Antioxidant, Anti-Inflammatory and Antidiabetic Proprieties of LC-MS/MS Identified Polyphenols from Coriander Seeds
Abstract
:1. Introduction
2. Results and Discussion
2.1. PCS Qualitative Analysis
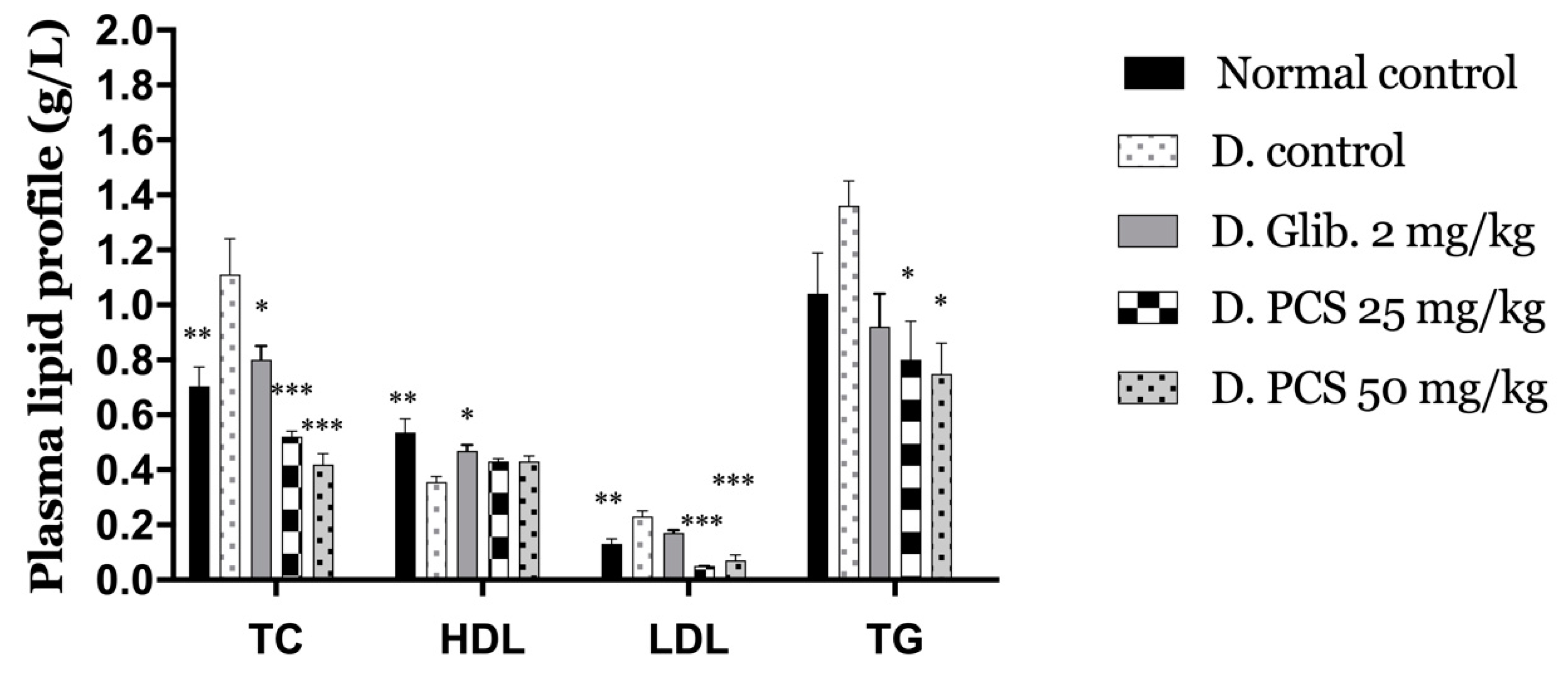
2.2. Evaluation of the Antidiabetic Activity
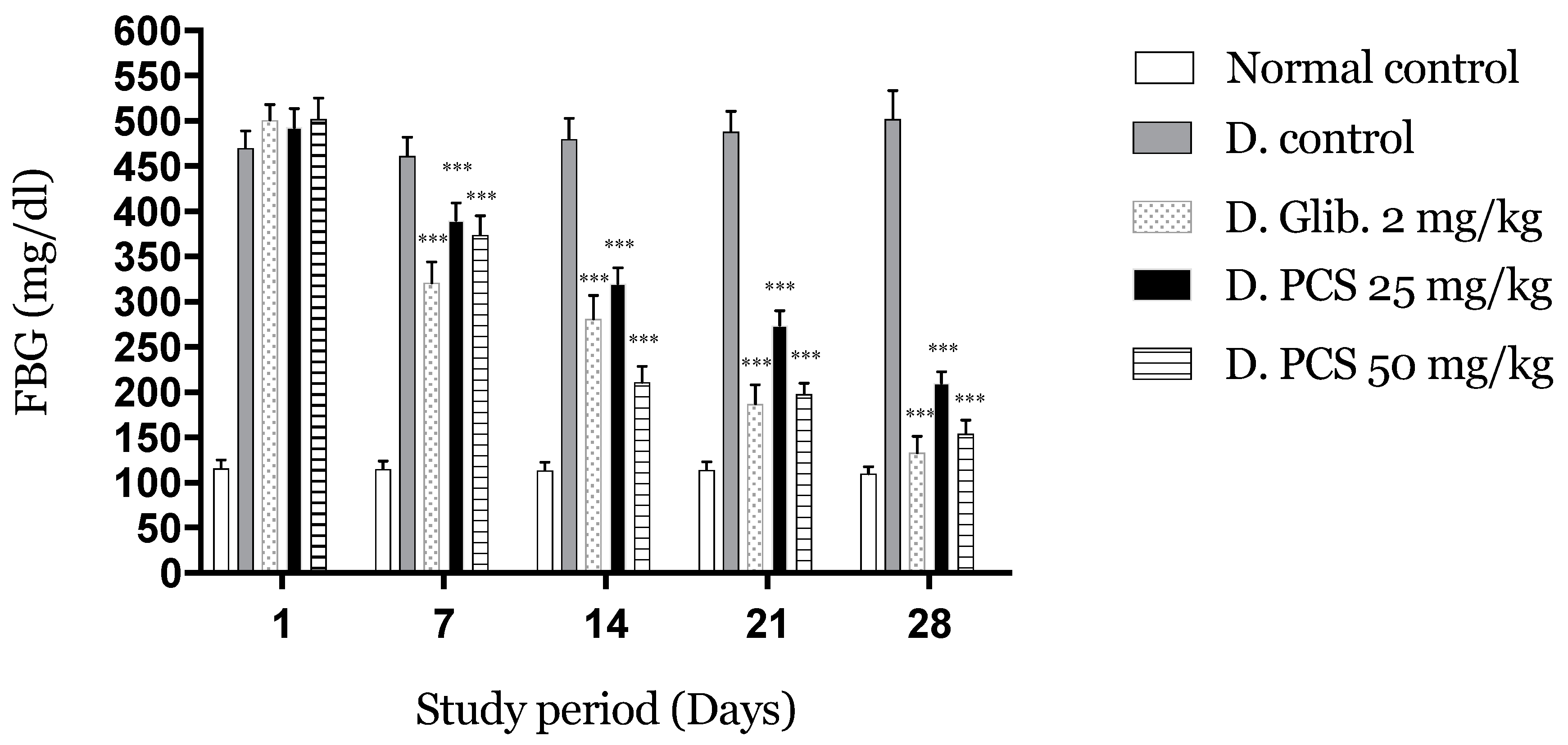
2.2.1. PCS Effects on FBG after Subacute Administration
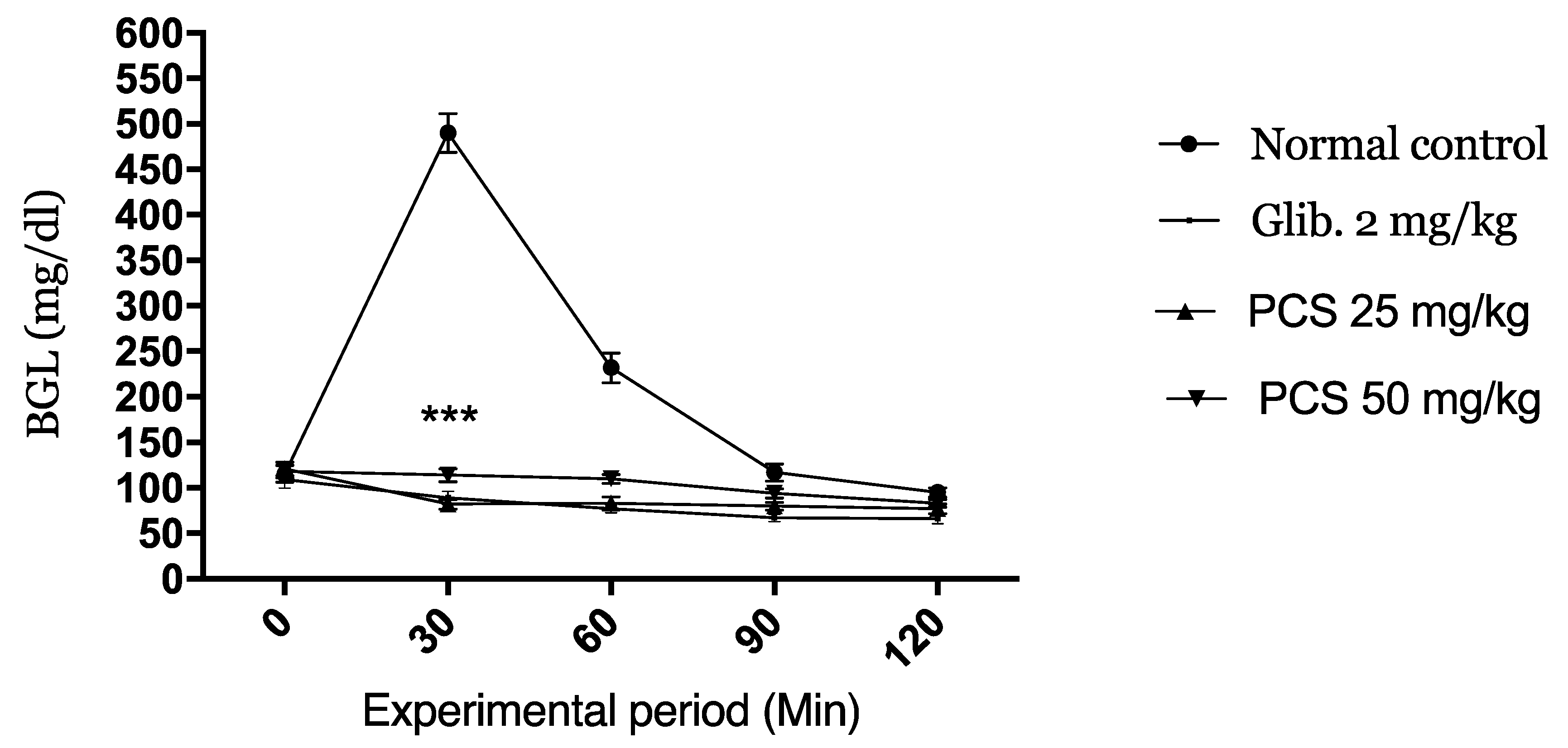
2.2.2. Oral Glucose Tolerance Test
2.3. Anti-Inflammatory Activity
2.4. Antioxidant Activity
2.4.1. Scavenging of the Free Radical DPPH
2.4.2. Discoloration Test of β-Carotene
3. Materials and Methods
3.1. Plant Materiel
3.2. Study Animal Selection
3.3. Polyphenols Extraction
3.4. Coriander Seed Polyphenol LC-MS/MS Analysis
3.5. Antidiabetic Activity Study
3.5.1. Preparation and Induction of Experimental Diabetes
3.5.2. Groups Repartition and Experimental Model
3.5.3. The Oral Glucose Tolerance Test (OGTT)
3.6. Carrageenan Induced Paw Edema and Anti-Inflammatory Evaluation
3.7. In Vitro Antioxidant Activity
3.7.1. DPPH Test
- I: inhibition Percentage.
- A0: Absorbance of the DPPH solution without a sample.
- A: Absorbance of the DPPH solution with the sample.
3.7.2. β-carotene Bleaching Test
- AA%: percentage of antioxidant activity.
- AE: absorbance after 2 h of the negative control.
- ABHT: absorbance after 2 h of BHT.
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 36 (Suppl. 1), S67–S74. Available online: https://care.diabetesjournals.org/content/36/Supplement_1/S67 (accessed on 4 May 2020). [CrossRef] [Green Version]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; Fernandes, J.D.R.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Parhofer, K.G. Diabetic dyslipidemia. Metab. Clin. Exp. 2014, 63, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar] [PubMed]
- Zuo, T.; Zhu, M.; Xu, W. Roles of Oxidative Stress in Polycystic Ovary Syndrome and Cancers. Oxid. Med. Cell. Longev. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza, G.A.; De Marqui, S.V.; Matias, J.N.; Guiguer, E.L.; Barbalho, S.M. Effects of Ginkgo biloba on Diseases Related to Oxidative Stress. Planta Med. 2020, 86, 376–386. [Google Scholar] [CrossRef] [Green Version]
- Mechchate, H.; Es-Safi, I.; Jawhari, F.Z.; Bari, A.; Grafov, A.; Bousta, D. Ethnobotanical survey about the management of diabetes with medicinal plants used by diabetic patients in Region of Fez-Meknes, Morocco. Ethnobot. Res. Appl. 2020, 19, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Han, J.; Bao, L.; Wang, W.; Ma, K.; Liu, H. Identification and α-Glucosidase Inhibitory Activity of Meroterpenoids from Hericium erinaceus. Planta Med. 2020, 86, 571–578. [Google Scholar] [CrossRef]
- Di Fabio, G.; Romanucci, V.; Zarrelli, M.; Giordano, M.; Zarrelli, A. C-4 Gem-Dimethylated Oleanes of Gymnema sylvestre and Their Pharmacological Activities. Molecules 2013, 18, 14892–14919. [Google Scholar] [CrossRef] [Green Version]
- Es-Safi, I.; Mechchate, H.; Amaghnouje, A.; El Moussaoui, A.; Cerruti, P.; Avella, M.; Grafov, A.; Bousta, D. Marketing and legal status of phytomedicines and food supplements in Morocco. J. Complement. Integr. Med. 2020. [Google Scholar] [CrossRef]
- Hamidpour, M.; Hamidpour, R.; Hamidpour, S.; Shahlari, M. Chemistry, Pharmacology, and Medicinal Property of Sage (Salvia) to Prevent and Cure Illnesses such as Obesity, Diabetes, Depression, Dementia, Lupus, Autism, Heart Disease, and Cancer. J. Tradit. Complement. Med. 2014, 4, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Kabbaoui, M.; Chda, A.; Mejrhit, N.; Aarab, L.; Bencheikh, R.; Tazi, A.; Farah, A. Antidiabetic Effect of Thymus Satureioides Aqueous Extract in Streptozotocin-Induced Diabetic Rats. Int. J. Pharm. Pharm. Sci. 2016, 8, 140. [Google Scholar] [CrossRef] [Green Version]
- Es-Safi, I.; Mechchate, H.; Amaghnouje, A.; Calarco, A.; Boukhira, S.; Noman, O.M.; A Mothana, R.; A Nasr, F.; Bekkari, H.; Bousta, D. Defatted Hydroethanolic Extract of Ammodaucus leucotrichus Cosson and Durieu Seeds: Antidiabetic and Anti-Inflammatory Activities. Appl. Sci. 2020, 10, 9147. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-Safi, I.; Bourhia, M.; Kyrylchuk, A.; Moussaoui, A.E.L.; Conte, R.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.E.; Grafov, A.; et al. In-Vivo Antidiabetic Activity and In-Silico Mode of Action of LC/MS-MS Identified Flavonoids in Oleaster Leaves. Molecules 2020, 25, 5073. [Google Scholar] [CrossRef]
- Silveira, A.C.; Dias, J.P.; Santos, V.M.; Oliveira, P.F.; Alves, M.G.; Rato, L.; Silva, B.M. The Action of Polyphenols in Diabetes Mellitus and Alzheimer’s Disease: A Common Agent for Overlapping Pathologies. Curr. Neuropharmacol. 2019, 17, 590–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadeem, M.; Anjum, F.M.; Khan, M.I.; Tehseen, S.; Elghorab, A.H.; Sultan, J.I. Nutritional and medicinal aspects of coriander (CoriandrumsativumL.). Br. Food J. 2013, 115, 743–755. [Google Scholar] [CrossRef]
- El-Hilaly, J.; Hmammouchi, M.; Lyoussi, B. Ethnobotanical studies and economic evaluation of medicinal plants in Taounate province (Northern Morocco). J. Ethnopharmacol. 2003, 86, 149–158. [Google Scholar] [CrossRef]
- Jourdan, A.J.L. Universal Pharmacopoeia; J. B. Baillière: Paris France, 1828. [Google Scholar]
- Tahraoui, A.; El-Hilaly, J.; Israili, Z.; Lyoussi, B. Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in south-eastern Morocco (Errachidia province). J. Ethnopharmacol. 2007, 110, 105–117. [Google Scholar] [CrossRef]
- Es-Safi, I.; Mechchate, H.; Amaghnouje, A.; Jawhari, F.Z.; Bari, A.; Cerruti, P.; Avella, M.; Andriy, A.; Andriy, D. Medicinal plants used to treat acute digestive system problems in the region of Fez-Meknes in Morocco: An ethnopharmacological survey. Ethnobot. Res. Appl. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Chithra, V.; Leelamma, S. Coriandrum sativum — mechanism of hypoglycemic action. Food Chem. 1999, 67, 229–231. [Google Scholar] [CrossRef]
- Eidi, A.; Eidi, M. Antidiabetic effects of sage (Salvia officinalis L.) leaves in normal and streptozotocin-induced diabetic rats. Diabetes Metab. Syndr. 2009, 3, 40–44. [Google Scholar] [CrossRef]
- Melo, E.D.A.; Filho, J.M.; Guerra, N.B. Characterization of antioxidant compounds in aqueous coriander extract (Coriandrum sativum L.). LWT 2005, 38, 15–19. [Google Scholar] [CrossRef]
- Mohite, S.; Salunkhe, A. Formulation and Evaluation of Emulgel Containing Coriandrum Sativum Seeds Oil for An-ti-Inflammatory Activity. J. Drug Deliv. Ther. 2019, 9, 124–130. [Google Scholar]
- Khani, A.; Rahdari, T. Chemical Composition and Insecticidal Activity of Essential Oil from Coriandrum sativum Seeds against Tribolium confusum and Callosobruchus maculatus. ISRN Pharmacol. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Hao, H.-H.; Shao, Z.-M.; Tang, D.; Lu, Q.; Chen, X.; Yin, X.-X.; Wu, J.; Chen, H. Preventive effects of rutin on the development of experimental diabetic nephropathy in rats. Life Sci. 2012, 91, 959–967. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Calixto-Campos, C.; Carvalho, T.T.; Hohmann, M.; Pinho-Ribeiro, F.A.; Fattori, V.; Manchope, M.F.; Zarpelon, A.C.; Baracat, M.M.; Georgetti, S.R.; Casagrande, R.; et al. Vanillic Acid Inhibits Inflammatory Pain by Inhibiting Neutrophil Recruitment, Oxidative Stress, Cytokine Production, and NFκB Activation in Mice. J. Nat. Prod. 2015, 78, 1799–1808. [Google Scholar] [CrossRef]
- Ángeles Martín, M.; Fernández-Millán, E.; Ramos, S.; Bravo, L.; Goya, L. Cocoa flavonoid epicatechin protects pancreatic beta cell viability and function against oxidative stress. Mol. Nutr. Food Res. 2014, 58, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; E Aston, C.; Lyons, T.J. Green Tea Supplementation Affects Body Weight, Lipids, and Lipid Peroxidation in Obese Subjects with Metabolic Syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef]
- Gerich, J.; Penhos, J.C.; Gutman, R.A.; Recant, L. Effect of Dehydration and Hyperosmolarity on Glucose, Free Fatty Acid and Ketone Body Metabolism in the Rat. Diabetes 1973, 22, 264–271. [Google Scholar] [CrossRef]
- American Diabetes Association Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2009, 32, S62–S67. Available online: https://care.diabetesjournals.org/content/32/Supplement_1/S62 (accessed on 4 May 2020). [CrossRef] [PubMed] [Green Version]
- Wilcox, G. Insulin and Insulin Resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Kumar, M.; Kaur, P.; Chandel, M.; Singh, A.P.; Jain, A.; Kaur, S. Antioxidant and hepatoprotective potential of Lawsonia inermis L. leaves against 2-acetylaminofluorene induced hepatic damage in male Wistar rats. BMC Complement. Altern. Med. 2017, 17, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucchesi, A.N.; Cassettari, L.L.; Spadella, C.T. Alloxan-Induced Diabetes Causes Morphological and Ultrastructural Changes in Rat Liver that Resemble the Natural History of Chronic Fatty Liver Disease in Humans. J. Diabetes Res. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.; Desai, P.B.; Kulkarni, S.S.; Hull, V.V.; Math, A.A.K.; Vernekar, S.N. Markers of renal function tests. N. Am. J. Med Sci. 2010, 2, 170–173. [Google Scholar]
- Chehade, J.M.; Gladysz, M.; Mooradian, A.D. Dyslipidemia in Type 2 Diabetes: Prevalence, Pathophysiology, and Management. Drugs 2013, 73, 327–339. [Google Scholar] [CrossRef]
- Girija, K.; Lakshman, K.; Udaya, C.; Sachi, G.S.; Divya, T. Anti–diabetic and anti–cholesterolemic activity of methanol extracts of three species of Amaranthus. Asian Pac. J. Trop. Biomed. 2011, 1, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Karim, N.; Ahmed, K.R.; Bukht, M.S.; Akter, J.; Chowdhury, H.A.; Hossain, S.; Anwar, N.; Selim, S.; Chowdhury, S.H.; Hossain, F.; et al. Pattern and predictors of dyslipidemia in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. 2013, 7, 95–100. [Google Scholar] [CrossRef]
- Vinegar, R.; Schreiber, W.; Hugo, R. Biphasic development of carrageenin edema in rats. J. Pharmacol. Exp. Ther. 1969, 166, 96–103. [Google Scholar]
- Ricciotti, E.; Fitzgerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.H.; De Groot, A.; Evstatieva, L.N. Screening of Plant Extracts for Antioxidant Activity: A Comparative Study on Three Testing Methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Bors, W.; Michel, C. Chemistry of the Antioxidant Effect of Polyphenols. Ann. N. Y. Acad. Sci. 2002, 957, 57–69. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-Induced Edema in Hind Paw of the Rat as an Assay for Antiinflammatory Drugs. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Prieto, M.A.; Rodríguez-Amado, I.; Vázquez, J.A.; Murado, M.A. β-Carotene Assay Revisited. Application To Characterize and Quantify Antioxidant and Prooxidant Activities in a Microplate. J. Agric. Food Chem. 2012, 60, 8983–8993. [Google Scholar] [CrossRef] [Green Version]
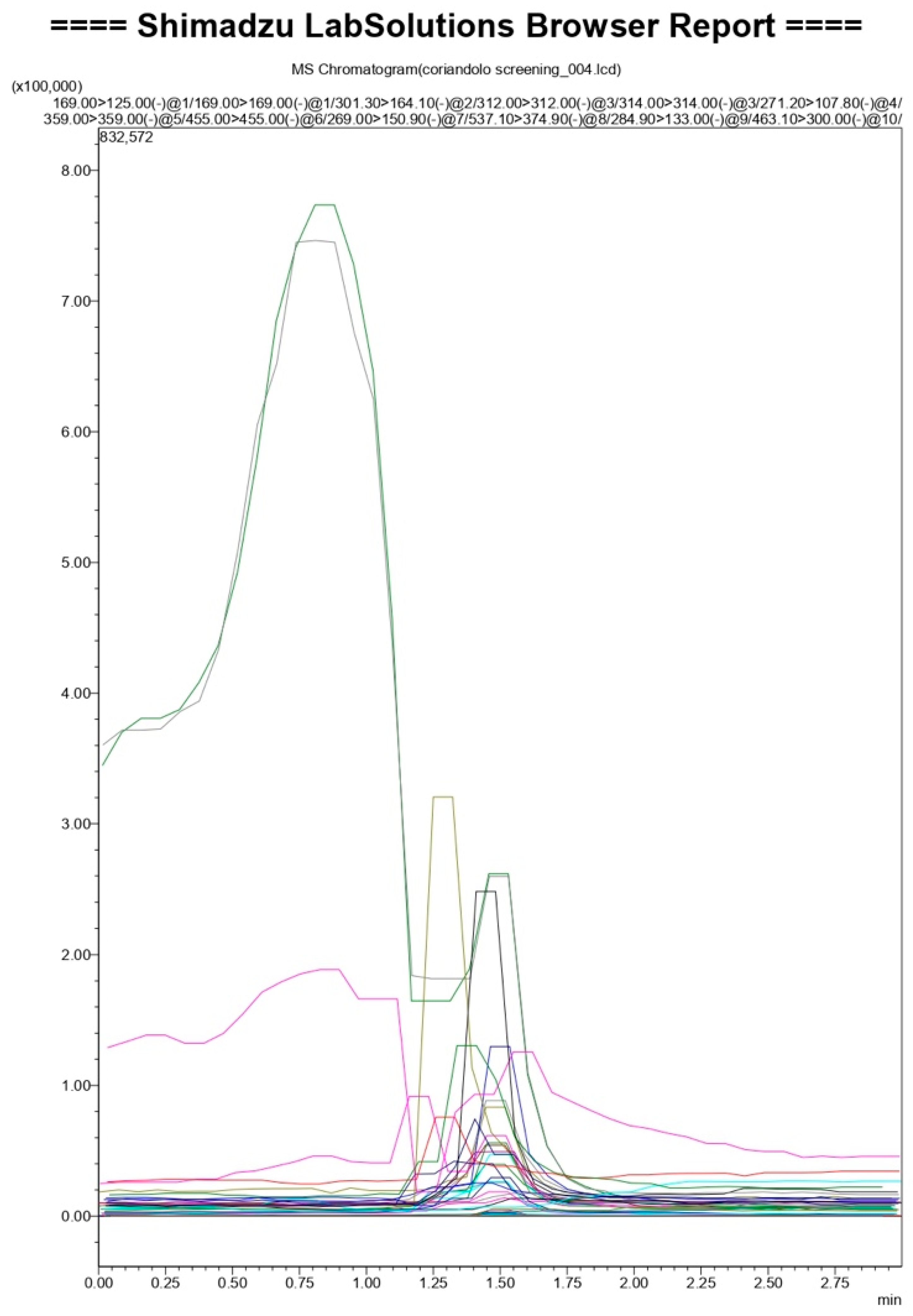
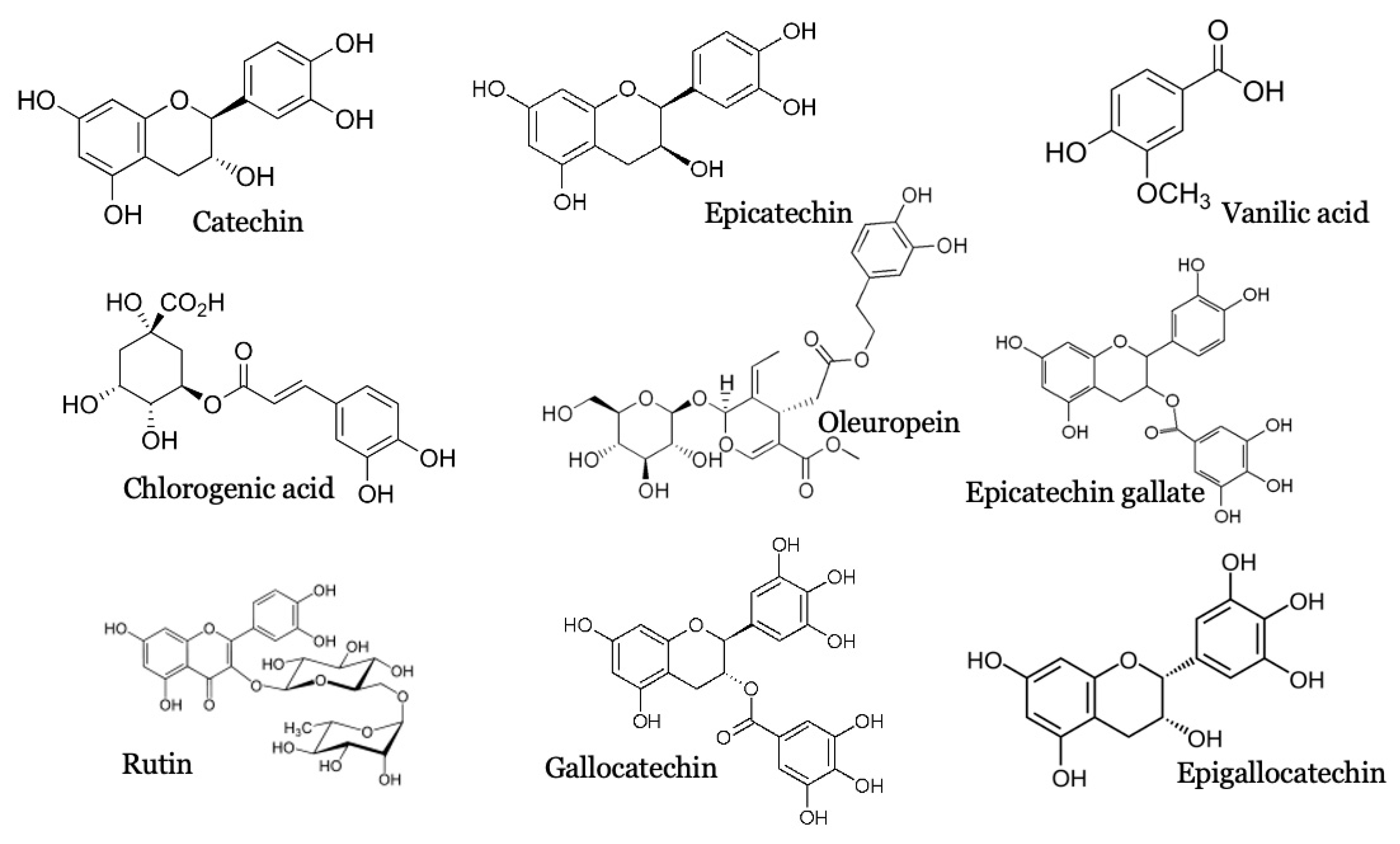

| Molecule | Analyzed Fragment |
|---|---|
| Vanillic acid | 167.00 > 123.00 |
| Chlorogenic acid | 353.00 > 190.00 |
| Catechin\Epicathechin | 289.00 > 179.00 |
| Oleuropein | 539.00 > 539.00 |
| Epicathechin gallate | 441.00 > 169.00 |
| Rutin | 609.00 > 301.00 |
| Gallocathechin\ Epigallocathechin | 305.00 > 125.00 |
| Treatment | Bodyweight Development | (g) | |||
|---|---|---|---|---|---|
| 1st Day | 7th Day | 14th Day | 21st Day | 28th Day | |
| Normal Control | 23.4 ± 1.8 | 24.7 ± 1.5 * | 25.2 ± 1.4 *** | 26.9 ± 1.3 *** | 27.2 ± 1.5 *** |
| Diabetic Control | 23.8 ± 1.6 | 21.7 ± 2.2 # | 20.1 ± 2.7 ### | 19.2 ± 2.5 ### | 17.7 ± 2.4 ### |
| D. Glib 2 mg/kg | 24.3 ± 1.6 | 23.1 ± 1.5 | 24.2 ± 1.7 ** | 25.8 ± 1.8 *** | 25.9 ± 1.7 *** |
| D. PCS 25 mg/kg | 22.5 ± 1.9 | 22.1 ± 1.6 | 23.6 ± 1.9 ** | 24.3 ± 1.9 ** | 26.2 ± 2.0 *** |
| D. PCS 50 mg/kg | 21.9 ± 2.0 | 21.2 ± 1.9 | 22.34 ± 2.4 * | 23.2 ± 2.6 ** | 24.8 ± 2.4 *** |
| Liver Biomarkers | Kidney Biomarkers | |||
|---|---|---|---|---|
| Treatments | ASAT(UI/L) | ALAT(UI/L) | Urea(g/L) | Creatinine(mg/L) |
| Normal control | 45.8 ± 6.1 | 311 ± 21.2 | 0.28 ± 0.03 | 3.4 ± 0.54 |
| Diabetic control | 134 ± 12.2 ### | 802 ± 98.3 ### | 0.63 ± 0.05 ### | 6.0 ± 0.62 ### |
| D. Glib 2 mg/kg | 48 ± 4.4 *** | 222 ± 15.2 *** | 0.28 ± 0.03 *** | 4.2 ± 0.35 ** |
| D. PCS 25 mg/kg | 32 ± 3.2 *** | 224 ± 15.6 *** | 0.29 ± 0.02 *** | 3.4 ± 0.30 *** |
| D. PCS 50 mg/kg | 32 ± 2.9 *** | 191 ± 13.2 *** | 0.34 ± 0.02 *** | 3.5 ± 0.36 *** |
| DPPH (IC50) (µg/mL) | β-carotene (Inhibition) (%) | |
|---|---|---|
| PCS | 0.0005 ± 6.98 × 10−6 | 53.73% |
| Positive control (BHT) | 0.14 ± 0.019 | _____ |
| Negative control | _____ | 3.37% |
Sample Availability: Samples of the compounds are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechchate, H.; Es-safi, I.; Amaghnouje, A.; Boukhira, S.; A. Alotaibi, A.; Al-zharani, M.; A. Nasr, F.; M. Noman, O.; Conte, R.; Amal, E.H.E.Y.; et al. Antioxidant, Anti-Inflammatory and Antidiabetic Proprieties of LC-MS/MS Identified Polyphenols from Coriander Seeds. Molecules 2021, 26, 487. https://doi.org/10.3390/molecules26020487
Mechchate H, Es-safi I, Amaghnouje A, Boukhira S, A. Alotaibi A, Al-zharani M, A. Nasr F, M. Noman O, Conte R, Amal EHEY, et al. Antioxidant, Anti-Inflammatory and Antidiabetic Proprieties of LC-MS/MS Identified Polyphenols from Coriander Seeds. Molecules. 2021; 26(2):487. https://doi.org/10.3390/molecules26020487
Chicago/Turabian StyleMechchate, Hamza, Imane Es-safi, Amal Amaghnouje, Smahane Boukhira, Amal A. Alotaibi, Mohammed Al-zharani, Fahd A. Nasr, Omar M. Noman, Raffaele Conte, El Hamsas El Youbi Amal, and et al. 2021. "Antioxidant, Anti-Inflammatory and Antidiabetic Proprieties of LC-MS/MS Identified Polyphenols from Coriander Seeds" Molecules 26, no. 2: 487. https://doi.org/10.3390/molecules26020487
APA StyleMechchate, H., Es-safi, I., Amaghnouje, A., Boukhira, S., A. Alotaibi, A., Al-zharani, M., A. Nasr, F., M. Noman, O., Conte, R., Amal, E. H. E. Y., Bekkari, H., & Bousta, D. (2021). Antioxidant, Anti-Inflammatory and Antidiabetic Proprieties of LC-MS/MS Identified Polyphenols from Coriander Seeds. Molecules, 26(2), 487. https://doi.org/10.3390/molecules26020487






