Sol-Gel Synthesis of Ceria-Zirconia-Based High-Entropy Oxides as High-Promotion Catalysts for the Synthesis of 1,2-Diketones from Aldehyde
Abstract
:1. Introduction
2. Results and Discussion
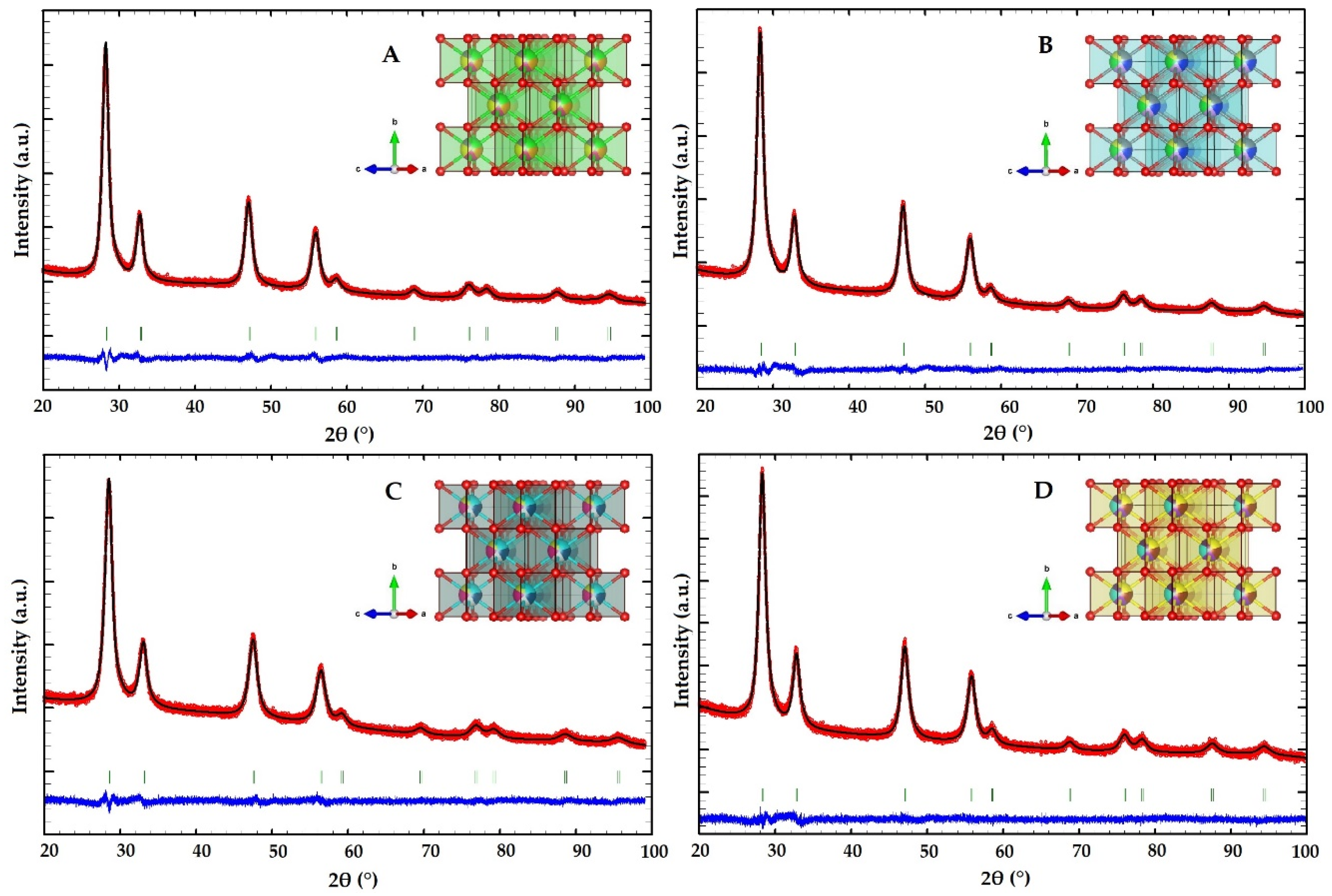
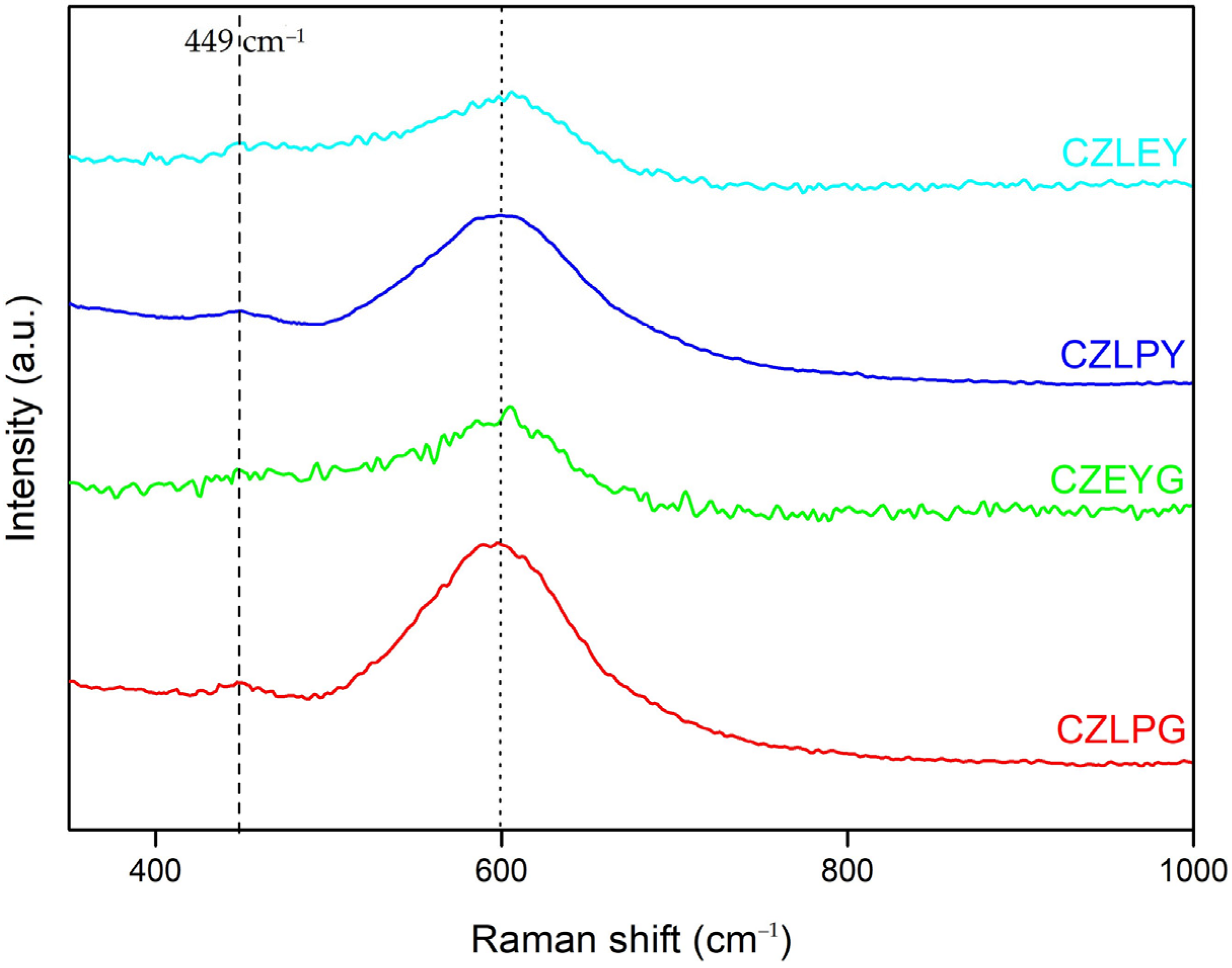
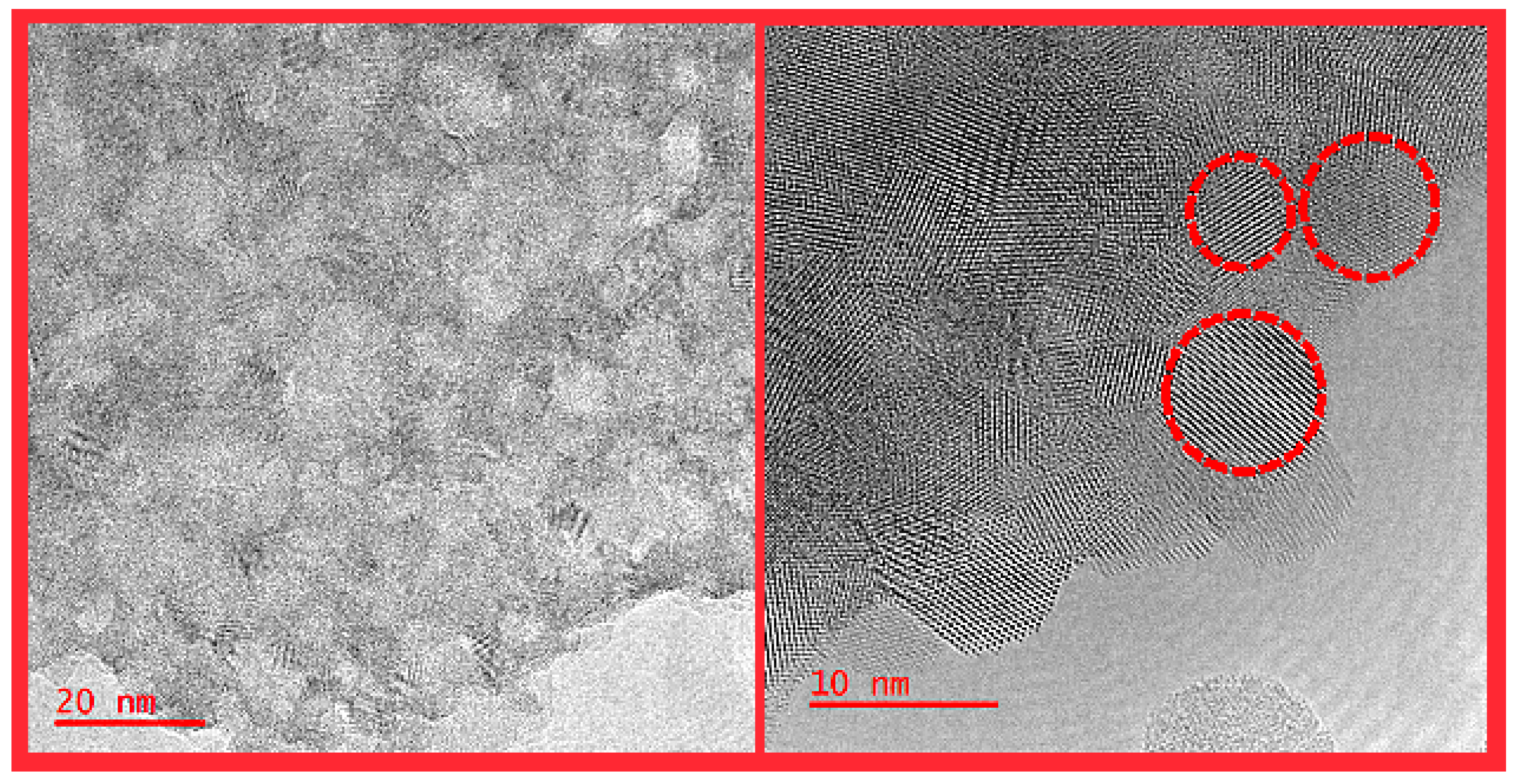
2.1. Structural Characterization of the HEOs
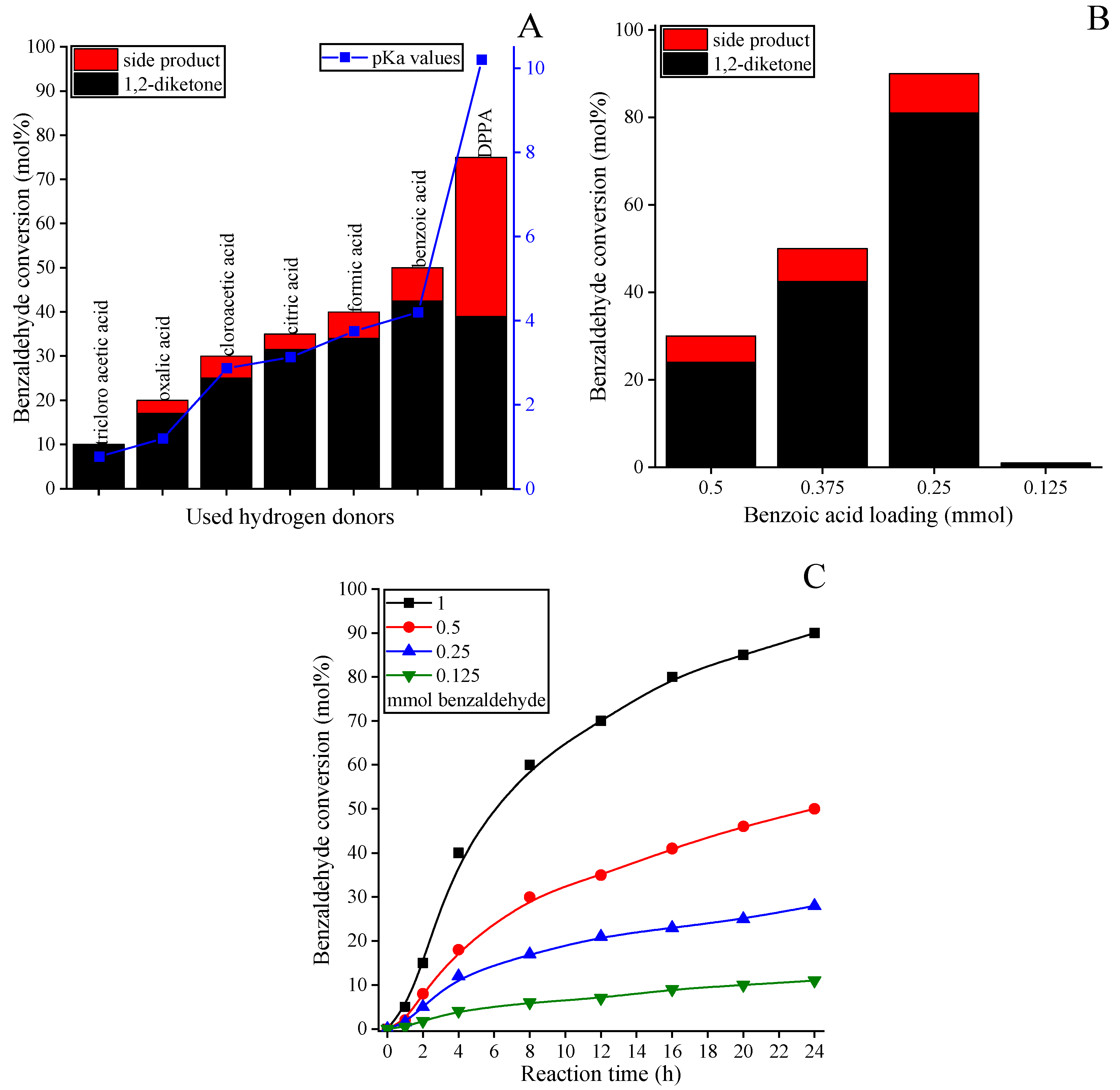
2.2. Catalytic Ability of the HEOs
3. Materials and Methods
3.1. Materials
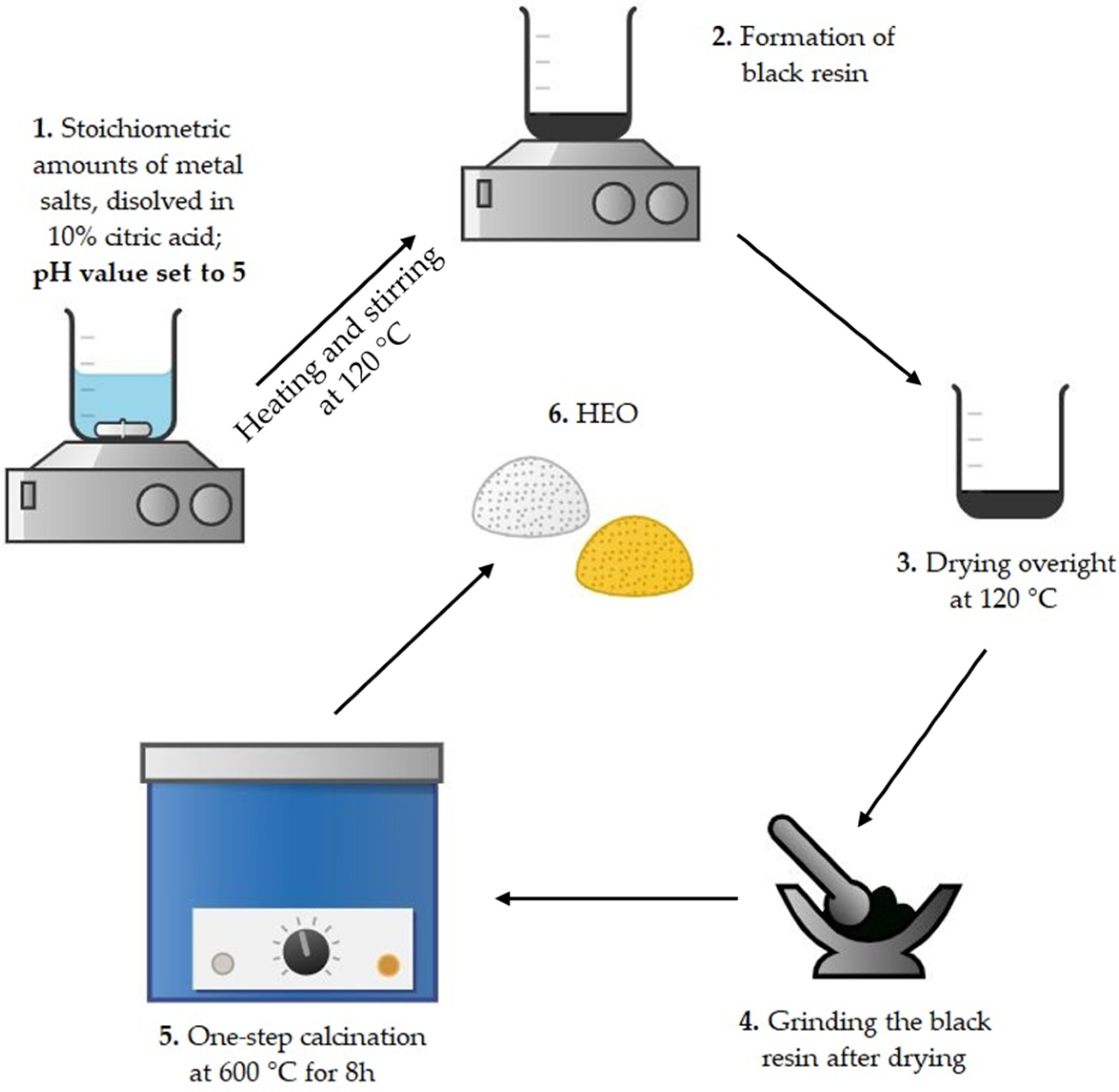
3.2. Synthesis of the HEOs
3.3. Methods
3.3.1. Powder X-ray Diffraction
3.3.2. Brunauer–Emmett–Teller Surface Area Measurement
3.3.3. Raman Spectroscopy
3.3.4. SEM, TEM, and STEM-EDX Measurements
3.3.5. ICP-MS Measurements
3.3.6. Temperature-Programmed Desorption of NH3
3.4. Catalytic Reaction Procedures
3.4.1. Pinacol-Type Oxidative Coupling of the Aldehydes
3.4.2. Hot Filtration Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.P. Entropy-stabilized oxides. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djenadic, R.; Sarkar, A.; Clemens, O.; Loho, C.; Botros, M.; Chakravadhanula, V.S.K.; Kübel, C.; Bhattacharya, S.S.; Gandhi, A.S.; Hahn, H. Multicomponent equiatomic rare earth oxides. Mater. Res. Lett. 2017, 5, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Gild, J.; Samiee, M.; Braun, J.L.; Harrington, T.; Vega, H.; Hopkins, P.E.; Vecchio, K.; Luo, J. High-entropy fluorite oxides. J. Eur. Ceram. Soc. 2018, 38, 3578–3584. [Google Scholar] [CrossRef]
- Chen, K.; Pei, X.; Tang, L.; Cheng, H.; Li, Z.; Li, C.; Zhang, X.; An, L. A five-component entropy-stabilized fluorite oxide. J. Eur. Ceram. Soc. 2018, 38, 4161–4164. [Google Scholar] [CrossRef]
- Sarkar, A.; Djenadic, R.; Wang, D.; Hein, C.; Kautenburger, R.; Clemens, O.; Hahn, H. Rare earth and transition metal based entropy stabilised perovskite type oxides. J. Eur. Ceram. Soc. 2018, 38, 2318–2327. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, T.; Gild, J.; Zhou, N.; Nie, J.; Qin, M.; Harrington, T.; Vecchio, K.; Luo, J. A new class of high-entropy perovskite oxides. Scr. Mater. 2018, 142, 116–120. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Stygar, M.; Mikuła, A.; Knapik, A.; Mroczka, K.; Tejchman, W.; Danielewski, M.; Martin, M. Synthesis and microstructure of the (Co,Cr,Fe,Mn,Ni)3O4 high entropy oxide characterized by spinel structure. Mater. Lett. 2018, 216, 32–36. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Z.; Du, S.; Zhang, Y.; Li, H.; Xiao, Z.; Chen, W.; Chen, R.; Wang, Y.; Zou, Y.; et al. Low-temperature synthesis of small-sized high-entropy oxides for water oxidation. J. Mater. Chem. A 2019, 7, 24211–24216. [Google Scholar] [CrossRef]
- Rost, C.M.; Rak, Z.; Brenner, D.W.; Maria, J.P. Local structure of the MgxNixCoxCuxZnxO(x = 0.2) entropy-stabilized oxide: An EXAFS study. J. Am. Ceram. Soc. 2017, 100, 2732–2738. [Google Scholar] [CrossRef]
- Berardan, D.; Meena, A.K.; Franger, S.; Herrero, C.; Dragoe, N. Controlled Jahn-Teller distortion in (MgCoNiCuZn)O-based high entropy oxides. J. Alloys Compd. 2017, 704, 693–700. [Google Scholar] [CrossRef]
- Stygar, M.; Dąbrowa, J.; Moździerz, M.; Zajusz, M.; Skubida, W.; Mroczka, K.; Berent, K.; Świerczek, K.; Danielewski, M. Formation and properties of high entropy oxides in Co-Cr-Fe-Mg-Mn-Ni-O system: Novel (Cr,Fe,Mg,Mn,Ni)3O4 and (Co,Cr,Fe,Mg,Mn)3O4 high entropy spinels. J. Eur. Ceram. Soc. 2020, 40, 1644–1650. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiang, H.; Dai, F.Z.; Peng, Z.; Zhou, Y. (La0.2Ce0.2Nd0.2Sm0.2Eu0.2)2Zr2O7: A novel high-entropy ceramic with low thermal conductivity and sluggish grain growth rate. J. Mater. Sci. Technol. 2019, 35, 2647–2651. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Szymczak, M.; Zajusz, M.; Mikuła, A.; Moździerz, M.; Berent, K.; Wytrwal-Sarna, M.; Bernasik, A.; Stygar, M.; Świerczek, K. Stabilizing fluorite structure in ceria-based high-entropy oxides: Influence of Mo addition on crystal structure and transport properties. J. Eur. Ceram. Soc. 2020, 40, 5870–5881. [Google Scholar] [CrossRef]
- Agasti, N.; Astle, M.A.; Rance, G.A.; Alves Fernandes, J.; Dupont, J.; Khlobystov, A.N. Cerium Oxide Nanoparticles Inside Carbon Nanoreactors for Selective Allylic Oxidation of Cyclohexene. Nano Lett. 2020, 20, 1161–1171. [Google Scholar] [CrossRef]
- Rashed, M.N.; Masuda, K.; Ichitsuka, T.; Koumura, N.; Sato, K.; Kobayashi, S. Zirconium Oxide-Catalyzed Direct Amidation of Unactivated Esters under Continuous-Flow Conditions. Adv. Synth. Catal. 2021, 363, 2529–2535. [Google Scholar] [CrossRef]
- Qaroush, A.K.; Alsoubani, F.A.; Al-Khateeb, A.M.; Nabih, E.; Al-Ramahi, E.; Khanfar, M.F.; Assaf, K.I.; Eftaiha, A.F. An efficient atom-economical chemoselective CO2 cycloaddition using lanthanum oxide/tetrabutyl ammonium bromide. Sustain. Energy Fuels 2018, 2, 1342–1349. [Google Scholar] [CrossRef]
- Boronat, M.; Corma, A.; Renz, M.; Viruela, P.M. Predicting the activity of single isolated Lewis acid sites in solid catalysts. Chem.-A Eur. J. 2006, 12, 7067–7077. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.J.; Rondón, M.; Suárez, E. Stereocontrolled photocyclization of 1,2-diketones: Application of a 1,3-acetyl group transfer methodology to carbohydrates. J. Org. Chem. 2008, 73, 3384–3391. [Google Scholar] [CrossRef]
- Schmitt, D.C.; Lam, L.; Johnson, J.S. Three-component coupling approach to trachyspic acid. Org. Lett. 2011, 13, 5136–5139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Cruz, O.; Zolotukhin, M.G.; Fomine, S.; Alexandrova, L.; Aguilar-Lugo, C.; Ruiz-Treviño, F.A.; Ramos-Ortíz, G.; Maldonado, J.L.; Cadenas-Pliego, G. High- Tg functional aromatic polymers. Macromolecules 2015, 48, 1026–1037. [Google Scholar] [CrossRef]
- Al-Kahraman, Y.M.S.A.; Yasinzai, M.; Singh, G.S. Evaluation of some classical hydrazones of ketones and 1,2-diketones as antileishmanial, antibacterial and antifungal agents. Arch. Pharm. Res. 2012, 35, 1009–1013. [Google Scholar] [CrossRef]
- Spikes, G.H.; Sproules, S.; Bill, E.; Weyhermu, T.; Wieghardt, K. One- and Two-Electron Reduced 1, 2-Diketone Ligands in [ CrIII (L•)3](S = 0) and Na2(Et2O)2[VIV(LRed)3](S = 1/2). Inorg. Chem. 2008, 47, 10935–10944. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wan, X. Ruthenium-catalyzed oxidation of alkynes to 1,2-diketones under room temperature and one-pot synthesis of quinoxalines. Tetrahedron Lett. 2013, 54, 642–645. [Google Scholar] [CrossRef]
- Jong, C.L.; Park, H.J.; Jin, Y.P. Rapid microwave-promoted solvent-free oxidation of α-methylene ketones to α-diketones. Tetrahedron Lett. 2002, 43, 5661–5663. [Google Scholar] [CrossRef]
- Sawaki, Y.; Ogata, Y. Kinetics of the Base-Catalyzed Decomposition of.alpha.-hydroperoxy ketones. J. Am. Chem. Soc. 1975, 97, 6983–6989. [Google Scholar] [CrossRef]
- Chen, B.; Wu, X.F. Palladium-catalyzed synthesis of 1,2-diketones from aryl halides and organoaluminum reagents using tert-butyl isocyanide as the co source. Org. Lett. 2020, 22, 636–641. [Google Scholar] [CrossRef]
- Min, H.; Palani, T.; Park, K.; Hwang, J.; Lee, S. Copper-catalyzed direct synthesis of diaryl 1,2-diketones from Aryl iodides and propiolic acids. J. Org. Chem. 2014, 79, 6279–6285. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.X.; Zeng, Y.F.; Zhang, S.S.; Li, Q.; Wang, H. Mild Mn(OAc)3-Mediated Aerobic Oxidative Decarboxylative Coupling of Arylboronic Acids and Arylpropiolic Acids: Direct Access to Diaryl 1,2-Diketones. Org. Lett. 2015, 17, 2972–2975. [Google Scholar] [CrossRef] [PubMed]
- Caron, A.; Morin, É.; Collins, S.K. Bifunctional Copper-Based Photocatalyst for Reductive Pinacol-Type Couplings. ACS Catal. 2019, 9, 9458–9464. [Google Scholar] [CrossRef]
- Mori, S.; Takubo, M.; Yanase, T.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Palladium on Carbon-Catalyzed synthesis of benzil derivatives from 1,2-Diarylalkynes with DMSO and molecular oxygen as dual oxidants. Adv. Synth. Catal. 2010, 352, 1630–1634. [Google Scholar] [CrossRef]
- Hernández, W.Y.; Centeno, M.A.; Romero-Sarria, F.; Odriozola, J.A. Synthesis and characterization of Ce1-xEuxO2-x/2 mixed oxides and their catalytic activities for CO oxidation. J. Phys. Chem. C 2009, 113, 5629–5635. [Google Scholar] [CrossRef]
- Krishna, K.; Bueno-López, A.; Makkee, M.; Moulijn, J.A. Potential rare earth modified CeO2 catalysts for soot oxidation. I. Characterisation and catalytic activity with O2. Appl. Catal. B Environ. 2007, 75, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Hernández, W.Y.; Laguna, O.H.; Centeno, M.A.; Odriozola, J.A. Structural and catalytic properties of lanthanide (La, Eu, Gd) doped ceria. J. Solid State Chem. 2011, 184, 3014–3020. [Google Scholar] [CrossRef]
- Sarkar, A.; Loho, C.; Velasco, L.; Thomas, T.; Bhattacharya, S.S.; Hahn, H.; Djenadic, R. Multicomponent equiatomic rare earth oxides with a narrow band gap and associated praseodymium multivalency. Dalt. Trans. 2017, 46, 12167–12176. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.H.; Hass, K.C.; McBride, J.R. Raman study of CeO2: Second-order scattering, lattice dynamics, and particle-size effects. Phys. Rev. B 1993, 48, 178–185. [Google Scholar] [CrossRef]
- Artini, C.; Pani, M.; Carnasciali, M.M.; Buscaglia, M.T.; Plaisier, J.R.; Costa, G.A. Structural features of Sm- and Gd-doped ceria studied by synchrotron X-ray diffraction and -raman spectroscopy. Inorg. Chem. 2015, 54, 4126–4137. [Google Scholar] [CrossRef]
- Paunović, N.; Dohcevic-Mitrovic, Z.; Scurtu, R.; Aškrabić, S.; Prekajski, M.; Matović, B.; Popović, Z.V. Suppression of inherent ferromagnetism in Pr-doped CeO2 nanocrystals. Nanoscale 2012, 4, 5469–5476. [Google Scholar] [CrossRef]
- Guo, M.; Lu, J.; Wu, Y.; Wang, Y.; Luo, M. UV and visible Raman studies of oxygen vacancies in rare-earth-doped ceria. Langmuir 2011, 27, 3872–3877. [Google Scholar] [CrossRef] [PubMed]
- Filtschew, A.; Hofmann, K.; Hess, C. Ceria and Its Defect Structure: New Insights from a Combined Spectroscopic Approach. J. Phys. Chem. C 2016, 120, 6694–6703. [Google Scholar] [CrossRef]
- Matei-Rutkovska, F.; Postole, G.; Rotaru, C.G.; Florea, M.; Pârvulescu, V.I.; Gelin, P. Synthesis of ceria nanopowders by microwave-assisted hydrothermal method for dry reforming of methane. Int. J. Hydrogen Energy 2016, 41, 2512–2525. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Y.; Wang, Q.; Wang, K.; Huang, X.; Stenzel, D.; Sarkar, A.; Azmi, R.; Bergfeldt, T.; Bhattacharya, S.S.; et al. Lithium containing layered high entropy oxide structures. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Hu, Y.; Wang, M.; Li, H. Influence of doping elements on catalytic performance of CeO2-ZrO2 solid solutions. J. Rare Earths 2008, 26, 530–535. [Google Scholar] [CrossRef]
- Jampaiah, D.; Tur, K.M.; Ippolito, S.J.; Sabri, Y.M.; Tardio, J.; Bhargava, S.K.; Reddy, B.M. Structural characterization and catalytic evaluation of transition and rare earth metal doped ceria-based solid solutions for elemental mercury oxidation. RSC Adv. 2013, 3, 12963–12974. [Google Scholar] [CrossRef]
- Florea, M.; Postole, G.; Matei-Rutkovska, F.; Urda, A.; Neaţu, F.; Massin, L.; Gelin, P. Influence of Gd and Pr doping on the properties of ceria: Texture, structure, redox behaviour and reactivity in CH4/H2O reactions in the presence of H2S. Catal. Sci. Technol. 2018, 8, 1333–1348. [Google Scholar] [CrossRef]
- Tok, A.I.Y.; Du, S.W.; Boey, F.Y.C.; Chong, W.K. Hydrothermal synthesis and characterization of rare earth doped ceria nanoparticles. Mater. Sci. Eng. A 2007, 466, 223–229. [Google Scholar] [CrossRef]
- Tiseanu, C.; Parvulescu, V.I.; Boutonnet, M.; Cojocaru, B.; Primus, P.A.; Teodorescu, C.M.; Solans, C.; Dominguez, M.S. Surface versus volume effects in luminescent ceria nanocrystals synthesized by an oil-in-water microemulsion method. Phys. Chem. Chem. Phys. 2011, 13, 17135–17145. [Google Scholar] [CrossRef] [PubMed]
- Beckers, I.; Krasniqi, B.; Kumar, P.; Escudero, D.; De Vos, D. Ligand-controlled selectivity in the Pd-catalyzed C-H/C-H cross-coupling of indoles with molecular oxygen. ACS Catal. 2021, 11, 2435–2444. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Chen, X.; Dai, S.; Bao, Z.; Yang, Q.; Ren, Q.; Zhang, Z. Cooperative Interplay of Brønsted Acid and Lewis Acid Sites in MIL-101(Cr) for Cross-Dehydrogenative Coupling of C-H Bonds. ACS Appl. Mater. Interfaces 2021, 13, 10845–10854. [Google Scholar] [CrossRef]
- Bijelić, J.; Stanković, A.; Medvidović-Kosanović, M.; Marković, B.; Cop, P.; Sun, Y.; Hajra, S.; Sahu, M.; Vukmirović, J.; Marković, D.; et al. Rational Sol-Gel-Based Synthesis Design and Magnetic, Dielectric, and Optical Properties Study of Nanocrystalline Sr3Co2WO9Triple Perovskite. J. Phys. Chem. C 2020, 124, 12794–12807. [Google Scholar] [CrossRef]
- Bijelić, J.; Tatar, D.; Hajra, S.; Sahu, M.; Kim, S.J.; Jagličić, Z.; Djerdj, I. Nanocrystalline Antiferromagnetic High-κ Dielectric Sr2NiMO6 (M = Te, W) with Double Perovskite Structure Type. Molecules 2020, 25, 3996. [Google Scholar] [CrossRef]
- El Yacoubi, A.; Massit, A.; El Moutaoikel, S.; Rezzouk, A.; Chafik El Idrissi, B. Rietveld Refinement of the Crystal Structure of Hydroxyapatite Using X-ray Powder Diffraction. Am. J. Mater. Sci. Eng. 2017, 5, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]



| Compound | Chemical Formula |
|---|---|
| CZLEY | Ce0.2Zr0.2La0.2Eu0.2Y0.2O2 |
| CZLPY | Ce0.2Zr0.2La0.2Pr0.2Y0.2O2 |
| CZEYG | Ce0.2Zr0.2Eu0.2Y0.2Gd0.2O2 |
| CZLPG | Ce0.2Zr0.2La0.2Pr0.2Gd0.2O2 |
| Compound | Pore Volume (cm3/g) | SBET (m2/g) | Average Crystallite Size (nm) | Acidity (a.u./g) |
|---|---|---|---|---|
| CZLEY | 0.14 | 48 | 6 | 31 |
| CZLPY | 0.27 | 103 | 6 | 77 |
| CZEYG | 0.08 | 51 | 5 | 54 |
| CZLPG | 0.33 | 60 | 6 | 44 |
| Aldehydes | Products | Aldehyde Conversion (mol%) | Product Yield (mol%) |
|---|---|---|---|
| Acetaldehyde | Diacetyl | 80 | 70 |
| Propionaldehyde | 3,4-hexanedione | 76 | 68 |
| Butyraldehyde | 4,5-octanedione | 71 | 61 |
| Benzaldehyde | Benzil | 90 | 81 |
| Furfural | Furil | 73 | 66 |
| Vanillin | 1,2-bis-benzo(1,3)diioxol-5-yl-ethane-1,2-dione | 67 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatar, D.; Kojčinović, J.; Marković, B.; Széchenyi, A.; Miletić, A.; Nagy, S.B.; Ziegenheim, S.; Szenti, I.; Sapi, A.; Kukovecz, Á.; et al. Sol-Gel Synthesis of Ceria-Zirconia-Based High-Entropy Oxides as High-Promotion Catalysts for the Synthesis of 1,2-Diketones from Aldehyde. Molecules 2021, 26, 6115. https://doi.org/10.3390/molecules26206115
Tatar D, Kojčinović J, Marković B, Széchenyi A, Miletić A, Nagy SB, Ziegenheim S, Szenti I, Sapi A, Kukovecz Á, et al. Sol-Gel Synthesis of Ceria-Zirconia-Based High-Entropy Oxides as High-Promotion Catalysts for the Synthesis of 1,2-Diketones from Aldehyde. Molecules. 2021; 26(20):6115. https://doi.org/10.3390/molecules26206115
Chicago/Turabian StyleTatar, Dalibor, Jelena Kojčinović, Berislav Marković, Aleksandar Széchenyi, Aleksandar Miletić, Sándor Balázs Nagy, Szilveszter Ziegenheim, Imre Szenti, Andras Sapi, Ákos Kukovecz, and et al. 2021. "Sol-Gel Synthesis of Ceria-Zirconia-Based High-Entropy Oxides as High-Promotion Catalysts for the Synthesis of 1,2-Diketones from Aldehyde" Molecules 26, no. 20: 6115. https://doi.org/10.3390/molecules26206115






