John, A.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 18. [Google Scholar]
- Iaroshenko, V.O.; Ali, S.; Mkrtchyan, S.; Gevorgyan, A.; Babar, T.M.; Semeniuchenko, V.; Hassan, Z.; Villinger, A.; Langer, P. Design and Synthesis of Condensed Thienocoumarins by Suzuki–Miyaura Reaction/Lactonization Tandem Protocol. Tetrahedron Lett. 2012, 53, 7135–7139. [Google Scholar] [CrossRef]
- Fondjo, E.S.; Tsemeugne, J.; De Dieu Tamokou, J.; Djintchui, A.N.; Kuiate, J.R.; Sondengam, B.L. Synthesis and Antimicrobial Activities of Some Novel Thiophene Containing Azo Compounds. Heterocycl. Commun. 2013, 19. [Google Scholar] [CrossRef]
- Al-Mousawi, S.M.; El-Apasery, M.A. Synthesis of Some Monoazo Disperse Dyes Derived from Aminothienochromene. Molecules 2013, 18, 8837–8844. [Google Scholar] [CrossRef] [PubMed]
- Djeukoua, K.S.D.; Fondjo, E.S.; Tamokou, J.-D.; Tsemeugne, J.; Simon, P.F.W.; Tsopmo, A.; Tchieno, F.M.M.; Ekom, S.E.; Pecheu, C.N.; Tonle, I.K.; et al. Synthesis, Characterization, Antimicrobial Activities and Electrochemical Behavior of New Phenolic Azo Dyes from Two Thienocoumarin Amines. Arkivoc 2020, 2019, 416–430. [Google Scholar] [CrossRef]
- Gewald, K. Zur Reaktion von α-Oxo-mercaptanen mit Nitrilen. Angew. Chem. 1961, 73, 114. [Google Scholar] [CrossRef]
- Hoberg, H. Halogenmethyl-aluminiumverbindungen. Angew. Chem. 1961, 73, 114–115. [Google Scholar] [CrossRef]
- Gewald, K. Heterocyclen aus CH-aciden Nitrilen, VII. 2-Amino-thiophene aus α-Oxo-mercaptanen und methylenaktiven Nitrilen. Chem. Ber. 1965, 98, 3571–3577. [Google Scholar] [CrossRef]
- Sopbué Fondjo, E.; Döpp, D.; Henkel, G. Reactions of Some Anellated 2-Aminothiophenes with Electron Poor Acetylenes. Tetrahedron 2006, 62, 7121–7131. [Google Scholar] [CrossRef]
- Yu, L.-S.-H.; Meng, C.-Y.; Wang, J.; Gao, Z.; Xie, J.-W. Substrate-Controlled Diastereoselectivity Switch in the Formation of Dihydrothieno[3,4-c]Coumarins via [4+1] Annulations. Adv. Synth. Catal. 2019, 361, 526–534. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, J.; Yun, H.; Baek, Y.; Lee, P.H. Rhodium-Catalyzed Intramolecular Transannulation Reaction of Alkynyl Thiadiazole Enabled 5,n-Fused Thiophenes. J. Org. Chem. 2017, 82, 1437–1447. [Google Scholar] [CrossRef]
- Weibenfels, M.; Hantschmann, A.; Steinfuhrer, T.; Birkner, E. Synthesis of 7-Substituted Ones Thieno[3,2-c]Coumarin-3-Carboxlyic Acid. Z. Chem. 1989, 29, 166. [Google Scholar] [CrossRef]
- El-Dean, A.M.K.; Zaki, R.M.; Geies, A.A.; Radwan, S.M.; Tolba, M.S. Synthesis and Antimicrobial Activity of New Heterocyclic Compounds Containing Thieno[3,2-c]Coumarin and Pyrazolo[4,3-c]Coumarin Frameworks. Russ. J. Bioorg. Chem. 2013, 39, 553–564. [Google Scholar] [CrossRef]
- Domracheva, I.; Kanepe-Lapsa, I.; Jackevica, L.; Vasiljeva, J.; Arsenyan, P. Selenopheno Quinolinones and Coumarins Promote Cancer Cell Apoptosis by ROS Depletion and Caspase-7 Activation. Life Sci. 2017, 186, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hafez, S.H.; Elkhateeb, A.; Gobouri, A.A.; Azab, I.H.E.; Kirsch, G. An Efficient Route for Synthesis and Reactions of Seleno-[2, 3-c]Coumarin. Heterocycles 2016, 92, 1054–1062. [Google Scholar] [CrossRef]



































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sawy, E.R.; Abdelwahab, A.B.; Kirsch, G. Synthetic Routes to Coumarin(Benzopyrone)-Fused Five-Membered Aromatic Heterocycles Built on the α-Pyrone Moiety. Part 1: Five-Membered Aromatic Rings with One Heteroatom. Molecules 2021, 26, 483. https://doi.org/10.3390/molecules26020483
El-Sawy ER, Abdelwahab AB, Kirsch G. Synthetic Routes to Coumarin(Benzopyrone)-Fused Five-Membered Aromatic Heterocycles Built on the α-Pyrone Moiety. Part 1: Five-Membered Aromatic Rings with One Heteroatom. Molecules. 2021; 26(2):483. https://doi.org/10.3390/molecules26020483
Chicago/Turabian StyleEl-Sawy, Eslam Reda, Ahmed Bakr Abdelwahab, and Gilbert Kirsch. 2021. "Synthetic Routes to Coumarin(Benzopyrone)-Fused Five-Membered Aromatic Heterocycles Built on the α-Pyrone Moiety. Part 1: Five-Membered Aromatic Rings with One Heteroatom" Molecules 26, no. 2: 483. https://doi.org/10.3390/molecules26020483
APA StyleEl-Sawy, E. R., Abdelwahab, A. B., & Kirsch, G. (2021). Synthetic Routes to Coumarin(Benzopyrone)-Fused Five-Membered Aromatic Heterocycles Built on the α-Pyrone Moiety. Part 1: Five-Membered Aromatic Rings with One Heteroatom. Molecules, 26(2), 483. https://doi.org/10.3390/molecules26020483






