Effects of Bisphenol A and Its Alternatives, Bisphenol F and Tetramethyl Bisphenol F on Osteoclast Differentiation
Abstract
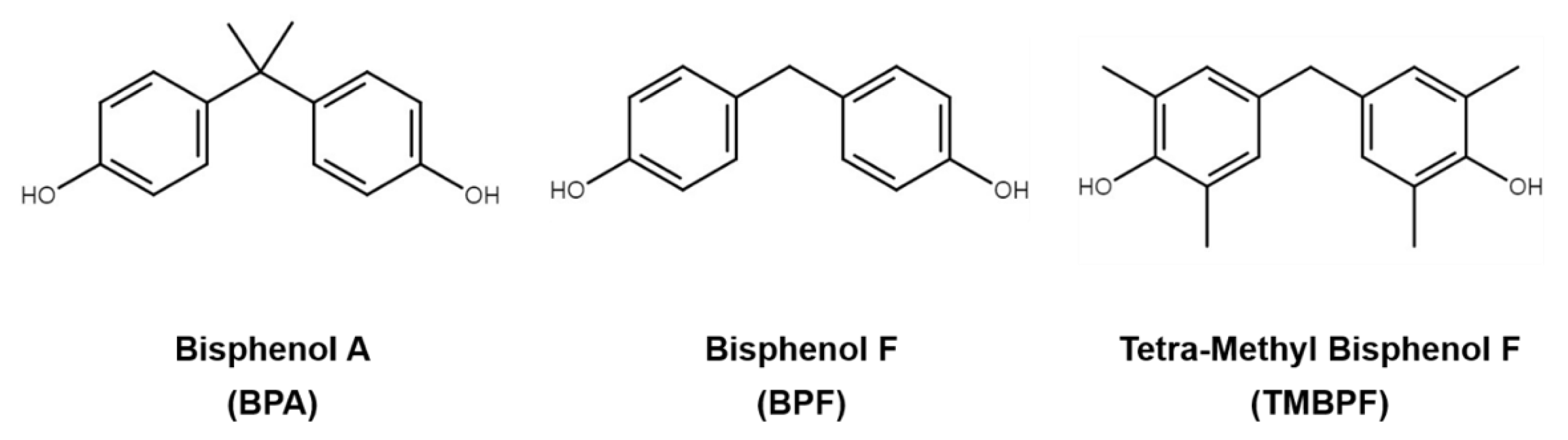
:1. Introduction
2. Results and Discussion
2.1. Effects of Bisphenol A and Its Alternatives on Cell Viability in Mouse Bone Marrow Macrophage RAW 264.7 Cells
2.2. Activation of Osteoclast Differentiation by Bisphenol A and Its Substitutes
2.3. Effect of TMBPF on Osteoclast Target-Related Genes Expression
2.4. Acceleration of the JNK and p38 Pathways by TMBPF in RAW 264.7 Cells
3. Materials and Methods
3.1. Reagents
3.2. Cell Culture and Osteoclast Differentiation
3.3. Cell Viability Assays
3.4. TRAP Staining and Activity Assays
3.5. RNA Preparation and RNA Quantitation by RT-PCR
3.6. Immunoblot Analysis
3.7. Statistical Analysis
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rodan, G.A. Bone homeostasis. Proc. Natl. Acad. Sci. USA 1998, 95, 13361–13362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, H.; Chen, X.; Gao, S.; Yu, X.; Xiao, J.; Zhang, B.; Liu, X.; Dai, M. Key Triggers of Osteoclast-Related Diseases and Available Strategies for Targeted Therapies: A Review. Front. Med. 2017, 4, 234. [Google Scholar] [CrossRef]
- Kurihara, N.; Reddy, S.V.; Menaa, C.; Anderson, D.; Roodman, G.D. Osteoclasts expressing the measles virus nucleocapsid gene display a pagetic phenotype. J. Clin. Investig. 2000, 105, 607–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodan, G.A.; Martin, T.J. Therapeutic approaches to bone diseases. Science 2000, 289, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Kotake, S.; Udagawa, N.; Hakoda, M.; Mogi, M.; Yano, K.; Tsuda, E.; Takahashi, K.; Furuya, T.; Ishiyama, S.; Kim, K.J.; et al. Activated human T cells directly induce osteoclastogenesis from human monocytes: Possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum. 2001, 44, 1003–1012. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Lomaga, M.A.; Yeh, W.C.; Sarosi, I.; Duncan, G.S.; Furlonger, C.; Ho, A.; Morony, S.; Capparelli, C.; Van, G.; Kaufman, S.; et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999, 13, 1015–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, B.R.; Josien, R.; Lee, S.Y.; Vologodskaia, M.; Steinman, R.M.; Choi, Y. The TRAF family of signal transducers mediates NF-kappaB activation by the TRANCE receptor. J. Biol. Chem. 1998, 273, 28355–28359. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, H.; Hirata, A.; Tsuji, T.; Yamamoto, T. Role of osteoclast extracellular signal-regulated kinase (ERK) in cell survival and maintenance of cell polarity. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2003, 18, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- David, J.P.; Sabapathy, K.; Hoffmann, O.; Idarraga, M.H.; Wagner, E.F. JNK1 modulates osteoclastogenesis through both c-Jun phosphorylation-dependent and -independent mechanisms. J. Cell Sci. 2002, 115, 4317–4325. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Udagawa, N.; Itoh, K.; Suda, K.; Murase, Y.; Nishihara, T.; Suda, T.; Takahashi, N. p38 MAPK-mediated signals are required for inducing osteoclast differentiation but not for osteoclast function. Endocrinology 2002, 143, 3105–3113. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, N. Regulation of NFATc1 in Osteoclast Differentiation. J. Bone Metab. 2014, 21, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, M.; Kogawa, M.; Wada, S.; Takayanagi, H.; Tsujimoto, M.; Katayama, S.; Hisatake, K.; Nogi, Y. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J. Biol. Chem. 2004, 279, 45969–45979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, L.A. Estrogen therapy for postmenopausal osteoporosis. Arq. Bras. De Endocrinol. E Metabol. 2006, 50, 705–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosla, S.; Melton, L.J.3rd; Riggs, B.L. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: Is a revision needed? J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2011, 26, 441–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambacciani, M.; Levancini, M. Hormone replacement therapy and the prevention of postmenopausal osteoporosis. Prz. Menopauzalny = Menopause Rev. 2014, 13, 213–220. [Google Scholar] [CrossRef]
- Kameda, T.; Mano, H.; Yuasa, T.; Mori, Y.; Miyazawa, K.; Shiokawa, M.; Nakamaru, Y.; Hiroi, E.; Hiura, K.; Kameda, A.; et al. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J. Exp. Med. 1997, 186, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Imai, Y.; Matsumoto, T.; Sato, S.; Takeuchi, K.; Igarashi, K.; Harada, Y.; Azuma, Y.; Krust, A.; Yamamoto, Y.; et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 2007, 130, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, A.; Rutkowska, A.; Rachon, D. Health risk of exposure to Bisphenol A (BPA). Rocz. Panstw. Zakl. Hig. 2015, 66, 5–11. [Google Scholar]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- Steinmetz, R.; Brown, N.G.; Allen, D.L.; Bigsby, R.M.; Ben-Jonathan, N. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology 1997, 138, 1780–1786. [Google Scholar] [CrossRef]
- Steinmetz, R.; Mitchner, N.A.; Grant, A.; Allen, D.L.; Bigsby, R.M.; Ben-Jonathan, N. The xenoestrogen bisphenol A induces growth, differentiation, and c-fos gene expression in the female reproductive tract. Endocrinology 1998, 139, 2741–2747. [Google Scholar] [CrossRef]
- Acconcia, F.; Pallottini, V.; Marino, M. Molecular Mechanisms of Action of BPA. Dose-Response A Publ. Int. Hormesis Soc. 2015, 13, 1559325815610582. [Google Scholar] [CrossRef] [Green Version]
- Bolli, A.; Galluzzo, P.; Ascenzi, P.; Del Pozzo, G.; Manco, I.; Vietri, M.T.; Mita, L.; Altucci, L.; Mita, D.G.; Marino, M. Laccase treatment impairs bisphenol A-induced cancer cell proliferation affecting estrogen receptor alpha-dependent rapid signals. Iubmb Life 2008, 60, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. Jama 2008, 300, 1303–1310. [Google Scholar] [CrossRef]
- Li, D.K.; Zhou, Z.; Miao, M.; He, Y.; Wang, J.; Ferber, J.; Herrinton, L.J.; Gao, E.; Yuan, W. Urine bisphenol-A (BPA) level in relation to semen quality. Fertil. Steril. 2011, 95, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Shankar, A.; Teppala, S. Relationship between urinary bisphenol A levels and diabetes mellitus. J. Clin. Endocrinol. Metab. 2011, 96, 3822–3826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trasande, L.; Attina, T.M.; Blustein, J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. Jama 2012, 308, 1113–1121. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Ryu, J.H.; Jeon, R.; Kang, D.; Yoo, K.Y. Effects of bisphenol A on breast cancer and its risk factors. Arch. Toxicol. 2009, 83, 281–285. [Google Scholar] [CrossRef]
- Szafran, A.T.; Stossi, F.; Mancini, M.G.; Walker, C.L.; Mancini, M.A. Characterizing properties of non-estrogenic substituted bisphenol analogs using high throughput microscopy and image analysis. Plos One 2017, 12, e0180141. [Google Scholar]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.K. Concern about the Safety of Bisphenol A Substitutes. Diabetes Metab. J. 2019, 43, 46–48. [Google Scholar] [CrossRef]
- Eladak, S.; Grisin, T.; Moison, D.; Guerquin, M.J.; N’Tumba-Byn, T.; Pozzi-Gaudin, S.; Benachi, A.; Livera, G.; Rouiller-Fabre, V.; Habert, R. A new chapter in the bisphenol A story: Bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil. Steril. 2015, 103, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.; Song, H.; Choi, J.; Sim, S.; Kojima, H.; Park, J.; Iida, M.; Lee, Y. The mixture effects of bisphenol derivatives on estrogen receptor and androgen receptor. Environ. Pollut. 2020, 260, 114036. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Takeuchi, S.; Sanoh, S.; Okuda, K.; Kitamura, S.; Uramaru, N.; Sugihara, K.; Yoshinari, K. Profiling of bisphenol A and eight its analogues on transcriptional activity via human nuclear receptors. Toxicology 2019, 413, 48–55. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Moriguchi, Y.; Oshima, H.; Kawaguchi, M.; Miyazaki, K.; Nakamura, M. Measurement of estrogenic activity of chemicals for the development of new dental polymers. Toxicol. Vitr. Int. J. Publ. Assoc. Bibra 2001, 15, 421–425. [Google Scholar] [CrossRef]
- Yamasaki, K.; Takeyoshi, M.; Yakabe, Y.; Sawaki, M.; Imatanaka, N.; Takatsuki, M. Comparison of reporter gene assay and immature rat uterotrophic assay of twenty-three chemicals. Toxicology 2002, 170, 21–30. [Google Scholar] [CrossRef]
- Streicher, C.; Heyny, A.; Andrukhova, O.; Haigl, B.; Slavic, S.; Schuler, C.; Kollmann, K.; Kantner, I.; Sexl, V.; Kleiter, M.; et al. Estrogen Regulates Bone Turnover by Targeting RANKL Expression in Bone Lining Cells. Sci. Rep. 2017, 7, 6460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, K.Y.; Pang, K.L.; Mark-Lee, W.F. A Review on the Effects of Bisphenol A and Its Derivatives on Skeletal Health. Int. J. Med Sci. 2018, 15, 1043–1050. [Google Scholar] [CrossRef] [Green Version]
- Lind, T.; Lejonklou, M.H.; Dunder, L.; Kushnir, M.M.; Ohman-Magi, C.; Larsson, S.; Melhus, H.; Lind, P.M. Developmental low-dose exposure to bisphenol A induces chronic inflammation, bone marrow fibrosis and reduces bone stiffness in female rat offspring only. Environ. Res. 2019, 177, 108584. [Google Scholar] [CrossRef]
- Hwang, J.K.; Min, K.H.; Choi, K.H.; Hwang, Y.C.; Jeong, I.K.; Ahn, K.J.; Chung, H.Y.; Chang, J.S. Bisphenol A reduces differentiation and stimulates apoptosis of osteoclasts and osteoblasts. Life Sci. 2013, 93, 367–372. [Google Scholar] [CrossRef]
- Hayman, A.R. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity 2008, 41, 218–223. [Google Scholar] [CrossRef]
- Lejonklou, M.H.; Christiansen, S.; Orberg, J.; Shen, L.; Larsson, S.; Boberg, J.; Hass, U.; Lind, P.M. Low-dose developmental exposure to bisphenol A alters the femoral bone geometry in wistar rats. Chemosphere 2016, 164, 339–346. [Google Scholar] [CrossRef]
- Pelch, K.E.; Carleton, S.M.; Phillips, C.L.; Nagel, S.C. Developmental exposure to xenoestrogens at low doses alters femur length and tensile strength in adult mice. Biol. Reprod. 2012, 86, 69. [Google Scholar] [CrossRef]
- Fic, A.; Mlakar, S.J.; Juvan, P.; Mlakar, V.; Marc, J.; Dolenc, M.S.; Broberg, K.; Masic, L.P. Genome-wide gene expression profiling of low-dose, long-term exposure of human osteosarcoma cells to bisphenol A and its analogs bisphenols AF and S. Toxicol. Vitr. Int. J. Publ. Assoc. Bibra 2015, 29, 1060–1069. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, N. Signaling Pathways in Osteoclast Differentiation. Chonnam Med J. 2016, 52, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Staples, C.A.; Dorn, P.B.; Klecka, G.M.; O’Block, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Soto, A.M.; Schaeberle, C.; Maier, M.S.; Sonnenschein, C.; Maffini, M.V. Evidence of Absence: Estrogenicity Assessment of a New Food-Contact Coating and the Bisphenol Used in Its Synthesis. Environ. Sci. Technol. 2017, 51, 1718–1726. [Google Scholar] [CrossRef]
- Cohen, I.C.; Cohenour, E.R.; Harnett, K.G.; Schuh, S.M. BPA, BPAF and TMBPF Alter Adipogenesis and Fat Accumulation in Human Mesenchymal Stem Cells, with Implications for Obesity. Int. J. Mol. Sci. 2021, 22, 5363. [Google Scholar] [CrossRef]
- Maffini, M.V.; Canatsey, R.D. An expanded toxicological profile of tetramethyl bisphenol F (TMBPF), a precursor for a new food-contact metal packaging coating. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 135, 110889. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-M.; Lee, S.-M.; Choi, J.; Soung, N.-K.; Heo, J.-D. Effects of Bisphenol A and Its Alternatives, Bisphenol F and Tetramethyl Bisphenol F on Osteoclast Differentiation. Molecules 2021, 26, 6100. https://doi.org/10.3390/molecules26206100
Kim H-M, Lee S-M, Choi J, Soung N-K, Heo J-D. Effects of Bisphenol A and Its Alternatives, Bisphenol F and Tetramethyl Bisphenol F on Osteoclast Differentiation. Molecules. 2021; 26(20):6100. https://doi.org/10.3390/molecules26206100
Chicago/Turabian StyleKim, Hye-Min, Seon-Min Lee, Jungil Choi, Nak-Kyun Soung, and Jeong-Doo Heo. 2021. "Effects of Bisphenol A and Its Alternatives, Bisphenol F and Tetramethyl Bisphenol F on Osteoclast Differentiation" Molecules 26, no. 20: 6100. https://doi.org/10.3390/molecules26206100






