Analyzing the Carotenoid Composition of Melilot (Melilotus officinalis (L.) Pall.) Extracts and the Effects of Isolated (All-E)-lutein-5,6-epoxide on Primary Sensory Neurons and Macrophages
Abstract
:1. Introduction
2. Results
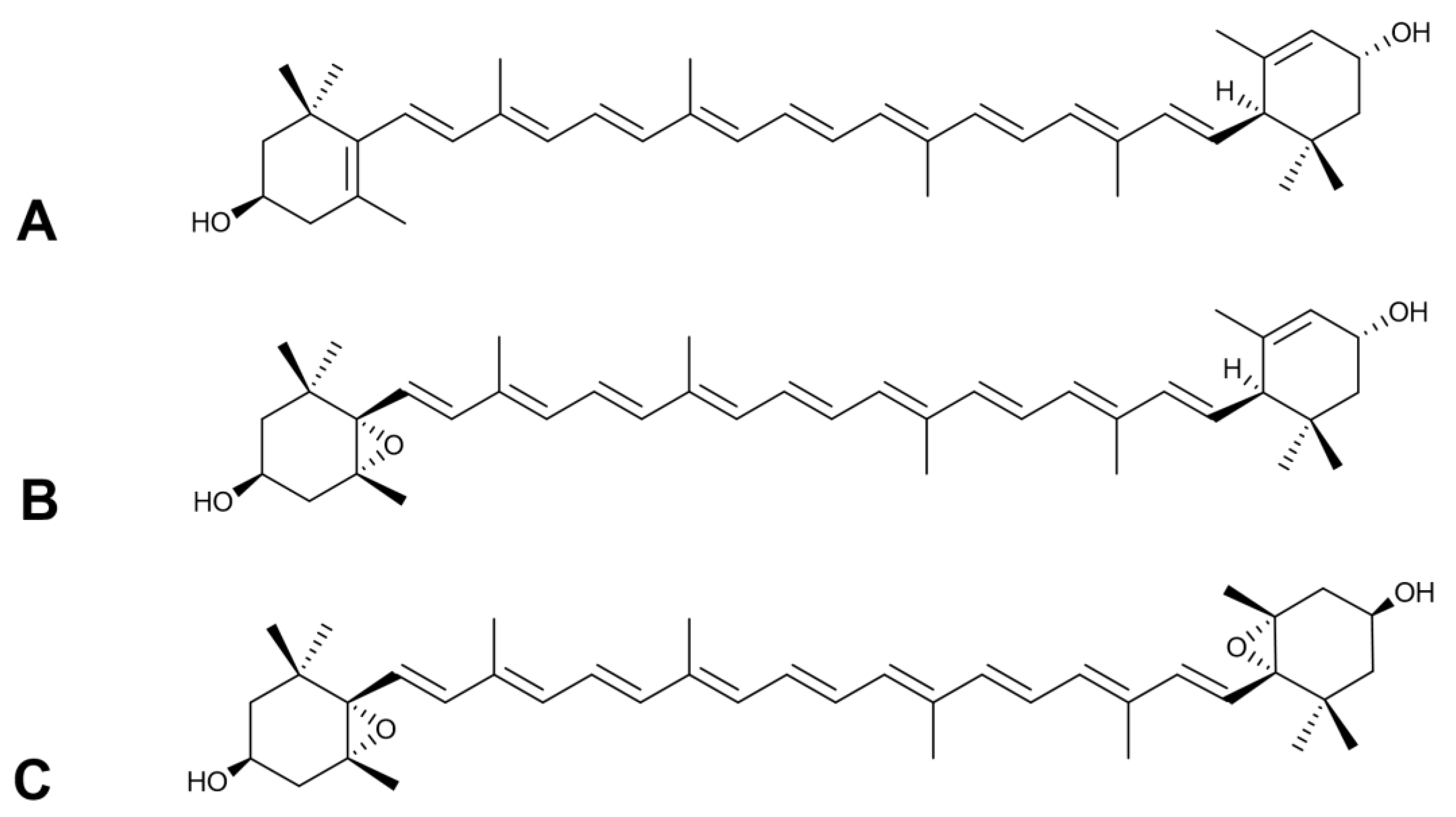
2.1. Carotenoid Composition of Meliloti Herba
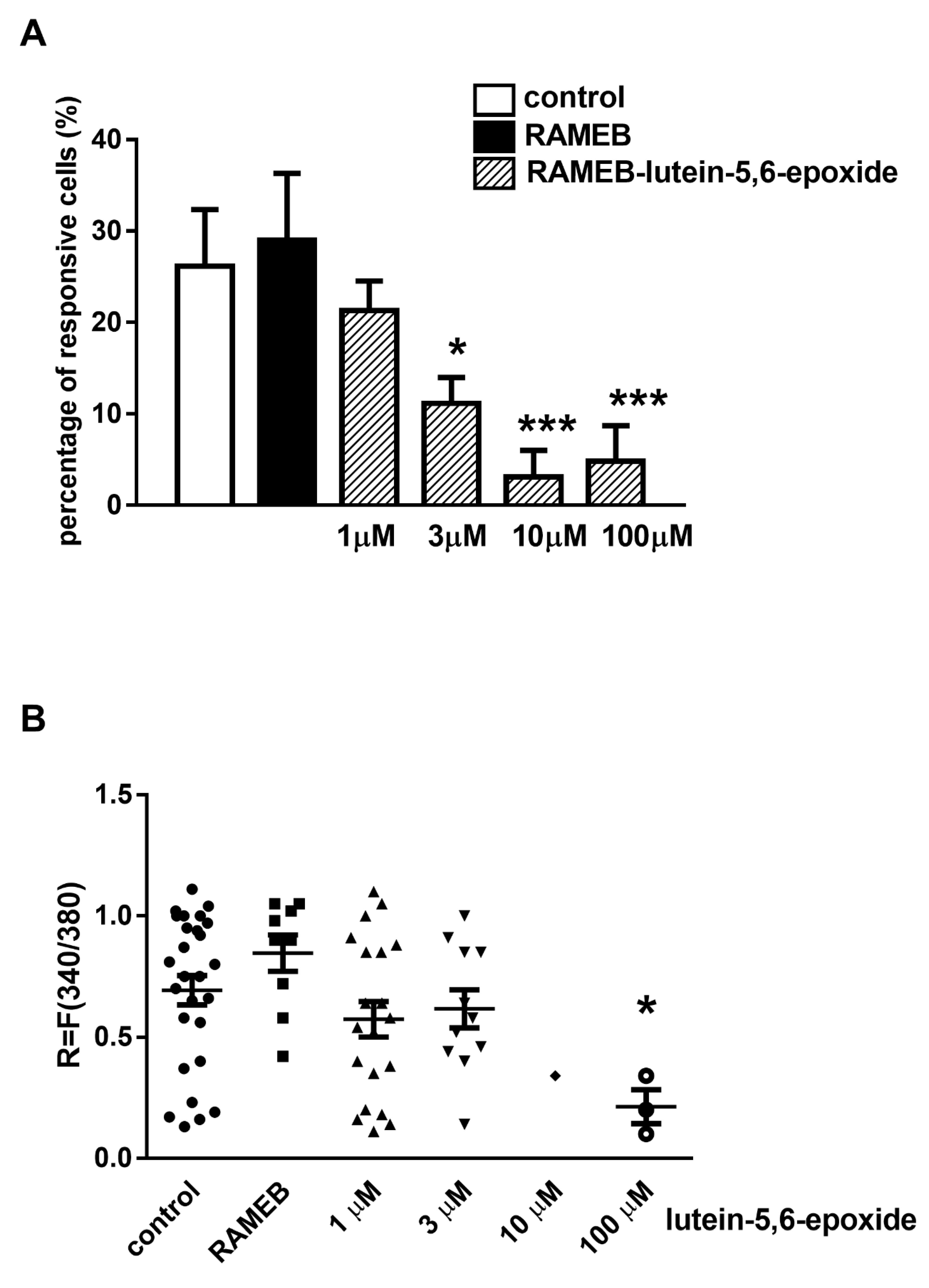
2.2. RAMEB-Lutein 5,6-Epoxide Decreases Mustard Oil-Evoked Ca2+-Influx in Cultured Primary Sensory Neurons
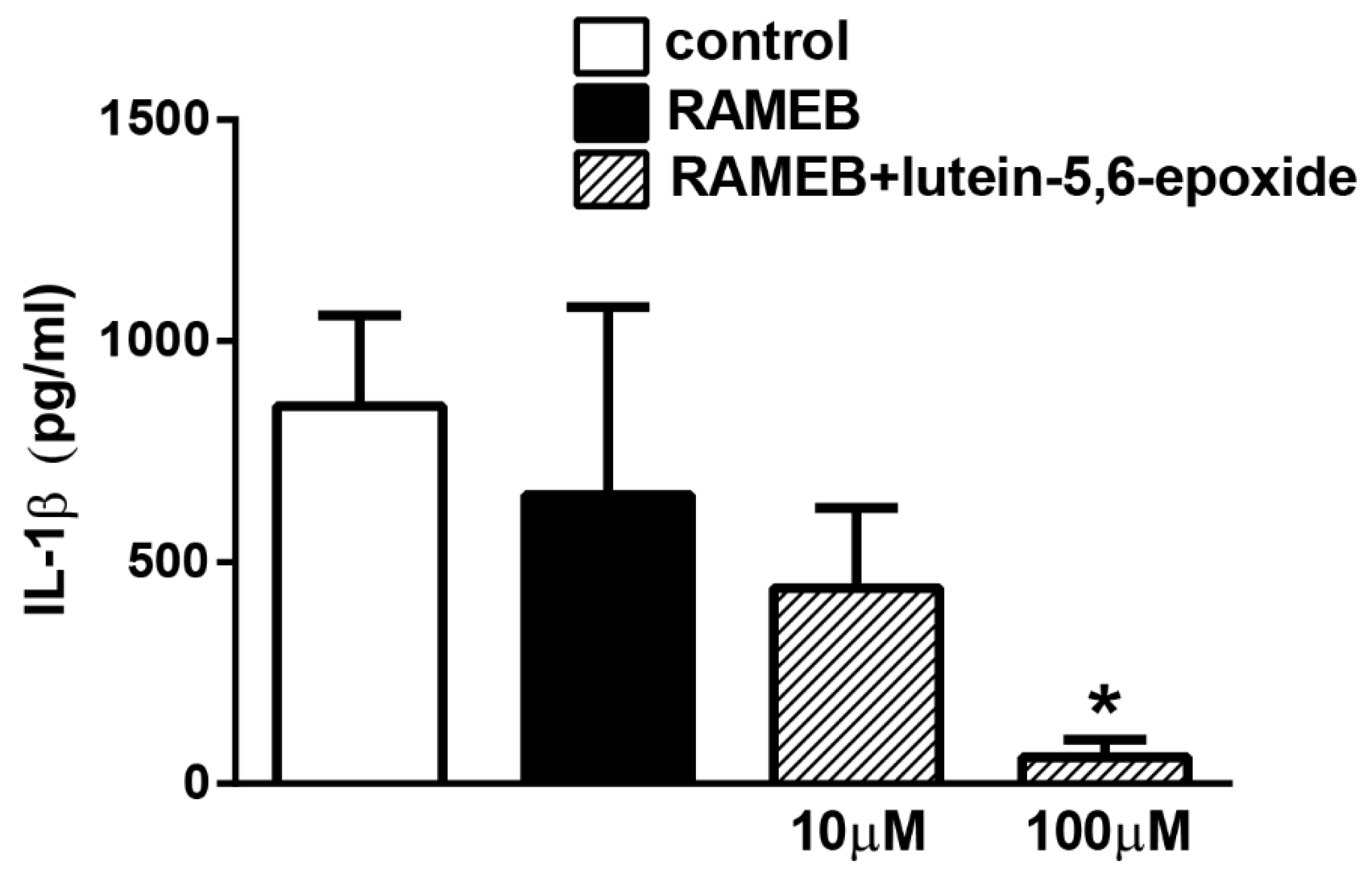
2.3. RAMEB-Lutein 5,6-Epoxide Reduces LPS-Induced IL-1β Production of Isolated Peritoneal Macrophages
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction of Carotenoids
4.2. Chemicals
4.3. Instrumentals
4.4. Experimental Conditions of HPLC Analysis
4.5. Experimental Conditions of CLC Separation
4.6. Identification of Carotenoids
4.7. Mass Spectrometry
4.7.1. Preparation of Different Matrices
4.7.2. MALDI-TOF/MS (Matrix-Assisted Laser Desorption/Ionization-Time of Flight Mass Spectrometry) Conditions and Measurements
4.8. In Vitro Investigation of the Effects of Isolated Lutein 5,6-Epoxide
4.8.1. Ethics Statement
4.8.2. Primary Cultures of Trigeminal Ganglion (TRG) Neurons
4.8.3. Ratio-Metric Technique of Intracellular Free Calcium Concentration [Ca2+]I Measurement with the Fluorescent Indicator Fura-2 AM
4.8.4. Primary Cultures of Peritoneal Macrophages and Measurement of Interleukin-1β (IL-1β)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- E/S/C/O/P. Meliloti herba—Melilot. In ESCOP Monographs, 2nd ed.; Thieme: Stuttgart, NY, USA, 2003; pp. 320–323. [Google Scholar]
- EMA Community Herbal Monograph on Melilotus officinalis (L.) Lam., Herba. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_Community_herbal_monograph/2010/01/WC500059265.pdf. (accessed on 20 May 2016).
- Chorepsima, S.; Tentolouris, K.; Dimitroulis, D.; Tentolouris, N. Melilotus: Contribution to wound healing in the diabetic foot. J. Herb. Med. 2013, 3, 81–86. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoids actions and their relation to health and disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef] [PubMed]
- Horváth, G.; Szőke, É.; Kemény, Á.; Bagoly, T.; Deli, J.; Szente, L.; Pál, S.; Sándor, K.; Szolcsányi, J.; Helyes, Z. Lutein inhibits the function of the Transient Receptor Potential A1 ion channel in different in vitro and in vivo models. J. Mol. Neurosci. 2012, 46, 1–9. [Google Scholar]
- Horváth, G.; Kemény, Á.; Barthó, L.; Molnár, P.; Deli, J.; Szente, L.; Bozó, T.; Pál, S.; Sándor, K.; Szőke, É.; et al. Effects of some natural carotenoids on TRPA1- and TRPV1-induced neurogenic inflammatory processes in vivo in the mouse skin. J. Mol. Neurosci. 2015, 56, 113–121. [Google Scholar]
- Gees, M.; Owsianik, G.; Nilius, B.; Voets, T. TRP channels. Compr. Physiol. 2012, 2, 563–608. [Google Scholar] [PubMed]
- Vay, L.; Gu, C.; McNaughton, P.A. The thermo-TRP ion channel family: Properties and therapeutic implications. Br. J. Pharmacol. 2012, 165, 787–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilius, B.; Szallasi, A. Transient receptor potential channels as drug targets: From the science of basic research to the art of medicine. Pharmacol. Rev. 2014, 66, 676–814. [Google Scholar] [CrossRef]
- Akopian, A.N.; Ruparel, N.B.; Patwardhan, A.; Hargreaves, K.M. Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J. Neurosci. 2008, 28, 1064–1075. [Google Scholar] [CrossRef]
- Salas, M.M.; Hargreaves, K.M.; Akopian, A.N. TRPA1-mediated responses in trigeminal sensory neurons: Interaction between TRPA1 and TRPV1. Eur. J. Neurosci. 2009, 29, 1568–1578. [Google Scholar] [CrossRef] [Green Version]
- Helyes, Z.; Németh, J.; Thán, M.; Bölcskei, K.; Pintér, E.; Szolcsányi, J. Inhibitory effect of anandamide on resiniferatoxin-induced sensory neuropeptide release in vivo and neuropathic hyperalgesia in the rat. Life Sci. 2003, 73, 2345–2353. [Google Scholar] [CrossRef]
- Helyes, Z.; Pintér, E.; Sándor, K.; Elekes, K.; Bánvölgyi, Á.; Keszthelyi, D.; Szőke, É.; Tóth, D.M.; Sándor, Z.; Kereskai, L.; et al. Impaired defense mechanism against inflammation, hyperalgesia, and airway hyperreactivity in somatostatin 4 receptor gene-deleted mice. Proc. Natl. Acad. Sci. USA 2009, 106, 13088–13093. [Google Scholar] [CrossRef] [Green Version]
- Geppetti, P.; Materazzi, S.; Nicoletti, P. The transient receptor potential vanilloid 1: Role in airway inflammation and disease. Eur. J. Pharmacol. 2006, 533, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef]
- Sjögren, B.; Svenningsson, P. Depletion of the lipid raft constituents, sphingomyelin and ganglioside, decreases serotonin binding at human 5-HT7(a) receptors in HeLa cells. Acta Physiol. 2007, 190, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Szőke, É.; Börzsei, R.; Tóth, D.M.; Lengl, O.; Helyes, Z.; Sándor, Z.; Szolcsányi, J. Effect of lipid raft disruption on TRPV1 receptor activation on trigeminal sensory neurons and transfected cell line. Eur. J. Pharmacol. 2010, 628, 67–74. [Google Scholar] [CrossRef]
- Sághy, É.; Szőke, É.; Payrits, M.; Helyes, Z.; Börzsei, R.; Erostyák, J.; Jánosi, T.Z.; Sétáló, G., Jr.; Szolcsányi, J. Evidence for the role of lipid rafts and sphingomyelin in Ca2+-gating of Transient Receptor Potential channels in trigeminal sensory neurons and peripheral nerve terminals. Pharmacol. Res. 2015, 100, 101–116. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Highly soluble cyclodextrin derivatives: Chemistry, properties, and trends in development. Adv. Drug Deliv. Rev. 1999, 36, 17–28. [Google Scholar] [CrossRef]
- Evrard, B.; Bertholet, P.; Gueders, M.; Flament, M.-P.; Piel, G.; Delattre, L.; Gayot, A.; Leterme, P.; Foidart, J.-M.; Cataldo, D. Cyclodextrins as a potential carrier in drug nebulization. J. Control. Release 2004, 96, 403–410. [Google Scholar] [CrossRef]
- Fonknechten, G.; Wuthrich, P.; Tsouderos, Y.; Varin, C. Patent Fr. Demande FR 2827516 A1 Lab. Servier, Jan 2003. Available online: https://patentimages.storage.googleapis.com/2a/1b/6c/ac99192108a651/WO2003015751A1.pdf (accessed on 18 January 2021).
- Fenyvesi, É. Approved pharmaceutical products containing cyclodextrins. Cyclodext. News 2013, 27, 1. [Google Scholar]
- Molnár, P.; Szabolcs, J. Alkaline permanganate oxidation of carotenoid epoxides and furanoids. Acta Chim. Acad. Sci. Hung. 1979, 99, 155–173. [Google Scholar]
- Schiedt, K.; Liaaen-Jensen, S. Isolation and Analysis. In Carotenoids; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland; Boston, MA, USA; Berlin, Germany, 1995; Volume 1A, pp. 81–108. [Google Scholar]
- Molnár, P. Research of the (E/Z)-isomerization of carotenoids in Pécs since the 1970s. Arch. Biochem. Biophys. 2009, 483, 156–164. [Google Scholar] [CrossRef]
- Molnár, P.; Kawase, M.; Motohashi, N. Isolation, crystallization and handling of carotenoids and (E/Z)-isomerization of carotenoids. In Functional Polyphenols and Carotenoids with Antioxidative Action; Motohashi, N., Ed.; RSFLASH: Kerala, India, 2005; pp. 111–131. [Google Scholar]
- Zechmeister, L. Cis-Trans Isomeric Carotenoids. Vitamins A and Arylpolyenes; Springer: Wien, Austria, 1962. [Google Scholar]
- Pfander, H.; Riesen, R. High-Performance Liquid Chromatography. In Carotenoids; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland; Boston, MA, USA; Berlin, Germany, 1995; Volume 1A, pp. 145–190. [Google Scholar]
- Enzell, C.R.; Back, S. Mass Spectrometry. In Carotenoids; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland; Boston, MA, USA; Berlin, Germany, 1995; Volume 1B, pp. 261–320. [Google Scholar]
- Englert, G. NMR Spectroscopy. In Carotenoids; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland; Boston, MA, USA; Berlin, Germany, 1995; Volume 1B, pp. 147–260. [Google Scholar]
- Deli, J.; Molnár, P.; Tóth, G.; Szabolcs, J.; Radics, L. Determination of the geometrical configuration of naturally occurring mono-cis lutein epoxides. Phytochemistry 1988, 27, 547–549. [Google Scholar] [CrossRef]
- Molnár, P.; Szabolcs, J.; Radics, L. Naturally occurring di-cis-violaxanthins from Viola tricolor: Isolation and identification by 1H-NMR spectroscopy of four di-cis-isomers. Phytochemistry 1986, 25, 195–199. [Google Scholar] [CrossRef]
- Horváth, G.; Molnár, P.; Farkas, Á.; Szabó, L.G.; Turcsi, E.; Deli, J. Separation and identification of carotenoids in flowers of Chelidonium majus L. and inflorescences of Solidago canadensis L. Chromatographia 2010, 71, S103–S108. [Google Scholar] [CrossRef]
- Young, A.J.; Philip, D.; Ruban, A.V.; Horton, P.; Frank, H.A. The xanthophyll cycle and carotenoid-mediated dissipation of excess excitation energy in photosynthesis. Pure Appl. Chem. 1997, 69, 2125–2130. [Google Scholar] [CrossRef]
- Rock, C.L. Carotenoids and cancer. In Carotenoids; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland; Boston, MA, USA; Berlin, Germany, 2009; Volume 5, pp. 269–287. [Google Scholar]
- Johnson, E.J.; Krinsky, N.I. Carotenoids and coronary heart diseases. In Carotenoids; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland; Boston, MA, USA; Berlin, Germany, 2009; Volume 5, pp. 287–301. [Google Scholar]
- Khachik, F.; Bernstein, P.S.; Garland, D.L. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1802–1811. [Google Scholar]
- Schalch, W.; Landrum, J.T.; Bone, R.A. The eye. In Carotenoids; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland; Boston, MA, USA; Berlin, Germany, 2009; Volume 5, pp. 301–335. [Google Scholar]
- Bhatt, D.L. Anti-inflammatory agents and antioxidants as a possible “Third Great Wave” in cardiovascular secondary prevention. Am. J. Cardiol. 2008, 101, 4D–13D. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, H.; Zhang, X.; He, S.; Dong, W.; Wang, X.; Chen, Y.; Liu, X.; Guo, C. Lipopolysaccharide downregulates CD163 expression to inhibit PRRSV infection via TLR4-NF-κB pathway. Front. Microbiol. 2020, 11, 501. [Google Scholar] [CrossRef]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Special molecules, special properties. In Carotenoids; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland; Boston, MA, USA; Berlin, Germany, 2008; Volume 4, pp. 1–6. [Google Scholar]
- Plesca-Manea, L.; Parvu, A.E.; Parvu, M.; Puia, M. Effect of coumarin on acute experimental inflammation. Noutatea Med. 1999, 3, 17–21. [Google Scholar]
- Plesca-Manea, L.; Parvu, A.E.; Parvu, M.; Taamas, M.; Buia, R.; Puia, M. Effect of Melilotus officinalis on acute inflammation. Phytother. Res. 2002, 16, 316–319. [Google Scholar] [CrossRef]
- Hsin-Lan, L.; Tsai-Hua, K.; Chyuan-Yuan, S.; Bing-Huei, C. Functional components in Scutellaria barbata D. Don with anti-inflammatory activity on RAW 264.7 cells. J. Food Drug Anal. 2018, 26, 31–40. [Google Scholar]
- Iványi, R.; Németh, K.; Visy, J.; Szeman, J.; Szente, L.; Simonyi, M. Water soluble carotenoid/CD complexes: Preparation, characterization. In Proceedings of the 14th International Cyclodextrins Symposium, Kyoto, Japan, 8–11 May 2008; pp. 1–29. [Google Scholar]
- Markovics, A.; Szőke, É.; Sándor, K.; Börzsei, R.; Bagoly, T.; Kemény, Á.; Elekes, K.; Pintér, E.; Szolcsányi, J.; Helyes, Z. Comparison of the anti-inflammatory and anti-nociceptive effects of cortistatin-14 and somatostatin-14 in distinct in vitro and in vivo model systems. J. Mol. Neurosci. 2012, 46, 40–50. [Google Scholar] [CrossRef]
Sample Availability: The isolated carotenoid samples are available from the authors. |


| Carotenoid | Peak Number | tR (min) | % | UV-Vis λmax (nm) in High Performance Liquid Chromatography (HPLC) Solvent | ||
|---|---|---|---|---|---|---|
| Unidentifiable mixture | 1 | 6.9 | 1.0 | 401 | 424 | 444 |
| (all-E)-neoxanthin | 2 | 9.4 | 3.4 | 417 | 440 | 469 |
| (9Z)-neoxanthin | 3 | 11.3 | 0.8 | 413 | 436 | 465 |
| (all-E)-violaxanthin | 4 | 12.5 | 10.5 | 416 | 438 | 468 |
| (all-E)-lutein 5,6-epoxide | 5 | 17.2 | 33.8 | 415 | 438 | 468 |
| flavoxanthin + chrysanthemaxanthin | 6 | 18.7 | 3.8 | 398 | 420 | 447 |
| (all-E)-lutein | 7 | 21.8 | 32.5 | (418) | 443 | 471 |
| (13Z) + (13′Z)-lutein 5,6-epoxide | 8 | 22.8 | 1.5 | 409 | 432 | 460 |
| (9Z) + (9′Z)-lutein | 9 | 25.5 | 2.6 | (414) | 439 | 466 |
| (13Z) + (13′Z)-lutein | 10 | 26.3 | 1.2 | (412) | 437 | 463 |
| α-cryptoxanthin | 11 | 32.8 | 1.6 | (420) | 445 | 473 |
| (9Z) + (9′Z)-β-cryptoxanthin | 12 | 36.2 | 1.3 | (420) | 447 | 473 |
| β-carotene | 13 | 39.6 | 6.0 | (423) | 452 | 478 |
| Carotenoid | Peak Number | tR (min) | % | UV-Vis λmax (nm) in HPLC Solvent | ||
|---|---|---|---|---|---|---|
| (all-E)-neoxanthin | 1 | 9.0 | 3.4 | 417 | 441 | 469 |
| (all-E)-violaxanthin | 2 | 12.0 | 10.5 | 416 | 439 | 468 |
| (all-E)-lutein 5,6-epoxide | 3 | 16.6 | 33.8 | 415 | 438 | 467 |
| flavoxanthin + chrysanthemaxanthin | 4 | 18.2 | 3.8 | 398 | 421 | 447 |
| (all-E)-lutein | 5 | 21.2 | 32.5 | (418) | 443 | 470 |
| β-carotene | 6 | 38.9 | 6.0 | (423) | 452 | 478 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horváth, G.; Csikós, E.; Andres, E.V.; Bencsik, T.; Takátsy, A.; Gulyás-Fekete, G.; Turcsi, E.; Deli, J.; Szőke, É.; Kemény, Á.; et al. Analyzing the Carotenoid Composition of Melilot (Melilotus officinalis (L.) Pall.) Extracts and the Effects of Isolated (All-E)-lutein-5,6-epoxide on Primary Sensory Neurons and Macrophages. Molecules 2021, 26, 503. https://doi.org/10.3390/molecules26020503
Horváth G, Csikós E, Andres EV, Bencsik T, Takátsy A, Gulyás-Fekete G, Turcsi E, Deli J, Szőke É, Kemény Á, et al. Analyzing the Carotenoid Composition of Melilot (Melilotus officinalis (L.) Pall.) Extracts and the Effects of Isolated (All-E)-lutein-5,6-epoxide on Primary Sensory Neurons and Macrophages. Molecules. 2021; 26(2):503. https://doi.org/10.3390/molecules26020503
Chicago/Turabian StyleHorváth, Györgyi, Eszter Csikós, Eichertné Violetta Andres, Tímea Bencsik, Anikó Takátsy, Gergely Gulyás-Fekete, Erika Turcsi, József Deli, Éva Szőke, Ágnes Kemény, and et al. 2021. "Analyzing the Carotenoid Composition of Melilot (Melilotus officinalis (L.) Pall.) Extracts and the Effects of Isolated (All-E)-lutein-5,6-epoxide on Primary Sensory Neurons and Macrophages" Molecules 26, no. 2: 503. https://doi.org/10.3390/molecules26020503
APA StyleHorváth, G., Csikós, E., Andres, E. V., Bencsik, T., Takátsy, A., Gulyás-Fekete, G., Turcsi, E., Deli, J., Szőke, É., Kemény, Á., Payrits, M., Szente, L., Kocsis, M., Molnár, P., & Helyes, Z. (2021). Analyzing the Carotenoid Composition of Melilot (Melilotus officinalis (L.) Pall.) Extracts and the Effects of Isolated (All-E)-lutein-5,6-epoxide on Primary Sensory Neurons and Macrophages. Molecules, 26(2), 503. https://doi.org/10.3390/molecules26020503









