Abstract
Carbonyl-containing metabolites widely exist in biological samples and have important physiological functions. Thus, accurate and sensitive quantitative analysis of carbonyl-containing metabolites is crucial to provide insight into metabolic pathways as well as disease mechanisms. Although reversed phase liquid chromatography electrospray ionization mass spectrometry (RPLC-ESI-MS) is widely used due to the powerful separation capability of RPLC and high specificity and sensitivity of MS, but it is often challenging to directly analyze carbonyl-containing metabolites using RPLC-ESI-MS due to the poor ionization efficiency of neutral carbonyl groups in ESI. Modification of carbonyl-containing metabolites by a chemical derivatization strategy can overcome the obstacle of sensitivity; however, it is insufficient to achieve accurate quantification due to instrument drift and matrix effects. The emergence of stable isotope-coded derivatization (ICD) provides a good solution to the problems encountered above. Thus, LC-MS methods that utilize ICD have been applied in metabolomics including quantitative targeted analysis and untargeted profiling analysis. In addition, ICD makes multiplex or multichannel submetabolome analysis possible, which not only reduces instrument running time but also avoids the variation of MS response. In this review, representative derivatization reagents and typical applications in absolute quantification and submetabolome profiling are discussed to highlight the superiority of the ICD strategy for detection of carbonyl-containing metabolites.
1. Introduction
Carbonyl-containing metabolites are characterized by the presence of an acyl group (R-C=O), such as aldehydes, ketones, keto acids, ketosteroids, and saccharides. Carbonyl-containing metabolites widely exist in biological samples and participate in many metabolic pathways, such as glycolysis, TCA cycle, fatty acid β-oxidation, and hormone synthesis. These metabolites have important physiological functions. For example, as products of lipid peroxidation induced by oxidative stress, several endogenous aldehydes and ketones, such as acrolein, 4-hydroxy-2-nonenal (HNE), and malondialdehyde (MDA), can exacerbate oxidative damage [1], resulting in changes in cell structure and dysfunction [2] or triggering various inflammatory and immune responses [3,4]. In addition, ketosteroids are a class of steroid hormones generated by the metabolism of cholesterol in the adrenal cortex, gonads, and placenta [5]. The metabolic disruptions of ketosteroids are associated with various endocrine diseases [6] and cancer [7], for example, the excessive secretion of aldosterone caused by primary aldosteronism (PA) can lead to elevated blood pressure and adversely affect the cardiovascular system [8]. In addition, saccharides occupy an important position in central carbon metabolism, including glycolysis and pentose phosphate pathways, which maintain the most basic life activities in living organisms [9]. Abnormal glucose metabolism behavior allows tumor cells to escape the normal process of apoptosis and enhance their proliferation and migration capabilities, which is called the Warburg effect and is a key factor in the pathogenesis of tumors [10]. Therefore, carbonyl-containing metabolites are seen as potential biomarkers [11] of many diseases such as diabetes [12], cardiovascular diseases [13], neurodegenerative diseases [14], and lung cancer [15]. Thus, accurate and sensitive quantification of carbonyl-containing metabolites is crucial to elucidate their biological function in these diseases.
Although studies on changes in the concentration of carbonyl-containing metabolites in biological fluids and tissues can provide insight into metabolic pathways as well as disease mechanisms, quantitative analysis of them remains a challenge. Firstly, there are thousands of carbonyl-containing metabolites in biological fluids and tissues which often exist in combination with other functional groups. Due to their different chemical structures, there is high diversity of physicochemical properties, such as solubility, volatility, and stability. Secondly, the concentrations of carbonyl-containing metabolites are low in some biological matrixes, for example, the concentration of pyruvaldehyde in the blood of healthy adults is 0.65 μM [16], while the concentration of androsterone is only 0.00065 μM [17], which is only one-thousandth of that of pyruvaldehyde. Quantifying these relatively low abundance endogenous metabolites from biological fluids is challenging due to stringent requirements for sensitivity and specificity. Thirdly, the diverse range of carbonyl-containing metabolites and low concentrations are further complicated by the high dynamic range in concentration of metabolites by physiological status. For example, the levels of testosterone are typically related to gender and developmental stage and physiological concentrations of testosterone in the serum of adult women are only 5–10% of those in adult men and are much lower in infants and children of both sexes [18]. Finally, due to the inherent variations of sample matrix such as salt content in biological samples (e.g., urine and sweat), measurement of carbonyl-containing metabolites is further compromised by matrix effects, leading to reduced accuracy of quantification [19].
Various methods have been developed to measure carbonyl-containing metabolites, including immunoassay, nuclear magnetic resonance (NMR), and mass spectrometry (MS). Immunoassay [20] is the most used method for steroid hormone analysis, which is relatively simple and easy to perform. However, it lacks sensitivity and specificity, especially when quantifying different steroid metabolites with similar chemical structures and all at low concentrations [21]. Due to cross-reactivity and matrix interference, the inter-assay variability of immunoassay limits quantitative accuracy [22]. Furthermore, an immunoassay is based on a specific antigen–antibody reaction, making it difficult to achieve high-throughput simultaneous analysis of multiple metabolites [23].
NMR is a preferred method for the detection of a broad spectrum of metabolites. In a typical biological sample, almost all metabolites will produce 1H NMR peaks, if their concentrations are above the detection limit. The advantage of NMR is that biological fluids do not require any physical or chemical treatment before analysis. The non-destructive nature of NMR also makes it possible to observe the dynamics and sequestration of metabolites in tissue samples [24]. However, the 1H NMR spectrum of a biological sample is an overlay of the spectra of all metabolites in the sample, which may lead to inaccurate concentration determination. In addition, the ability to detect low concentrations of metabolites is limited as compared with an MS-based analysis [25]. The limit of detection of NMR is usually in the millimolar to micromolar range, making NMR unsuitable for some carbonyl-containing metabolites, such as ketosteroids with concentrations below the nanomolar level.
MS is a detection method with high specificity and sensitivity, which is usually combined with a high-resolution separation technique, such as gas chromatography or liquid chromatography. Due to differences in separation and ionization mechanisms, both separation techniques have their own pros and cons. GC-MS is highly sensitive and specific for separation and detection of volatile aldehyde and ketones, such as, formaldehyde and acetone. In addition, high reproducibility fragmentation pattern and retention time of metabolites and low instrumental-based variability in GC-MS allow for matching with an established metabolome database for metabolite identification [26]. However, derivatization is required for nonvolatile metabolites, which is tedious and labor intensive [27]. The majority of carbonyl-containing metabolites are polar, nonvolatile, and require derivatization prior to GC analysis; certain carbonyl-containing metabolites, such as keto acids, are inherently unstable to heat and light and are easily decarboxylated [28], making it difficult to analyze by GC-MS.
LC-MS has become the most versatile analytical approach for metabolomics, including untargeted metabolic profiling [29] and targeted metabolite quantification [30], as it permits the detection of a broad range of different chemical properties of metabolites in varying concentrations. The type of chromatography column and mobile phase are the key factors for LC separation for metabolomics. Reverse-phase (RP) columns have the advantages of high resolution, good repeatability, and extensive metabolite coverage, in particular, for the analysis of low-polarity metabolites [31]. Moreover, the mobile phase gradient for RPLC usually starts with a high percentage of water and less organic solvent, which makes it, currently, by far most commonly used in metabolomics as it is compatible with the analysis of aqueous biological samples [32]. The selection of the ionization mode is important because it determines the types of metabolites being detected. Electrospray ionization (ESI) is the preferred ionization mode, because it requires lower temperatures for ionization than other ionization modes, and therefore it can be utilized for thermally unstable metabolites [33].
Although RPLC-ESI-MS integrates the excellent separation capability of RPLC, good versatility of ESI and high specificity and sensitivity of MS detection, it is often challenging to directly analyze carbonyl-containing metabolites due to poor ionization efficiency of neutral carbonyl groups by ESI. In addition, the high polarity of some carbonyl-containing metabolites exhibits poor chromatographic retention in RPLC. In this regard, chemical derivatization prior to LC-MS detection is an emerging strategy for overcoming the obstacles to analyzing carbonyl-containing metabolites [34]. Chemical derivatization-based LC-MS has been developed since the 1980s [35] and the introduction of chemical moiety could convert the carbonyl-containing metabolites into MS-detectable and LC-separable derivatives. For example, Zhao et al. [36] used dansylhydrazine (Dns-Hz) as a derivatizing reagent for labeling of carbonyl-containing metabolites, so that acetaldehyde can be ionized after derivatization and its MS signals significantly increased. In addition, chemical derivatization facilitates chromatographic separation of isomeric metabolites, such as glucose 6-phosphate and fructose 6-phosphate [37].
There are two advantages of chemical derivatization in mass spectrometry quantification. First, chemical derivatization can enhance the MS response and LC separation of metabolite species that have low abundance, poor chromatographic behavior, or are difficult to ionize. Second, chemical isotope labeling could solve the problem of concentration-independent MS signal differences caused by ionization efficiency. This is due to instrument drift and matrix effects produced by the co-eluting components, which cause fluctuations in the ionization efficiency of metabolites in ESI, thereby, resulting in a variable response in LC-ESI-MS analysis [38]. In general, an internal standard (IS) is needed to ensure the accuracy and precision of quantification because of its identical physicochemical properties and similar chromatographic behavior as the metabolites. However, it is impractical to provide the stable isotope-labeled analogs for all target metabolites due to limited commercial availability, labor-intensive syntheses, and high cost [39]. Thus, a more reliable strategy is urgently required. The emergence of ICD can solve the problems outlined above. In this strategy, the stable isotope-coded moiety of derivatization reagent is used to label standard metabolites to form derivatives, which acts as ISs. This approach can generate isotope ISs for all the target metabolites simultaneously in the derivatization step.
Several excellent reviews have focused on isotope-coded derivatization strategies and their applications in global metabolomics or targeted small-molecular weight compounds [39,40,41,42,43]. In this review, we focus on the detection of carbonyl-containing metabolites and present the general principles and properties of representative derivatization reagents, as well as their advantages and shortcomings. In addition, typical applications in absolute quantification and submetabolome profiling are discussed to highlight the superiority of the ICD strategy for detection of carbonyl-containing metabolites.
2. Types of Derivatization Reagents for Carbonyl-Containing Metabolites
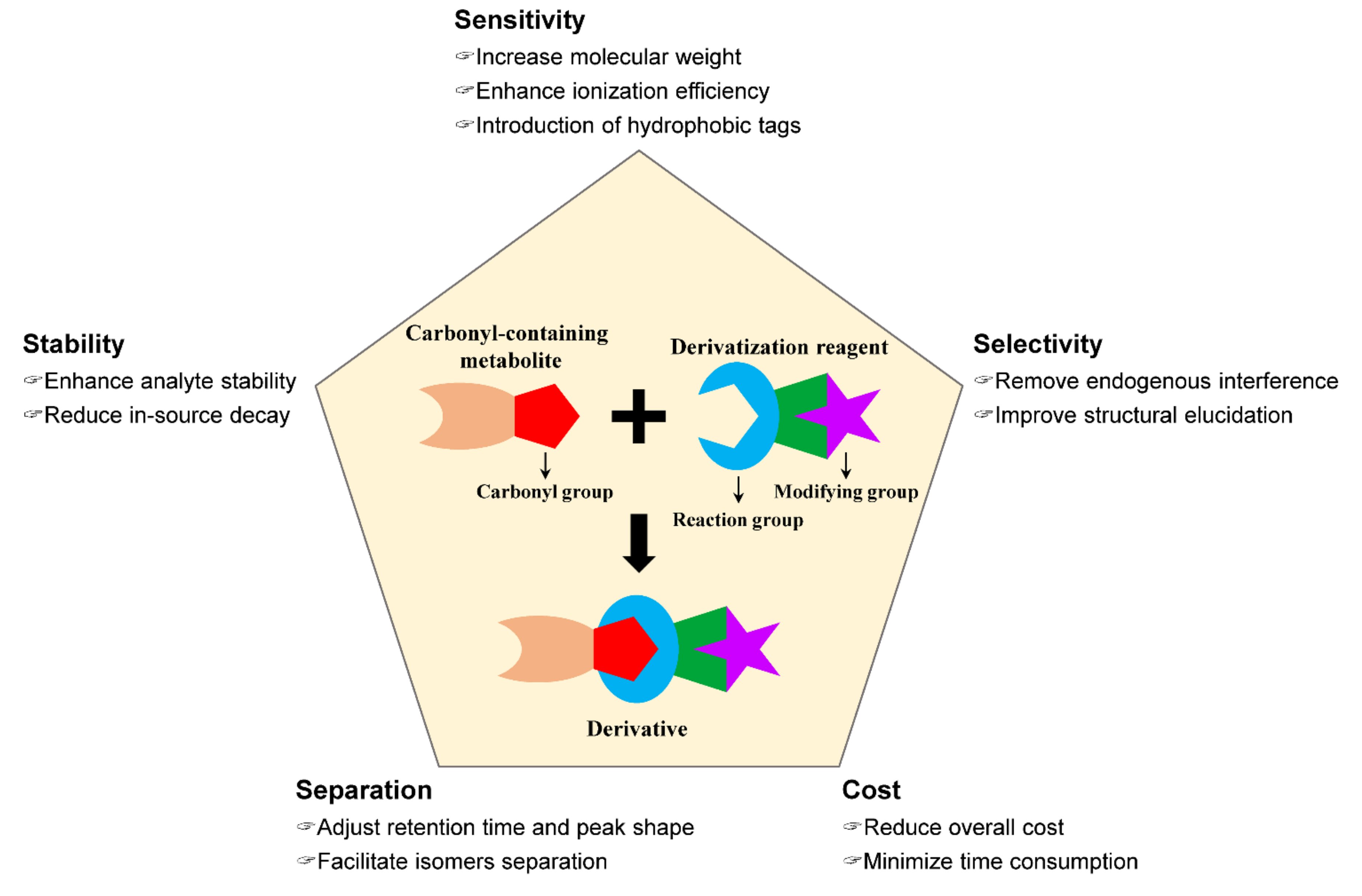
As described above, modification of carbonyl-containing metabolites by a chemical derivatization strategy can convert them into LC-separable and MS-detectable derivatives. As shown in Figure 1, an excellent chemical derivatization reagent should be able to (1) enhance the stability of analytes by modifying the unstable moiety of analytes and reduce its in-source decay during MS detection [44,45], (2) improve the detection selectivity through removing endogenous interference or aiding structural elucidation by characteristic fragmentation in collision-induced dissociation (CID) [46,47], (3) improve the chromatographic separation performance and peak shape of analytes by adjusting retention time [48,49] and facilitate isomers separation by chiral derivatization reagents [50,51,52,53], (4) enhance detection sensitivity by the introduction of hydrophobic tags or permanent charged moiety to enhance ionization efficiency and improve the fragmentation pathway of analyte in CID for tandem MS analysis [54], and (5) reduce overall cost and improve throughout [55].
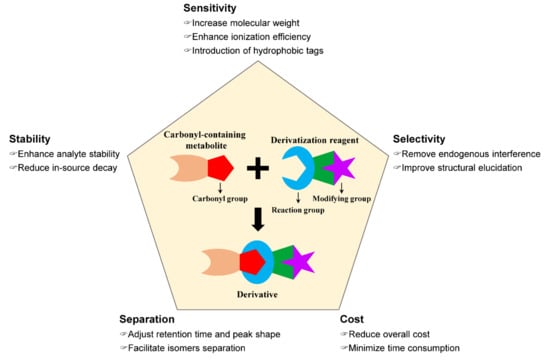
Figure 1.
Key factors of an excellent chemical derivatization reagent.
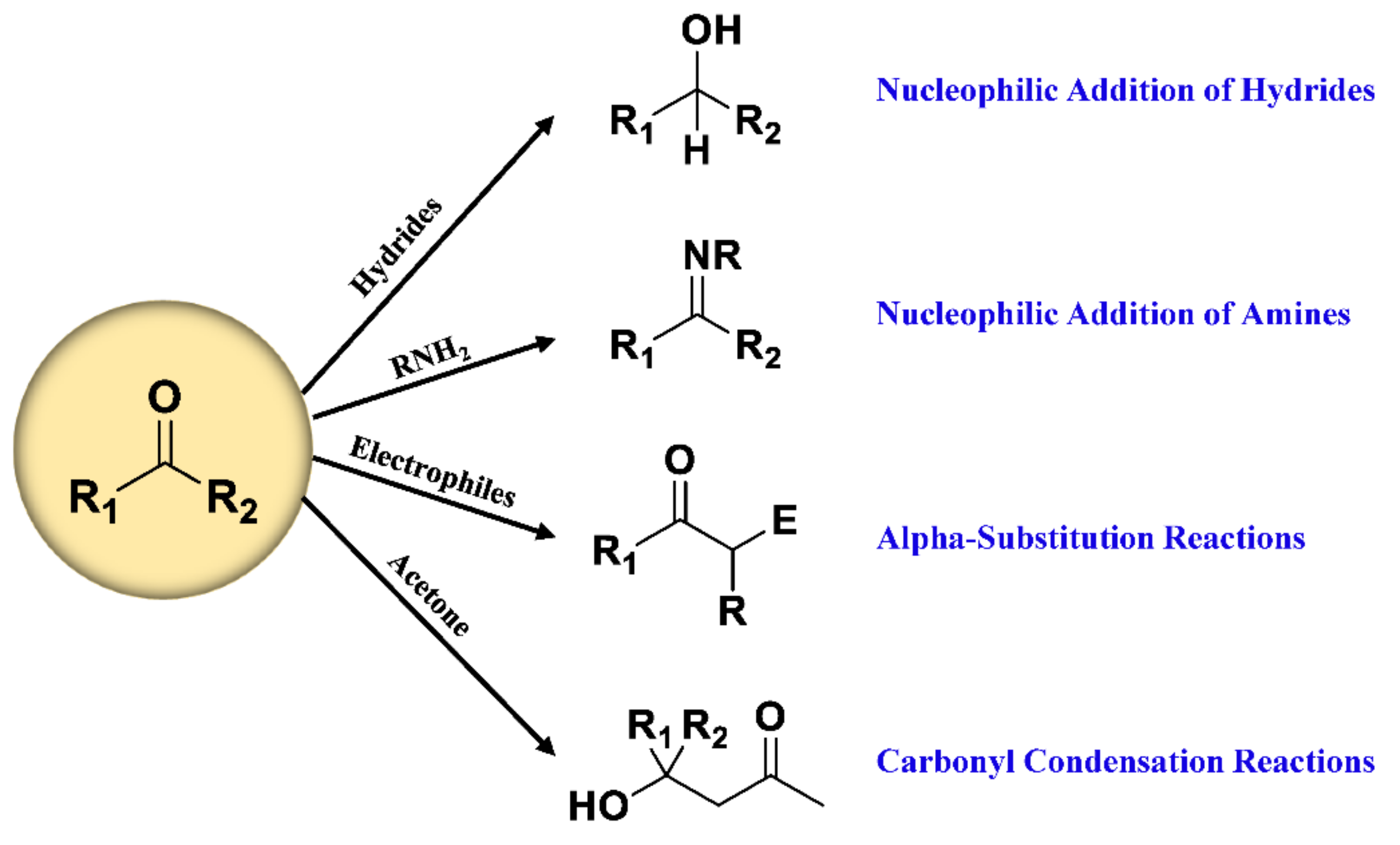
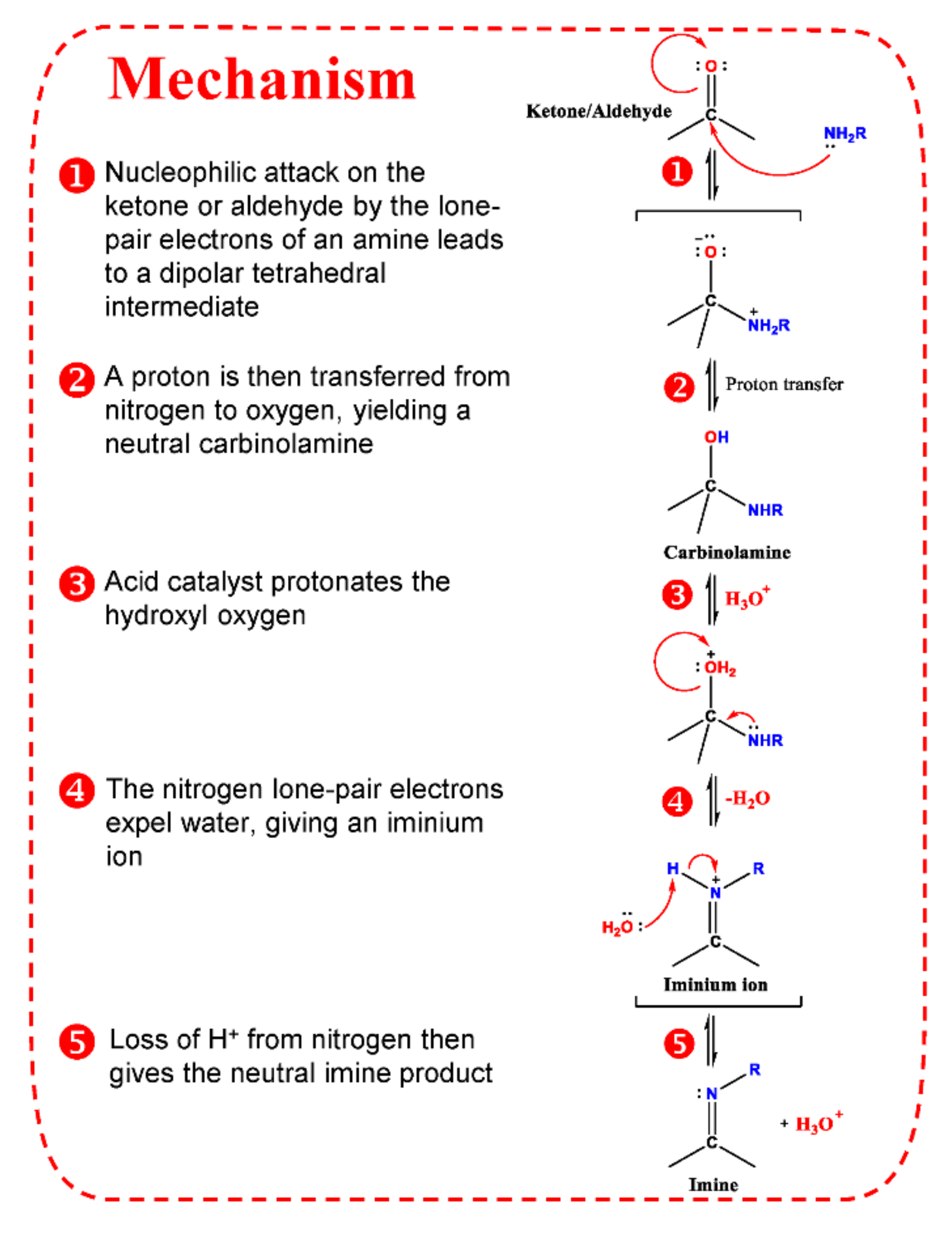
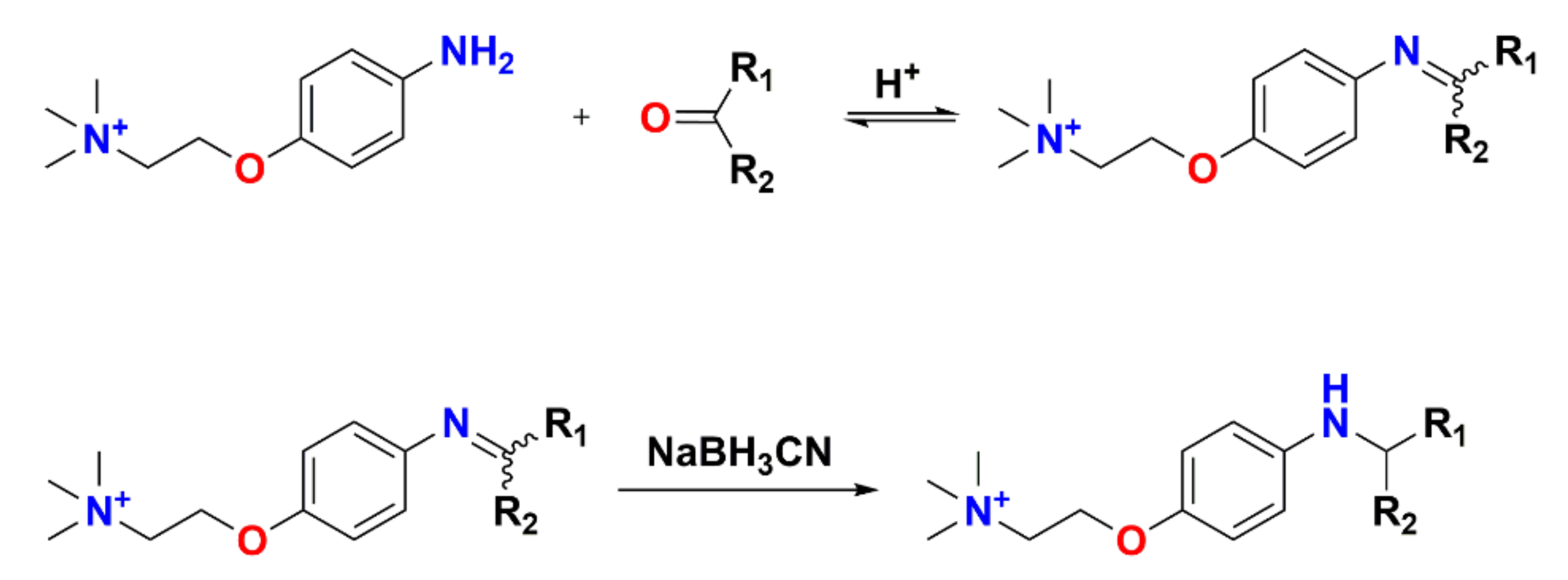
Therefore, many reagents have been developed based on classic carbonyl reactions (Figure 2) for detection of carbonyl-containing metabolites prior to LC-MS analysis. The essence of most used derivatization reagents are primary amines, which, as nucleophiles, react with carbonyl groups in the metabolites to yield imines. As illustrated in Figure 3, the rate-determining step of imine formation is the initiation of nucleophilic addition to yield a carbinolamine intermediate, which then loses water to give the imine. The substituents at the α-nucleophile moiety have a distinct effect on the stability of derivatives [56], and also determine the rate of derivatization reaction. Depending on the types of α-nucleophile moiety, derivatization reagent can be divided into four categories: hydrazines, hydrazides, hydroxylamines, and amines (Table S1).
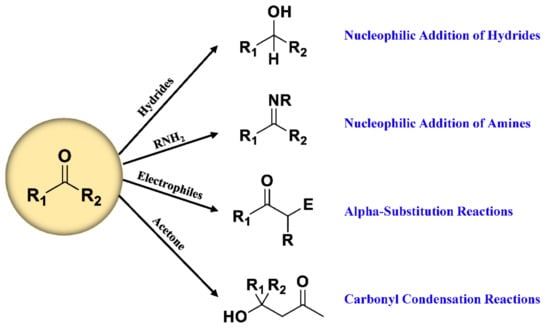
Figure 2.
Classic reactions of carbonyl compounds.
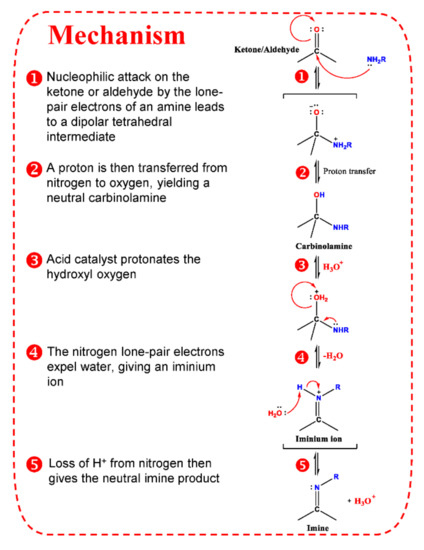
Figure 3.
Mechanism of imine formation.
2.1. Hydrazines
Hydrazine can react with carbonyl group to form hydrazones for better detection of carbonyl-containing metabolites. 2,4-Dinitrophenylhydrazine (DNPH) is the most widely used derivatization reagent, since it was first introduced [57,58]. Various DNPH labeling methods have been developed for the quantification of a wide range of carbonyl compounds, such as disinfection byproducts in water [59,60], toxins in food [61,62], harmful components in cigarette smoke [63], and photoproducts from solar-irradiated crude oil-seawater systems [64]. Chen et al. [65] were the first to optimize the DNPH derivatization condition in biological samples and established a rapid and sensitive isotope-dilution LC-MS/MS method for detection of urinary MDA with a limit of detection (LOD) of 1.6 pmol/mL−1. The derivatized MDA can be effectively retained on the C18 column and can be easily positively charged in the ESI ionization process.
Some carbonyl-containing compounds are highly reactive and easily degraded, such as α-keto acids. For these type of compounds, Zimmermann et al. [44] proposed a derivatization reagent, phenylhydrazine (PH), to stabilize these metabolites as soon as sample collection and prior to storage. PH can quench α-keto acids from further degradation and derivatization simultaneously at −20 °C, and therefore it is capable of absolute quantification of α-keto acids in biological fluids. The aromatic ring from PH added onto the α-keto acids can enhance the MS signal and increase the chromatographic retention times. The most challenging analysis of carbonyl-group containing metabolites is sugars with hemiacetal structure. Although carbohydrate derivatization takes longer to convert from the cyclic form to the open-chain form, the PH-derivatized sugars have a massive increase in sensitivity and, more importantly, they can produce a satisfied chromatographic resolution for isomers, such as glucose 6-phosphate and fructose 6-phosphate [37].
3-Nitrophenylhydrazine (3-NPH) is also an efficient derivatization reagent for quantitation of low-molecular weight (LMW) sugars [66]. Compared with other hydrazine-type derivatization reagents, there is no need to remove excessive 3-NPH in the reacted system as 3-NPH does not produce any background signals in negative ion ESI-MS detection mode, and therefore does not interfere with quantitation of LMW sugars. Han et. al. first used 3-NPH for quantitation of both LMW sugars and carboxylic metabolites [66,67]. Moreover, 3-NPH has been used to quantify flavor-active aldehydes, ketones, and organic acids in a wide concentration range up to six orders of magnitude [68].
Another derivatization reagent that has been used for both fatty aldehydes [69] and fatty acids [70] is 2,4-bis(diethylamino)-6-hydrazino-1,3,5-triazine (T3). The derivatization reaction of T3 with fatty aldehydes was quicker and milder than previously mentioned reagents, such as DNPH, owing to the high nucleophilicity of the hydrazine group in T3, and thus minimized the risk of fatty aldehydes degradation. In addition, derivatization with T3 significantly increased the sensitivity of fatty aldehydes due to the increased alkalinity of the derivatives, and also improved chromatographic performance due to the enhanced the hydrophobicity of the fatty aldehydes [69].
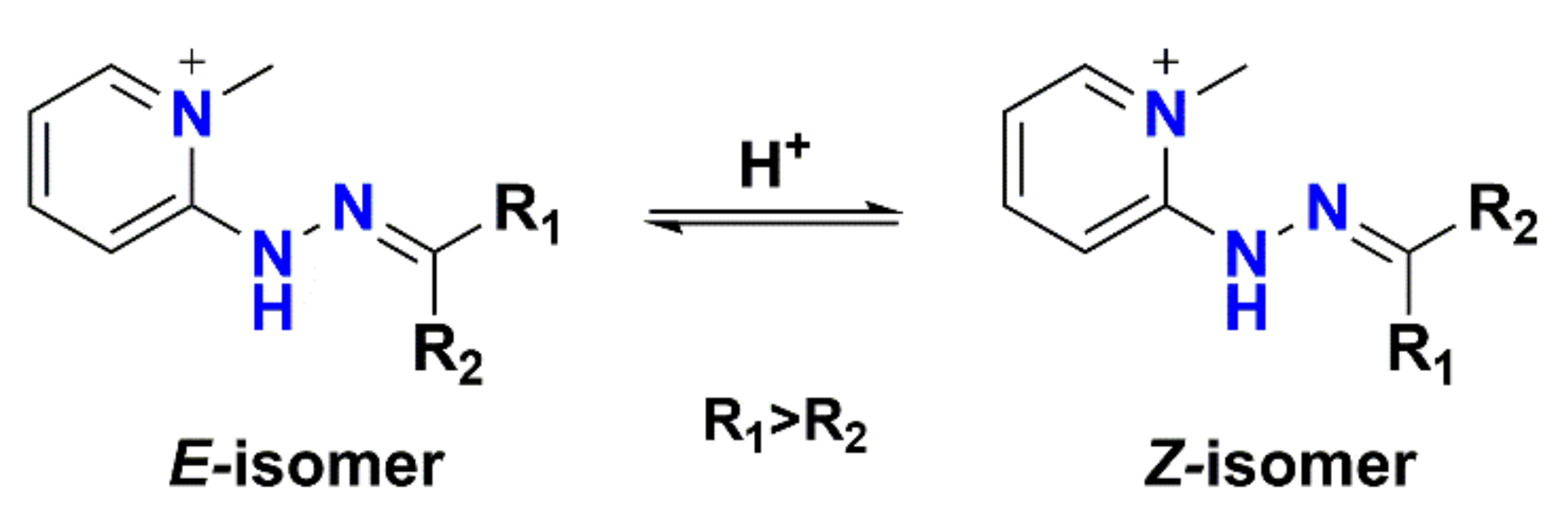
Furthermore, 2-hydrazino-1-methylpyridine (HMP) and 2-hydrazinopyridine (2-HP) are also commonly used for derivatizing carbonyl-containing metabolites. Since HMP contains a fixed charged quaternary amine, derivatization with HMP can increase the detection sensitivity of cortisol and cortisone by 1000 times. Cortisol-bisHMP and cortisone-bisHMP are mainly formed after derivatization with HMP and each metabolite generates four peaks from four isomers (E, E- E, Z- Z, E- and Z, Z-isomers) in the chromatogram. Scheme 1 shows the E/Z-isomers of HMP-derivatized metabolites. To improve the detection sensitivity and quantitative accuracy, Magda et al. [71] modified the LC method to collapse all four peaks into one peak. Higashi et al. [72] proposed, for the first time, to enhance the sensitivity of ketosteroids through 2-HP derivatization. Later, this reagent was used for sensitive quantification of steroid hormones in human cerebrospinal fluid [73] and plasma [74]. Nadarajah et al. [75] tested five derivatization reagents (Girard’s reagent T (GRT), 1-benzyl-pyrrolidine-3-carboxylic acid hydrazide (1-BPH), 4-aminobenzoic hydrazide (4-ABH), isoniazid (INH), and 2-HP) for analysis of ketosteroids in saliva and 2-HP was superior not only in chromatographic behavior but also in signal response.
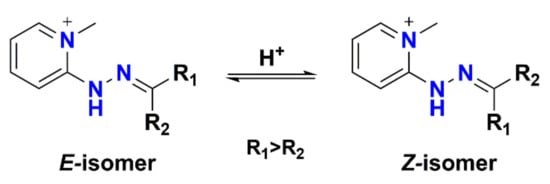
Scheme 1.
The E-/Z-isomers of HMP-derivatized carbonyl-containing metabolites.
2.2. Hydrazides
Hydrazide reagents, such as Girard’s reagents and sulfonyl hydrazides, can react with a carbonyl group under weak acid conditions to form Schiff base. Girard’s reagents are types of hydrazide reagents with a quaternary ammonium group; Girard’s reagent T (GT) and Girard’s reagent P (GP) are used the most.
Dury et al. [76] proposed a highly sensitive and specific LC-MS/MS method for accurate quantitation of six neurosteroids based on a GT ketone-specific derivatization. Although six neurosteroids lack ionization efficiency in ESI-MS and are structurally highly similar, derivatization with GT successfully enhanced MS detection sensitivity and achieved better chromatographic separation. 4-Hydrazino-N,N,N-trimethyl-4-oxobutanaminium iodide (HTMOB) is a modified Girard’s reagent with three methylene linkages between the quaternary amine and hydrazine group. This modification strategy improved the ESI-MS/MS fragmentation characteristics of HTMOB derivatives. Thus, as compared with the GT derivatives, a 3.3- to 7.0-fold increase in the signal intensities of HTMOB derivatives was observed [77].
Similar to GT, GP introduces a fixed charge on a quaternary ammonium group, which relies on pyridinium moiety to increase ketosteroid solubility in reversed-phase solvents and give a strong response in positive mode ESI-MS detection [78]. Crick et al. [79] compared several modified Girard’s reagents and showed that the sensitivity of quaternary ammonium was similar to that of tertiary amine, but the quaternary ammonium aided the transfer of the positive charge from the pyridinium moiety to the steroid backbone, leading to the enhancement of the efficiency of MSn fragmentation. In addition, derivatization of formylated nucleosides with GP exhibited the best detection sensitivity as compared with GT and 4-(2-(trimethylammonio)ethoxy)benzenaminium halide (4-APC) [80]. Later, Feng’s group synthesized four derivatization reagents (2-(2-hydrazinyl-2-oxoethyl)isoquinolin-2-ium bromide, HIQB; N,N,N-triethyl-2-hydrazinyl-2-oxoethanaminium bromide, THB; GT; GP) which all contained a quaternary ammonium group to enhance the detection sensitivity of carbonyl compounds. Among all the four reagents, HIQB labeling exhibited the highest hydrophobicity and the lowest LODs for almost all analytes. Moreover, the characterized fragment ions produced by the HIQB derivatives were higher in molecular weight and had a better MS response than those of other three reagents, so the HIQB derivatives could be less affected by the interference from low molecular weight metabolites. Taken together, HIQB has been chosen for profiling and quantification of carbonyl compounds using the stable isotope labeling double precursor ion scan MS strategy [81].
Unlike Girard’s reagents, 7-(diethylamino)coumarin-3-carbohydrazide (CHH) and “tandem mass tag hydrazine” (TMTH) contain a chargeable tertiary amino group which can also enhance the derivative signal in ESI-MS. The TMTH reagent combined with a carbonyl-reactive hydrazine group with tandem mass tags (TMT) was originally developed by Thomson et al. [82] and can yield characteristic mass reporter ions in an ESI-MS/MS analysis. Rigdova et al. [83] derivatized thirty oxosteroids with TMTH, with improved sensitivity that ranged from 14-fold to 2755-fold as compared with their underivatized steroids. CHH was the first derivatization reagent used for the simultaneous detection of both aliphatic and lipid-bound aldehydes and ketones to overcome their different properties [84]. The high hydrophobicity of CHH makes it possible to simultaneously extract water-soluble, lipid-bound, and highly hydrophobic aldehydes and ketones and prevent their loss during the extraction process. Moreover, characteristic neutral losses and fragment ions produced by the CHH derivatives during the CID process can be used to clearly identify the carbonyl compounds. In addition, Brkljacic et al. [85] designed a glutamic acid-related hydrazide reagent, which had higher sensitivity than DNPH owing to its higher ionization efficiency. Moreover, the fragmentations of glutamic acid-related hydrazones were structure specific and highly reproducible, which facilitated its use in sensitive MRM analysis.
Another type of hydrazide reagent is sulfonyl hydrazide, such as, p-toluenesulfonylhydrazine (TSH) and dansylhydrazine (Dns-Hz). TSH has been considered to be a suitable substitute for DNPH with similar reactivity. In addition, TSH can improve solubility of derivatives for better compatibility with aqueous biological samples. In addition, TSH has high volatility, which easily decomposes into gas-phase products at elevated temperatures, therefore, there is no risk of contamination of the MS by excessive TSH. However, the overall sensitivity of the analytes is reduced due to the formation of E- and Z- geometrical isomers of derivatives, which can be baseline separated in the UHPLC chromatograms. Some metabolites such as mercaptopyruvic acid significantly decrease during the derivatization process due to rapid oxidation and dimerization. Despite these limitations, TSH derivatization can distinguish a series of isomer pairs, for example, ketoleucine and ketoisoleucine, or acetone and propionaldehyde [86].
Dns-Hz is a fluorescent derivatization reagent, which has been widely used for fluorescence detection, and can also analyze the lipophilic reactive carbonyls (RCs) in biological samples by LC-ESI-MS/MS [87]. Compared with TSH, derivatization of RCs with Dns-Hz has several advantages. Most notably, the dimethylamino group of Dns-Hz, which is a tertiary amine, is easily protonated under acidic conditions, thereby, enhancing the ionization characteristics of RCs, and the Dns-Hz derivatives can produce a simple and clear product ion with an m/z value of 236.1 by CID. The drawback of Dns-Hz is that the capability of isomer separation may be relatively weak. For instance, the HNE and 4-hydroxy-2-hexenal (HHE), as a pair of stereoisomers, could not be separated in LC after derivatization.
2.3. Hydroxylamines
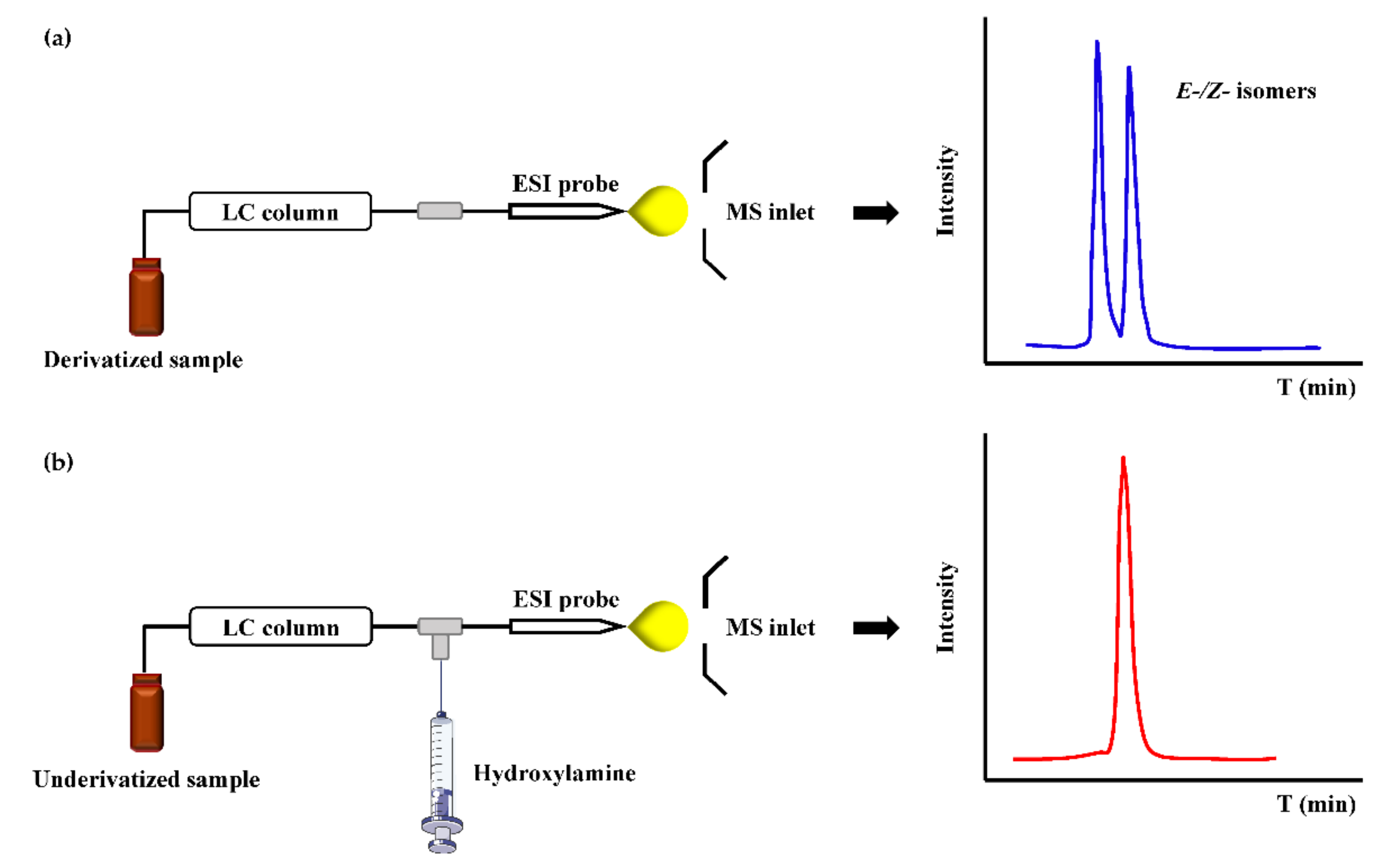
Unlike hydrazine-based reagents to form hydrazones, hydroxylamine (HA) reagents, including O-substituted hydroxylamine (-ONH2) and N-substituted hydroxylamine (-NHOH), can react with a carbonyl group to form oximes or nitrones. NH2OH is the simplest hydroxylamine reagent, and it has always been devoted to label ketosteroids. However, the poor hydrophobicity of NH2OH itself has limitation to improve the retention of metabolites. Liu et al. [88] quantified ten steroid hormones by hydroxylamine derivatization using HPLC-MS and a LOQ was achieved in the range of 0.05–5 ng/mL which was increased by 3.9–202.6 times after derivatization. Because hydroxylamine reacts fast and shows no contaminations to MS, Chen et al. [89] developed a fully automated in-tube SPME/LC-PCD-MS method, and demonstrated rapid and accurate quantification of urinary hexanal and heptanal by simple sample preparation and avoiding LC separation of E-/Z- isomers of oximes (Figure 4). The only catch is that the sensitivity needs to be further improved by employing more advanced mass spectrometers.
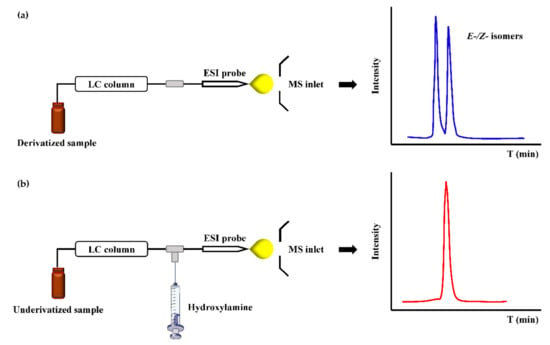
Figure 4.
The scheme and chromatogram of oxime derivatives obtained by (a) pre-column derivatization and (b) post-column derivatization.
Apart from the poor hydrophobicity of NH2OH, it also suffers from producing complex metabolites resulting from Beckmann rearrangement reaction [90]. Derivatization with O-substituted hydroxylamine can overcome the rearrangement changes, which has attracted the attention of analytical chemists. Methoxyamine (MOA), which is similar to NH2OH, has also been dedicated to labeling steroid hormones containing carbonyl groups and has achieved detecting 29 steroid hormones at the level of pg/mL, which increased the sensitivity by three orders of magnitude after derivatization [91]. Owing to its poor retention, excessive methoxyamine can easily be diverted to waste without compromising the ionization efficiency of the derivative products [92].
O-(3-trimethylammonium propyl) hydroxylamine bromide is a quaternary aminooxy (QAO) reagent, which has an aminooxy (-ONH2) functional group as a carbonyl reactive group, and also processes a permanent positive charge, giving a better ionization efficiency in ESI-MS. As compared with HA, the signal-to-noise (S/N) ratio of QAO reagent for testosterone analysis is about 10 times higher than that of HA [93]. Furthermore, the use of QAO reagent can improve the reliability of hormone analysis in the complex matrix due to the formation of chromatographically separated derivative isomers [94].
N-[2-(aminooxy)ethyl]-N,N-dimethyl-1-dodecylammonium (QDA) is also a quaternary ammonium tag, which can greatly improve detection sensitivity of ESI-MS. Meanwhile, QDA has a hydrophobic domain for better retention on column [95]. The key principle of this reagent is based on the use of N,N-dimethyl-p-phenylenediamine as a catalyst to accelerate the derivatization reaction and, subsequently, the quench reaction at -80 °C. This method can minimize the degradation of unstable metabolites [96].
A brominated O-substituted hydroxylamine, namely 1-((ammoniooxy)methyl)-2-bromobenzene chloride (BBHA) has been selected as a new probe for a mild and rapid derivatization of carbonyl compounds [97]. On the basis of the existence of the characteristic 79Br/81Br isotopic pattern, carbonyl compounds can be specifically detected in complex mixtures. Different modification groups on the O-substituted hydroxylamine can achieve different detection purposes. For example, aminooxy-N-(3-perfluorooctyl-propyl) acetamide [98] is a fluorous O-substituted hydroxylamine tag and can selectively react with carbonyls to yield oximes, which can be extracted from the biological fluids using a fluorine spin column to simplify analysis. In addition, the fluorous O-substituted hydroxylamine tag can increase the hydrophobicity of the analytes, achieving a 30-fold increase in sensitivity. The method achieved both chromatographic separations and MS/MS detection of the structural isomers of acetoacetate and hydroxybutyrate.
Conway et al. [99] proposed a new strategy which used a chemoselective probe immobilized to magnetic beads to efficiently separate carbonyl metabolites from biological matrix, thereby, reducing MS background interference and enhancing sensitivity by up to six orders of magnitude. In addition, the biorthogonal cleavage of the chemoselective probe can be cleaved from the magnetic beads under mild conditions to avoid degradation of labile carbonyl metabolites [100].
Although the chromatographically separated E-/Z- isomers formed by the reaction of asymmetric carbonyl metabolites with O-substituted hydroxylamines have been considered useful for structure identification in some cases, it brings huge challenges to MS sensitivity. Guan et al. [101] firstly designed and synthesized two novel N-substituted coumaroyl hydroxylamines (4-hydroxylaminopropyl-7-methoxylcoumarin (HAMC) and 7-hydroxy-4-(4-(hydroxyamino)butyl)-2H-chromen-2-one (HAHC)) with long aliphatic chain, which enhanced the ionization efficiency of derivatives. Compared with other O-substituted hydroxylamines, HAMC and HAHC provided a mild and efficient labeling in less than 30 min at room temperature, and also overcame the shortcomings of forming E-/Z- isomers. These novel N-substituted hydroxylamines have been applied for the determination of furfuraldehydes in foodstuffs, and their further applications on the analysis of more aliphatic aldehydes are promising [102].
2.4. Amines
Amines are another type of derivatization reagent for carbonyl metabolites, which are usually used in combination with a reducing reagent, such as sodium cyanoborohydride (NaBH3CN) and 2-picoline borane (pic-BH3), to reduce the Schiff base derivative to a single, more stable secondary amine product. Since many ketones tend to react more slowly than aldehydes [103], amines are often used to derivatize aldehydes. The reaction can proceed completely to prevent hydrolysis, and hence the method allows samples to be stored over longer periods.
Aniline has been used to react with sugars for better detection for many years [104]. The derivatization reaction of sugars should be carried out at a low pH since it promotes the opening of the sugar ring, while aniline is a weak base that can still have enough unprotonated form to react with sugar at a low pH. As a derivatization reagent, aniline can achieve the best separation for structural isomers such as four common hexoses (glucose, fructose, mannose, and galactose) [105] and also enables targeting of multiple functional groups that are more suitable for global metabolic profiling [106].
3-Amino-9-ethylcarbazole (AEC) has been used to derivatize sugar phosphates in biological fluids based on reductive amination [107]. However, the derivatization reaction for biological samples, often in the liquid phase, is usually accompanied by shortcomings, such as time-consuming, excessive derivatization reagents and low derivatization efficiency. Qin et al. [108] modified the method by firstly employing SiO2@PD_Ti4+ microspheres to selectively enrich sugar phosphates and remove the excess number of other metabolites prior to derivatization, and then react with AEC based on solid-phase derivatization. This modification has significantly shortened derivatization time and reduced the interference of the residual derivatization reagents on the ionization efficiency, leading to three orders of magnitude improvement on the sensitivity for detection of sugar phosphates and making it possible to determine trace sugar phosphates in small amounts of biological fluids.
Unlike aniline, 4-(2-(trimethylammonio) ethoxy) benzenaminium halide (4-APC) is a selective derivatization reagent for the analysis of aldehydes [109]. The nucleophilic group of 4-APC has aniline moiety and its pKa is low enough to retain nucleophilic property to ensure fast reduction of the resulting imine by NaBH3CN, as shown in Scheme 2. Due to the difficulty in the formation of the initial imine between the ketone and the aniline moiety, 4-APC can selectively label aldehydes. In addition, a quaternary tetraalkylammonium group can connect to the para position of the aniline through an ethoxy spacer to provide a permanent positive charge for enhancing MS sensitivity, while minimizing the induction of the nucleophilicity of NH2 by the positive charge. Labeling with 4-APC can dramatically increase the MS sensitivity of aldehydes, such as hexanal, by more than 1000 times [110]. Another derivatization reagent, 4-(2-((4-bromophenethyl)dimethylammonio)ethoxy)benzenaminium dibromide (4-APEBA), has been designed based on 4-APC to address some additional stringent issues in a metabolite analysis, such as biomarker analysis of lipid peroxidation [111]. 4-APEBA contains a bromophenethyl group which can significantly enhance the recognition of metabolites through the characteristic 79Br/81Br isotopic pattern. Meanwhile, the molecular weight of 4-APEBA is higher than that of 4-APC, resulting in a higher S/N ratio of the 4-APEBA derivatives in biological fluids.
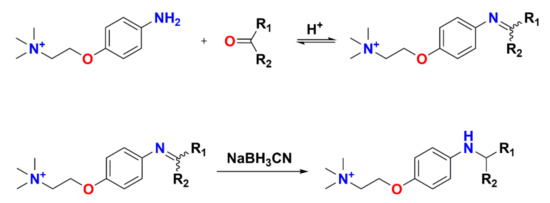
Scheme 2.
Pathway for imine formation and reduction using aldehydes and 4-APC.
Sun et al. [112] developed a new derivatization reagent, 4-(1-methyl-1H-phenanthro [9,10-d]imidazol-2-yl)phenlamine (MPIA). MPIA can label aldehydes through reductive amination under weak acid condition to produce secondary amine derivatives without E-/Z- isomers and few byproducts. As compared with the widely used DNPH, MPIA derivatives show higher sensitivity, which is probably attributed to the strong proton affinity of the lone pairs on imidazole nitrogen atom and the high hydrophobicity of MPIA derivatives.
Different from monoamines, diamines react with α-dicarbonyl compounds to form cyclic compounds, so that a single derivatized product can be obtained without a reducing reagent.
It is well known that o-phenylenediamine (OPD) is the most commonly used derivatization reagent for quantitative α-dicarbonyl compounds [113,114,115,116]. OPD reacts with α-dicarbonyl compounds, such as methylglyoxal, to produce stable 2-methylquinoxaline with high MS detection sensitivity [117]. However, when it is used to determine the content of α-dicarbonyl compounds in biological samples with high levels of sugar, such as honey, OPD may undergo Maillard reaction with reducing sugars to form α-dicarbonyl compounds, thereby, affecting quantification. Hurtado-Sanchez et al. [118] compared three derivatization reagents (2,4,5-triamine-6-hydroxypyrimidine (TRI), 5,6-diamino-2,4-hydroxypyrimidine (DDP), and OPD) and pointed out that TRI was the most advantageous derivatization reagent for analyzing α-dicarbonyl compounds in samples with high sugar content, due to much less amount of interfering α-dicarbonyl compounds formed as compared with DDP and OPD.
Similarly, 3,4-diaminobenzophenone (DABP) has been proven to have better performance in determining the levels of malondialdehyde in biological fluids [119]. DABP can generate a stable derivative without using reducing agent, hence, less interference. DABP derivatives can achieve approximately 50–100 times higher in MS sensitivity as comparing with DNPH, and therefore no concentration step is required when directly determining the levels of malondialdehyde in urine and saliva samples.
2.5. Others
Other derivatization reagents containing unique functional groups have been employed for the detection of carbonyl compounds. According to the Schiff reaction of aldehydes with D-cysteine to form only a stable substituted thiazolidine carboxylic acids, Kim et al. [120] developed a simple and rapid method to determine eight aldehydes in beverages and avoid the drawback of hydrazine as derivatization reagents forming syn- and anti-isomers. Acetylacetone is a specific derivatization reagent to formaldehyde according to the principle of the Hantzach reaction [121]. As compared with the commonly used DNPH, the derivatization with acetylacetone takes place under mild conditions and forms stable derivatives, but it requires a longer reaction time of about 30 min. Acetylacetone has also been used to detect trace levels of free formaldehyde in cosmetics by LC-PCD-UV with limits of detection and quantification levels of 0.7 and 2.3 ng/mL−1 [122]. However, acetylacetone is more toxic and has weak fluorescence, which is not easy to store, which has prevented it from common use.
Cyclohexane-1,3-dione (CHD) is an alternative derivatization reagent since it is less toxic and irritant than acetylacetone and other derivatization reagents, such as 3-methyl-2-benzothiazolinone hydrazone (MBTH) and phenylhydrazine-4-sulfonic acid (PHSA) [123]. The derivatization using CHD has high selectivity and sensitivity without the need of pH adjustment, and each different aldehyde only forms one derivative without the formation of isomers. Moreover, the dimethyl CHD forms better derivatives with aldehydes due to their increased hydrophobicity [124].
Similar to acetylacetone, ammonium sulfite has also been used for post-column derivatization of aldehydes [125]. Ammonium sulfite can react with aldehydes to perform LC-MS analysis in negative ionization mode with relatively strong anti-interference ability. In addition, the characteristic fragments of the derivative can be obtained with extremely low collision energy, resulting in a significant reduction in false positive detections in mass spectrometry. Thus, compared with the LC-PCD-MS method that employs HA to react with aldehydes [89], ammonium sulfite derivatives suffer less matrix effects and can improve detection sensitivity by five-fold.
9,10-Phenanthrenequinone (PQ) has been selected as a derivatization reagent for aliphatic aldehydes and the benefit of this methods is the high selectivity for aliphatic aldehydes, hence, significantly reducing interferences from other carbonyl compounds including ketones and carboxylic acids [126]. The high selectivity of PQ enables the detection sensitivity of aliphatic aldehydes in the range of 4–100 pM, which is about 19–1000-fold higher than can be achieved by MPIA. In addition, PQ is commercially available, and hence can save the time to synthesize a derivatization agent, which is an overall advantage over previously mentioned reagents, for example, CHD-based reagents and MPIA.
N-(1-chloroalkyl)pyridinium quaternization (NAPIQ) is also a new derivatization method for labeling aliphatic aldehydes [127]. Unlike common precharged reagents, uncharged pyridine and thionyl chloride in NAPIQ are designed to add a permanently charged label to the aldehyde. Pyridine is far less competitive in ionization than charged derivatives, whereas excess thionyl chloride can be easily removed with deionized water, which converts thionyl chloride into less residual sulfur dioxide bubbles. Therefore, this method could detect aliphatic aldehydes directly after derivatization without further postprocessing. Moreover, NAPIQ has been designed to label ketosteroids with alcoholic or α,β-unsaturated ketone moieties and carbohydrates by changing the reaction solvent [128]. This simple derivatization method only generates one product and is suitable for ketosteroids containing more than one carbonyl groups. Thus, the sensitivity and selectivity of the NAPIQ strategy are higher than those of Girard reagents.
Carbohydrates are a complex class of metabolites, clearly, one method will not be adequate to detect all carbohydrates. Simultaneous quantitation of sixteen monosaccharides through 1-phenyl-3-methyl-5-pyrazolone (PMP) derivatization could achieve baseline separation of isomers within 10 min by taking advantage of the high hydrophobicity of PMP molecules [129]. Derivatization of xylose and arabinose with 3-NPH could not achieve effective separation of the two metabolites, however, they could be effectively separated using PMP derivatization. Owing to efficient separation, the LODs of most monosaccharide derivatives with PMP could reach ranges between femtomoles and attomoles, which was increased by more than 1000-fold as compared with picomoles obtained by UV or ion trap MS.
3. Application of the Stable Isotope-Coded Derivatization Method in LC-MS Analysis of Carbonyl-Containing Metabolites
Although modification of carbonyl-containing metabolites by a chemical derivatization strategy can overcome the obstacles of detectability and sensitivity, it is still insufficient with an accuracy issue due to instrument drift and matrix effects produced by the co-eluting components. The emergence of the ICD strategy provides a good solution to the problems encountered above. Thus, LC-MS methods that utilize ICD has been applied in metabolomics including quantitative and profiling analysis (Table 1).

Table 1.
Summary of ICD reagents for carbonyl-containing metabolites and their applications.
3.1. Absolute Quantification
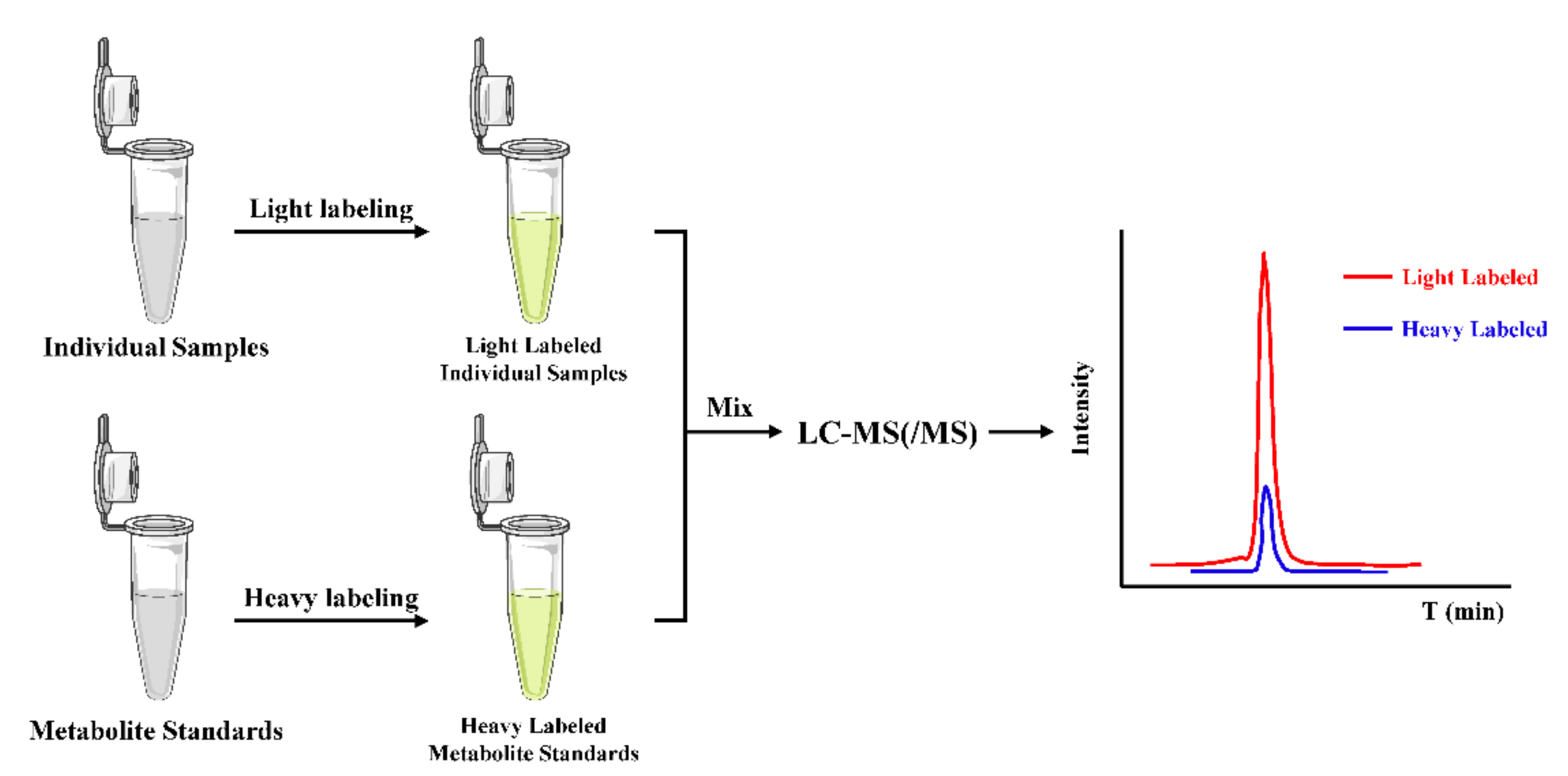
In the ICD strategy, as illustrated in Figure 5, the stable isotope-coded moiety of the derivatization reagent is used to label the standard metabolites of known amounts to form derivatives, which acts as ISs and can generate isotope ISs for all the target metabolites simultaneously in the derivatization step. Therefore, ICD could significantly improve the quantitative accuracy and precision by compensating for ESI matrix effects and instrument drift.
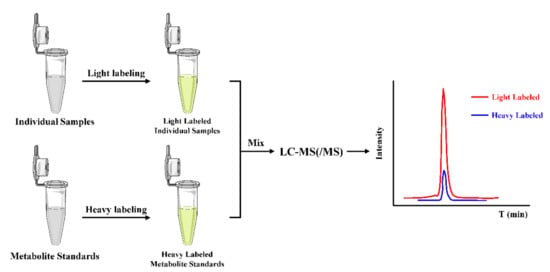
Figure 5.
General workflow of the ICD strategy for the absolute quantification.
As illustrated in Figure 5, the HMP/d3-HMP reagent pair have been utilized for the differentiation and quantification of levels of neurosteroids in brain in response to immobilization stress and antipsychotic drug administration [130]. The HMP derivatization has improved the detection sensitive by 500- and 3000-fold for allopregnanolone and pregnenolone and the use of d3-HMP can significantly reduce the run-to-run ionization differences and achieve high reproducibility and accuracy. However, the isotope effect may cause derivatives and their isotope ISs to be eluted at a different time in chromatographic separation. The shift in retention time may be great enough to cause a different matrix effect in LC-MS, especially when using electrospray ionization (ESI). Due to the deuterium isotope substitution, the d3-HMP derivatives always eluted about 0.1 min earlier than the HMP derivatives and showed a slightly undesirable effect on the quantitative analysis.
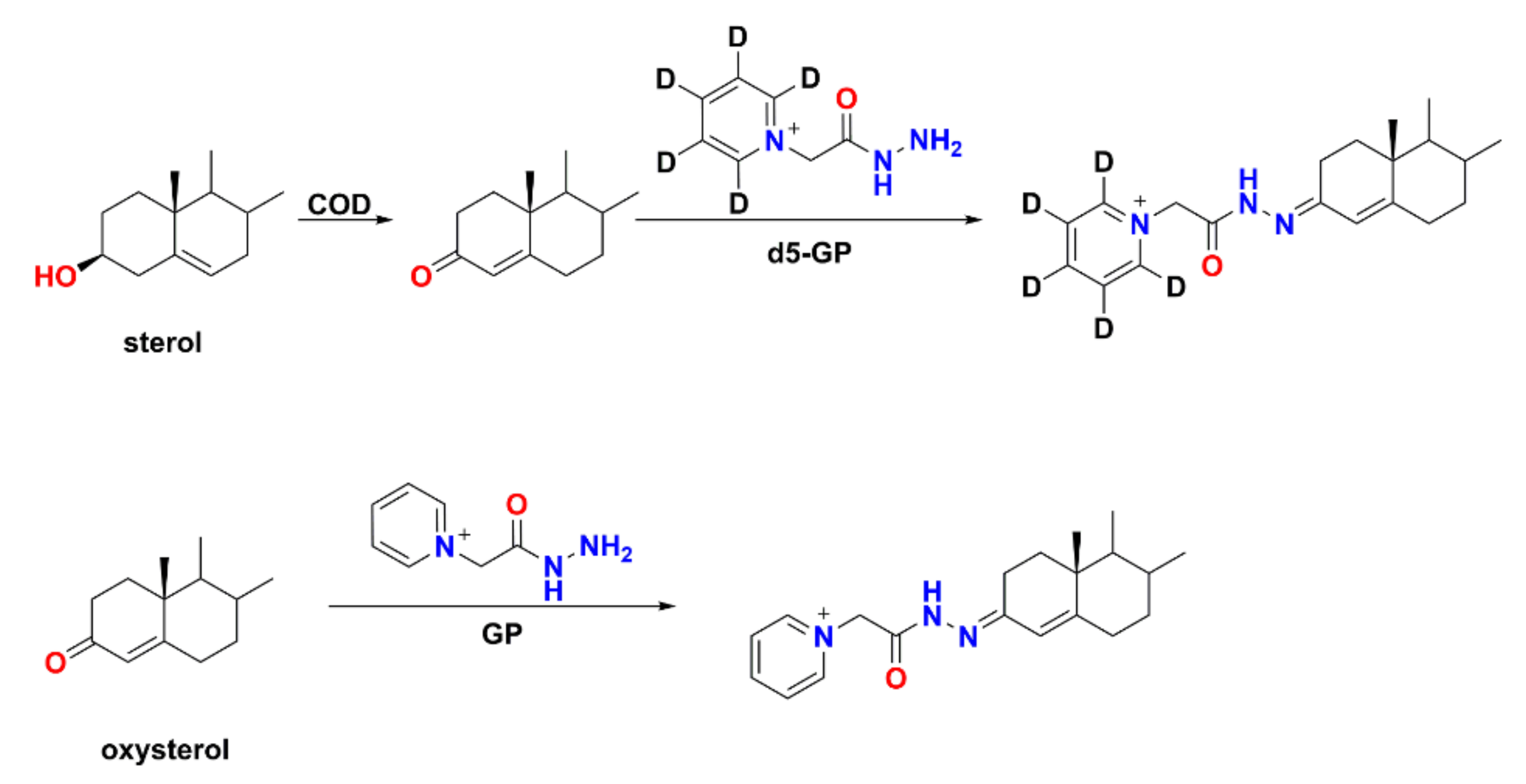
The GP/d5-GP reagent pair have been used to label steroid hormones in follicular fluid samples and steroid hormone standards derivatized with d5-GP act as internal standards for accurately quantifying steroid hormones by calibrating the variation of MS response due to the ion suppression and matrix effects [131]. GP/d5-GP derivatives also accomplish a significant improvement in the ionization efficiency by introducing a permanently charged moiety. The mass difference between d5-GP and GP is 5 Da, which minimizes the negative effects of naturally occurring stable isotopes, such as 13C. In addition, deuterium is labeled on the ring of d5-GP, hence, minimizing the H/D exchange, and providing great stability as molecular tag. The GP/d5-GP reagent pair have been used to simultaneously determine the levels of aldosterone in the left and right adrenal venous serum samples in a single run [136]. This approach can improve the reliability of the quantification and in the meantime shortened the analysis time, hence, enhancing the throughput of the analysis. Since GP can label sterols with a carbonyl group, the sterols with a hydroxyl group and oxysterols with a carbonyl group cannot be analyzed in a single LC-MS analysis using GP derivatization. However, GP/d5-GP can be used to quantify oxysterols and sterols simultaneously if sterols are oxidized by cholesterol oxidase (COD) first to contain a carbonyl group (Scheme 3) [137]. On the basis of this principle, it is possible to quantify up to four different samples in a single run by using differential mass tags and isobaric mass tags simultaneously through multiplex methods [138].
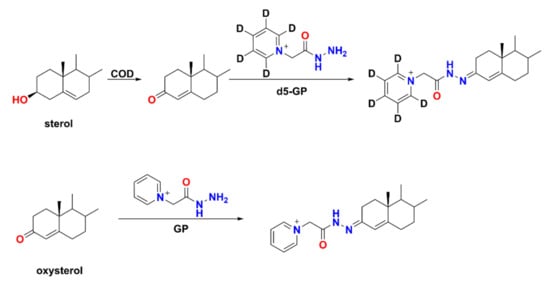
Scheme 3.
Reaction strategy for simultaneous quantification of sterol and oxysterol.
GP has been used to derivatize ketosterols, such as 7α-hydroxy-4-cholesten-3-one (7αC4), to form hydrazones; however, the application of this method is limited by low sensitivity as the LOQ is close to the maximum concentration in the circulation system [139]. The detection sensitivity of QAO derivatives is at least 2- to 3-fold higher than that of GP, therefore, De Barber et al. [132] utilized QAO and d3-QAO reagents to achieve sensitive isotope dilution quantification of 7αC4 and 7α, 12α-dihydroxy-4-cholesten-3-one (7α12αC4) in the plasma of cerebrotendinous xanthomatosis (CTX) patients. As compared with the GP derivatives, the equilibrium constant of oxime is higher, thereby, displaying a better stability of QAO derivatives at room temperature. As comparing with the commercially available QAO-d0-tagged ketosterol-d7 internal standard, QAO-d3-tagged ketosterol-d0 internal standard showed acceptable correlation. Thus, the isotope-coded derivatization reagent, d3-QAO, could be used to generate internal standard for quantification. Later, the method has been modified to reduce the analysis time by two minutes for each sample [140].
Among the developed derivatization reagents, DNPH is the most popular derivatization reagent for aldehyde, but for absolute quantification purposes, its isotope-coded derivatization reagents, d3-DNPH or 15N4-DNPH, are expensive and difficult to prepare. In this case, MPIA/d3-MPIA has been developed, which can be economically and simply prepared with low-cost d0/d3-iodomethane (CH3I/CD3I). Moreover, d0/d3-MPIA, as compared with DNPH, exhibits higher sensitivity with mild derivatization conditions for the quantification of aliphatic aldehydes in aquatic products [112].
Considering the deuterium isotope effect on the quantification, the use of 13C/15N isotope could avoid potential retention time variations induced by deuterium labeling. Sobsey et al. [133] developed a new method for quantification of malondialdehyde using 3-NPH as the derivatization reagent, which has achieved enhanced sensitivity and increased the linearity range with shorter analysis time than the previous DNPH-LC-UV method. While using 13C6-3-NPH to generate an internal standard (13C6-3-NPH-MDA), the quantitative accuracy and precision of MDA in human plasma can be achieved without interference from matrix effect.
In addition, 14N/15N-ammonium acetate coupled with PQ is the first commercially available ICD reagent pair, which avoids the complicated and time-consuming synthesis process. EI-Maghrabey et al. [134] used this cost-effective ICD reagent and developed a method with very sensitivity and high accuracy to determine two highly reactive 4-hydroxy-2-alkenals, i.e., HHE and HNE, in human serum at ultratrace levels. The LOD for both HHE and HNE of the method is about 20–32000 and 4–8700 times lower than all the reported methods. The benefit of using 14N/15N as isotopic signature is that it can completely avoid the deuterium isotope effect, resulting in effectively minimized matrix effects and increased sensitivity during LC-ESI-MS/MS analysis.
There are some other commercially available ICD reagents, such as d0/d8-acetylacetone [121], d0/d5-pyridine, and aniline/13C6-aniline. Formaldehyde has been derivatized to 3,5-diacetyl-1,4-dihydrolutidine (DDL) and d12-DDL by d0/d8-acetylacetone for its accurate quantification in personal-care products [121]. In addition, N-(1-chloroalkyl)pyridinium quaternization has been developed to identify and quantify aliphatic aldehydes in human thyroid carcinoma and para-carcinoma tissue [127]. Pyridine-d5 labeled aliphatic aldehydes have been prepared as internal standards. Furthermore, d0/d5-pyridine could also be used to simultaneously label different functional groups including hydroxyls and carbonyls [141]. Similarly, aniline/13C6-aniline could also label multiple functional groups to simultaneously quantify metabolites involved in central carbon and energy metabolism [104,106].
3.2. Submetabolome Profiling
Another important application of the ICD strategy is submetabolome profiling. By using the ICD reagent pair, carbonyl-containing metabolites will exhibit a pair of peaks with equal intensity in MS or MS/MS spectra, which can be easily recognized in a mixture.
Dns-Hz/13C2-Dns-Hz [36] is a typical ICD reagent pair for labeling carbonyl for submetabolome profiling and has been used to profile the carbonyl-containing metabolites in human urine, which detected 2030 ± 39 pairs per run with high reproducibility. This approach has been demonstrated to be valuable for profiling important carbonyl-containing metabolites in complex biological fluids. This ICD reagent combined with other labeling reagent could achieve a broader coverage of carbonyl, amine, carboxyl, and hydroxyl submetabolomes [142].
QDA/13CD3-QDA is another ICD reagent pair for profiling carbonyl-containing metabolites in tissue and cell extracts [95]. The hydrophobic moiety of QDA provides better retention property for carbonyl-containing metabolites, which is capable of removal interferences from hydrophilic metabolites, while the stable isotope signatures enable simple identification of carbonyl metabolites. This method is suitable for quantification of low abundance carbonyl metabolites [96].
Another example of ICD reagent is d0/d20-2,4-bis(diethylamino)-6-hydrazino-1,3,5-triazine (T3/D3) [69], which has been utilized for both the discovery and comprehensive characterization of endogenous fatty aldehydes in rat plasma and brain tissue. A total of 43 and 19 fatty aldehydes were significantly changed in plasma and brain tissues between the control group and the model group, which revealed the capability of T3/D3 in profiling of fatty aldehydes.
4-APC/d4-4-APC ICD pairs has also been used to profile aldehyde-containing compounds [110]. Since 4-APC and d4-4-APC could produce two characteristic neutral fragments, 87 Da and 91 Da, double neutral loss scan (DNLS) could be performed for identification of potential aldehyde-containing compounds. The DNLS has inherent high selectivity as compared with the full scan mode, therefore, this method can improve the detection sensitivity. This reagent, coupled with black phosphorous, as MALDI matrix, exhibits strong capability in the quantitative analysis of aldehydes in complex samples including saliva, urine, and serum [143]. 4-APC/d4-4-APC has also been used in combination with BQB/d7-BQB for simultaneous profiling of aldehydes and thiols in beer samples [144].
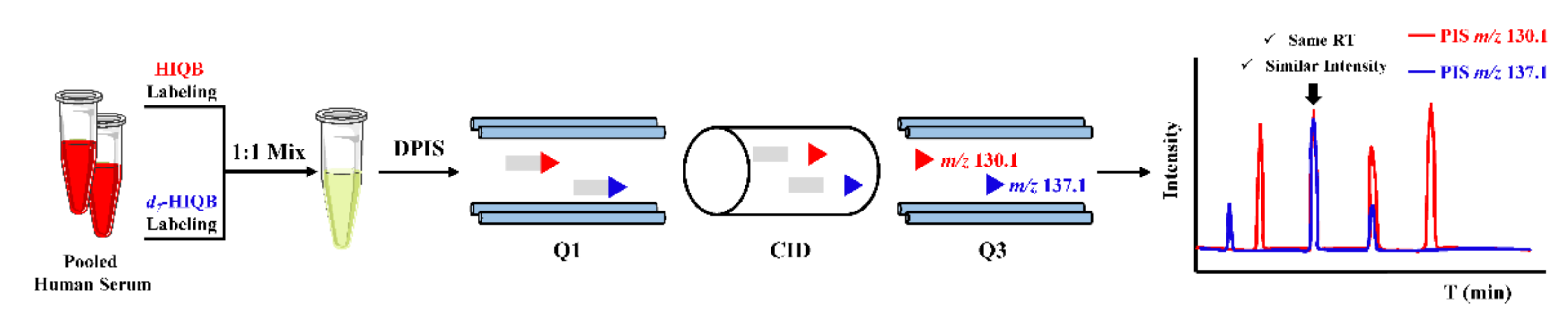
Stable isotope labeling double precursor ion scan MS analysis (IL-DPIS-MS) strategy is an alternative method for untargeted metabolic profiling without HRMS or prior knowledge of a metabolites list. It was firstly proposed by Feng’s group, in 2017, for profiling and relative quantitation of carbonyl compounds in human serum derivatized with HIQB/d7-HIQB [81]. As shown in Figure 6, two characteristic product ions of m/z 130.1 and 137.1 were obtained from HIQB/d7-HIQB labeled carbonyl compounds under CID and were utilized for double precursor ion scans to characterize carbonyl compounds, which significantly improved the detection selectivity and accuracy as compared with the full scan mode. A total of 44 carbonyl compounds could be identified in serum. These detected metabolites could differentiate myelogenous leukemia (ML) patients from healthy controls, which could serve as biomarkers for ML. Moreover, aberrant serum carbonyl compound profiles can also reflect poor cognitive performance of subclinical carotid atherosclerosis (SCA) patients [13].
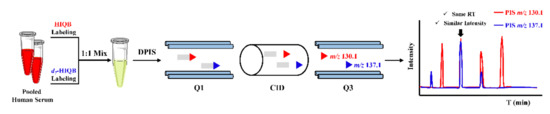
Figure 6.
The scheme for profiling of carbonyl compounds using HIQB/d7-HIQB.
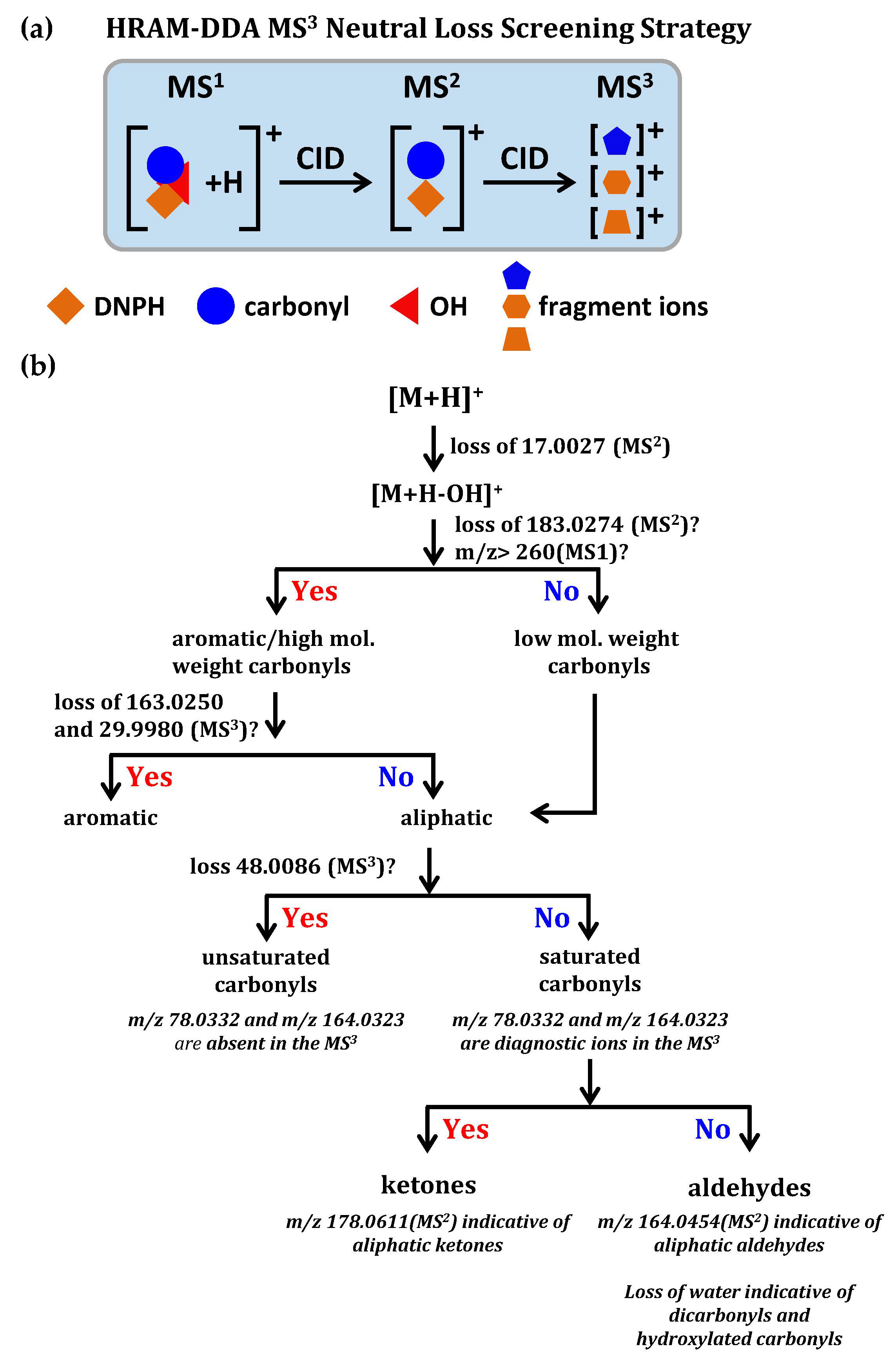
Dator et al. [135] firstly used the high resolution accurate mass (HRAM) screening strategy combined with diagnostic neutral loss of hydroxyl radical triggering MS3 fragmentation for characterizing known and unknown DNPH-derivatized carbonyls, as shown in Figure 7. The HRAM screening strategy has reliably determined the neutral loss of DNPH-derivatized carbonyls, which was due to the loss of hydroxyl radical (17.0027 Da), rather than other interferences, such as NH3 (17.0266 Da). Observation of the neutral loss of hydroxyl radical triggered MS3 fragmentation to produce characteristic ions (m/z 78.0332 and 164.0323) in the MS3, which minimized false positive identification. In addition, this method coupled with d3-DNPH allowed relative quantification of carbonyl compounds in saliva [135].
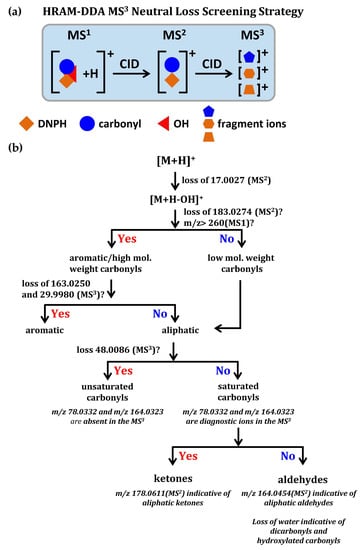
Figure 7.
(a) Scheme of HRAM-DDA MS3 neutral loss screening strategy; (b) classification scheme for characterizing known and unknown DNPH-derivatized carbonyls. Adapted with permission from [135]. Copyright 2017 American Chemical Society.
4. Conclusions
Carbonyl-containing metabolites widely exist in biological samples and have important physiological and pathological functions. Thus, quantitatively accurate analysis of carbonyl-containing metabolites with high sensitivity is crucial to provide insight into metabolic pathways as well as disease mechanisms. However, quantitative analysis remains a challenge due to the high diversity of physicochemical properties, and the high dynamic concentration range of carbonyl-containing metabolites presented in variations of sample matrix. Although RPLC-ESI-MS is widely used for the measurement of these metabolites, it is still challenging to directly analyze carbonyl-containing metabolites using RPLC-ESI-MS due to poor ionization efficiency of neutral carbonyl groups in ESI. In this regard, modification of carbonyl-containing metabolites by a chemical derivatization strategy can convert them into MS-detectable and LC-separable derivatives. Almost all the derivatization reagents are primary amines, which, as nucleophiles, react with carbonyl groups in the metabolites to yield imines. Since substituents at the α-nucleophile moiety having a distinct effect on the stability of derivatives, the types of derivatization reagents play an important role in the rate of derivatization reaction, which can be divided into four categories: hydrazines, hydrazides, hydroxylamines, and amines. However, a pair of E-/Z- isomers can be formed as derivatization products and exhibit broad peaks or be separated in the LC chromatograms, which obstruct quantification. To overcome this obstacle, a post-column derivatization strategy or reductive amination method using a reducing agent could be an efficient solution. Although modification of carbonyl-containing metabolites by a chemical derivatization strategy can overcome the obstacles of detectability and sensitivity, it is still insufficient to achieve accurate quantification, since instrument drift and matrix effects, produced by the co-eluting components, result in fluctuations of ionization efficiency of metabolites in ESI. The emergence of ICD provides a good solution to the problems encountered above. Thus, LC-MS methods that utilize ICD have been applied in metabolomics including quantitative and profiling analysis. In addition, ICD makes multiplex isobaric or differential mass tags possible, which enables multiple samples to be detected in a single run, whereby reducing instrument running time, and also avoids the variation of MS response due to instrument drift. A simultaneous ICD strategy for multichannel submetabolome analysis has several unbeatable advantages, including high coverage of metabolites and quantitative high throughput, which makes molecular phenotyping of a population possible.
Supplementary Materials
The following are available online, Table S1: Derivatization reagents for carbonyl-containing metabolites.
Author Contributions
Writing—original draft preparation, Y.S.; conceptualized and supervision, H.T.; writing—review and editing, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Shanghai Municipal Science and Technology Major Project (2017SHZDZX01), the Ministry of Science and Technology of China (2018YFE0201603, 2020YFE0201600, and 2017YFC0906800), and the National Natural Science Foundation of China (81590953 and 31821002).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Uchida, K. Role of reactive aldehyde in cardiovascular diseases. Free Radic. Biol. Med. 2000, 28, 1685–1696. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Domingues, R.M.; Domingues, P.; Melo, T.; Perez-Sala, D.; Reis, A.; Spickett, C.M. Lipoxidation adducts with peptides and proteins: Deleterious modifications or signaling mechanisms? J. Proteom. 2013, 92, 110–131. [Google Scholar] [CrossRef]
- Fedorova, M.; Bollineni, R.C.; Hoffmann, R. Protein Carbonylation as a Major Hallmark of Oxidative Damage: Update of Analytical Strategies. Mass Spectrom. Rev. 2014, 33, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.P.; Soldin, O.P.; Guo, T.D.; Soldin, S.J. Steroid hormones: Relevance and measurement in the clinical laboratory. Clin. Lab. Med. 2004, 24, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.P.; Soldin, S.J.; Tractenberg, R.E.; Guo, T.D.; Kundra, P.; Verbalis, J.G.; Jonklaas, J. Use of steroid profiles in determining the cause of adrenal insufficiency. Steroids 2007, 72, 71–84. [Google Scholar] [CrossRef]
- Santen, R.J.; Yue, W.; Wang, J.-P. Estrogen metabolites and breast cancer. Steroids 2015, 99, 61–66. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Dekkers, T.; Peitzsch, M.; Dietz, A.S.; Bidlingmaier, M.; Treitl, M.; Williams, T.A.; Bornstein, S.R.; Haase, M.; Rump, L.C.; et al. Mass Spectrometry-Based Adrenal and Peripheral Venous Steroid Profiling for Subtyping Primary Aldosteronism. Clin. Chem. 2016, 62, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Ma, S.; Huang, Y.; Yuan, H.; Zhao, Y. LC-MS/MS-based non-isotopically paired labeling (NIPL) Strategy for the qualification and quantification of monosaccharides. Talanta 2021, 231, 122336. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Perluigi, M.; Coccia, R.; Butterfield, D.A. 4-Hydroxy-2-Nonenal, a Reactive Product of Lipid Peroxidation, and Neurodegenerative Diseases: A Toxic Combination Illuminated by Redox Proteomics Studies. Antioxid. Redox Signal. 2012, 17, 1590–1609. [Google Scholar] [CrossRef] [PubMed]
- Sas, K.M.; Karnovsky, A.; Michailidis, G.; Pennathur, S. Metabolomics and Diabetes: Analytical and Computational Approaches. Diabetes 2015, 64, 718–732. [Google Scholar] [CrossRef]
- Wu, D.M.; Zhang, J.J.; Guo, N.; Zheng, S.J.; Zhu, Q.F.; Feng, Y.Q.; Peng, S.S.; Wu, Y.H.; Tuo, M.H. Cognitive impairment correlates with serum carbonyl compound profiles in subclinical carotid atherosclerosis. Neuroreport 2018, 29, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Xie, C.S.; Markesbery, W.R. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol. Aging 2001, 22, 187–194. [Google Scholar] [CrossRef]
- Poli, D.; Goldoni, M.; Corradi, M.; Acampa, O.; Carbognani, P.; Internullo, E.; Casalini, A.; Mutti, A. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME-GC/MS. J. Chromatogr. B 2010, 878, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argiles, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Kancheva, L.; Hill, M.; Vcelakova, H.; Vrbikova, J.; Pelikanova, T.; Starka, L. The identification and simultaneous quantification of neuroactive androstane steroids and their polar conjugates in the serum of adult men, using gas chromatography-mass spectrometry. Steroids 2007, 72, 792–801. [Google Scholar] [CrossRef]
- Kushnir, M.M.; Rockwood, A.L.; Roberts, W.L.; Pattison, E.G.; Bunker, A.M.; Fitzgerald, R.L.; Meikle, A.W. Performance characteristics of a novel tandem mass spectrometry assay for serum testosterone. Clin. Chem. 2006, 52, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Li, L. Matrix effect on chemical isotope labeling and its implication in metabolomic sample preparation for quantitative metabolomics. Metabolomics 2015, 11, 1733–1742. [Google Scholar] [CrossRef]
- Sun, M.; Manolopoulou, J.; Spyroglou, A.; Beuschlein, F.; Hantel, C.; Wu, Z.D.; Bielohuby, M.; Hoeflich, A.; Liu, C.; Bidlingmaier, M. A microsphere-based duplex competitive immunoassay for the simultaneous measurements of aldosterone and testosterone in small sample volumes: Validation in human and mouse plasma. Steroids 2010, 75, 1089–1096. [Google Scholar] [CrossRef]
- Ohlsson, C.; Nilsson, M.E.; Tivesten, A.; Ryberg, H.; Mellstrom, D.; Karlsson, M.K.; Ljunggren, O.; Labrie, F.; Orwoll, E.S.; Lee, D.M.; et al. Comparisons of Immunoassay and Mass Spectrometry Measurements of Serum Estradiol Levels and Their Influence on Clinical Association Studies in Men. J. Clin. Endocrinol. Metab. 2013, 98, E1097–E1102. [Google Scholar] [CrossRef]
- Kushnir, M.M.; Blamires, T.; Rockwood, A.L.; Roberts, W.L.; Yue, B.F.; Erdogan, E.; Bunker, A.M.; Meikle, A.W. Liquid Chromatography-Tandem Mass Spectrometry Assay for Androstenedione, Dehydroepiandrosterone, and Testosterone with Pediatric and Adult Reference Intervals. Clin. Chem. 2010, 56, 1138–1147. [Google Scholar] [CrossRef]
- Fanelli, F.; Belluomo, I.; Di Lallo, V.D.; Cuomo, G.; De Iasio, R.; Baccini, M.; Casadio, E.; Casetta, B.; Vicennati, V.; Gambineri, A.; et al. Serum steroid profiling by isotopic dilution-liquid chromatography-mass spectrometry: Comparison with current immunoassays and reference intervals in healthy adults. Steroids 2011, 76, 244–253. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C. Systems biology—Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef]
- Stringer, K.A.; McKay, R.T.; Karnovsky, A.; Quemerais, B.; Lacy, P. Metabolomics and its Application to Acute Lung Diseases. Front. Immunol. 2016, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Advances in metabolite identification. Bioanalysis 2011, 3, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; dos Santos, D.C.M.; Garcia, A.; Barbas, C. Analytical protocols based on LC-MS, GC-MS and CE-MS for nontargeted metabolomics of biological tissues. Bioanalysis 2014, 6, 1657–1677. [Google Scholar] [CrossRef]
- Gil, A.; Siegel, D.; Permentier, H.; Reijngoud, D.-J.; Dekker, F.; Bischoff, R. Stability of energy metabolitesAn often overlooked issue in metabolomics studies: A review. Electrophoresis 2015, 36, 2156–2169. [Google Scholar] [CrossRef] [PubMed]
- Gika, H.; Virgiliou, C.; Theodoridis, G.; Plumb, R.S.; Wilson, I.D. Untargeted LC/MS-based metabolic phenotyping (metabonomics/metabolomics): The state of the art. J. Chromatogr. B 2019, 1117, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Rochat, B. From targeted quantification to untargeted metabolomics: Why LC-high-resolution-MS will become a key instrument in clinical labs. Trac-Trends Anal. Chem. 2016, 84, 151–164. [Google Scholar] [CrossRef]
- Yin, P.Y.; Xu, G.W. Current state-of-the-art of nontargeted metabolomics based on liquid chromatography-mass spectrometry with special emphasis in clinical applications. J. Chromatogr. A 2014, 1374, 1–13. [Google Scholar] [CrossRef]
- Gika, H.G.; Theodoridis, G.A.; Plumb, R.S.; Wilson, I.D. Current practice of liquid chromatography-mass spectrometry in metabolomics and metabonomics. J. Pharm. Biomed. Anal. 2014, 87, 12–25. [Google Scholar] [CrossRef]
- Santa, T. Derivatization reagents in liquid chromatography/electrospray ionization tandem mass spectrometry. Biomed. Chromatogr. 2011, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.Q.; Jiao, Y.; Xu, F.G. Chemical derivatization-based LC-MS for metabolomics: Advantages and challenges. Bioanalysis 2016, 8, 1881–1883. [Google Scholar] [CrossRef]
- Anderegg, R.J. Derivatization in mass spectrometry: Strategies for controlling fragmentation. Mass Spectrom. Rev. 1988, 7, 395–424. [Google Scholar] [CrossRef]
- Zhao, S.; Dawe, M.; Guo, K.; Li, L. Development of High-Performance Chemical Isotope Labeling LC-MS for Profiling the Carbonyl Submetabolome. Anal. Chem. 2017, 89, 6758–6765. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.L.; Worth, A.J.; Mesaros, C.; Snyder, N.W.; Glickson, J.D.; Blair, I.A. Diisopropylethylamine/hexafluoroisopropanol-mediated ion-pairing ultra-high-performance liquid chromatography/mass spectrometry for phosphate and carboxylate metabolite analysis: Utility for studying cellular metabolism. Rapid Commun. Mass Spectrom. 2016, 30, 1835–1845. [Google Scholar] [CrossRef] [PubMed]
- Tudela, E.; Munoz, G.; Munoz-Guerra, J.A. Matrix effect marker for multianalyte analysis by LC-MS/MS in biological samples. J. Chromatogr. B 2012, 901, 98–106. [Google Scholar] [CrossRef]
- Higashi, T.; Ogawa, S. Isotope-coded ESI-enhancing derivatization reagents for differential analysis, quantification and profiling of metabolites in biological samples by LC/MS: A review. J. Pharm. Biomed. Anal. 2016, 130, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, L. Chemical derivatization in LC-MS-based metabolomics study. Trac-Trends Anal. Chem. 2020, 131, 11. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Li, S.; Zhu, Q.F.; Wang, Q.; Hussain, D.; Feng, Y.Q. Derivatization for liquid chromatography-electrospray ionization-mass spectrometry analysis of small-molecular weight compounds. Trac-Trends Anal. Chem. 2019, 119, 13. [Google Scholar] [CrossRef]
- Huang, T.J.; Armbruster, M.R.; Coulton, J.B.; Edwards, J.L. Chemical Tagging in Mass Spectrometry for Systems Biology. Anal. Chem. 2019, 91, 109–125. [Google Scholar] [CrossRef]
- El-Maghrabey, M.H.; Kishikawa, N.; Kuroda, N. Current trends in isotope-coded derivatization liquid chromatographic-mass spectrometric analyses with special emphasis on their biomedical application. Biomed. Chromatogr. 2020, 34, 29. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Sauer, U.; Zamboni, N. Quantification and Mass Isotopomer Profiling of alpha-Keto Acids in Central Carbon Metabolism. Anal. Chem. 2014, 86, 3232–3237. [Google Scholar] [CrossRef] [PubMed]
- Gowacki, R.; Stachniuk, J.; Borowczyk, K.; Jakubowski, H. Quantification of homocysteine and cysteine by derivatization with pyridoxal 5’-phosphate and hydrophilic interaction liquid chromatography. Anal. Bioanal. Chem. 2016, 408, 1935–1941. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chu, J.M.; Huang, W.; Xiong, J.; Xing, X.W.; Zhou, X.; Feng, Y.Q.; Yuan, B.F. Hydrophilic Material for the Selective Enrichment of 5-Hydroxymethylcytosine and Its Liquid Chromatography-Tandem Mass Spectrometry Detection. Anal. Chem. 2013, 85, 6129–6135. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Qi, C.-B.; Liu, T.; Xiao, H.-M.; Cheng, Q.-Y.; Jiang, H.-P.; Yuan, B.-F.; Feng, Y.-Q. Formation and Determination of Endogenous Methylated Nucleotides in Mammals by Chemical Labeling Coupled with Mass Spectrometry Analysis. Anal. Chem. 2017, 89, 4153–4160. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Hu, Y.L.; Veillon, L.; Snovida, S.I.; Rogers, J.C.; Saba, J.; Mechref, Y. Quantitative LC-MS/MS Glycomic Analysis of Biological Samples Using AminoxyTMT. Anal. Chem. 2016, 88, 7515–7522. [Google Scholar] [CrossRef]
- Berdyshev, E.V.; Gorshkova, I.A.; Garcia, J.G.N.; Natarajan, V.; Hubbard, W.C. Quantitative analysis of sphingoid base-1-phosphates as bisacetylated derivatives by liquid chromatography-tandem mass spectrometry. Anal. Biochem. 2005, 339, 129–136. [Google Scholar] [CrossRef]
- Lv, H.J.; Luo, S.Q.; Tian, R.C.; Zhang, W.H.; Sun, B.S.; Cui, Y. Analysis of endogenous epinephrine and norepinephrine enantiomers in rat plasma and application to a stereoselective pharmacokinetics. J. Pharm. Biomed. Anal. 2020, 177, 8. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Jiang, X.; Zhou, J.L.; Shi, Z.Q.; Liu, L.F.; Xin, G.Z. A readily O-16-/O-18-isotopically-paired chiral derivatization approach for the quantification of 2-HG metabolic panel by liquid chromatography-Tandem mass spectrometry. Anal. Chim. Acta 2019, 1077, 174–182. [Google Scholar] [CrossRef]
- Cheng, Q.Y.; Xiong, J.; Wang, F.; Yuan, B.F.; Feng, Y.Q. Chiral derivatization coupled with liquid chromatography/mass spectrometry for determining ketone metabolites of hydroxybutyrate enantiomers. Chin. Chem. Lett. 2018, 29, 115–118. [Google Scholar] [CrossRef]
- Cheng, Q.Y.; Xiong, J.; Huang, W.; Ma, Q.; Ci, W.M.; Feng, Y.Q.; Yuan, B.F. Sensitive Determination of Onco-metabolites of D- and L-2-hydroxyglutarate Enantiomers by Chiral Derivatization Combined with Liquid Chromatography/Mass Spectrometry Analysis. Sci Rep. 2015, 5, 11. [Google Scholar] [CrossRef]
- Xu, F.; Zou, L.; Liu, Y.; Zhang, Z.; Ong, C.N. Enhancement of the capabilities of liquid chromatography-mass spectrometry with derivatization: General principles and applications. Mass Spectrom. Rev. 2011, 30, 1143–1172. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cai, W.J.; Wang, W.; Sun, M.X.; Feng, Y.Q. Rapid Analysis of Monosaccharides in Sub-milligram Plant Samples Using Liquid Chromatography-Mass Spectrometry Assisted by Post-column Derivatization. J. Agric. Food. Chem. 2020, 68, 2588–2596. [Google Scholar] [CrossRef] [PubMed]
- Kolmel, D.K.; Kool, E.T. Oximes and Hydrazones in Bioconjugation: Mechanism and Catalysis. Chem. Rev. 2017, 117, 10358–10376. [Google Scholar] [CrossRef]
- Allen, C.F.H. The identification of carbonyl compounds by use of 2,4-dinitrophenylhydrazine. J. Am. Chem. Soc. 1930, 52, 2955–2959. [Google Scholar] [CrossRef]
- Brady, O.L. The use of 2 4-dinitrophenylhydrazine as a reagent for carbonyl compounds. J. Chem. Soc. 1931, 61, 756–759. [Google Scholar] [CrossRef]
- Zweiner, C.; Glauner, T.; Frimmel, F.H. Method optimization for the determination of carbonyl compounds in disinfected water by DNPH derivatization and LC-ESI-MS-MS. Anal. Bioanal. Chem. 2002, 372, 615–621. [Google Scholar] [CrossRef]
- Banos, C.E.; Silva, M. Comparison of several sorbents for continuous in situ derivatization and preconcentration of low-molecular mass aldehydes prior to liquid chromatography-tandem mass spectrometric determination in water samples. J. Chromatogr. A 2009, 1216, 6554–6559. [Google Scholar] [CrossRef]
- Siegel, D.; Rasenko, T.; Koch, M.; Nehis, I. Determination of the Alternaria mycotoxin tenuazonic acid in cereals by high-performance liquid chromatography-electrospray ionization ion-trap multistage mass spectrometry after derivatization with 2,4-dinitrophenylhydrazine. J. Chromatogr. A 2009, 1216, 4582–4588. [Google Scholar] [CrossRef]
- Tolgyesi, A.; Stroka, J.; Tamosiunas, V.; Zwickel, T. Simultaneous analysis of Alternaria toxins and citrinin in tomato: An optimised method using liquid chromatography-tandem mass spectrometry. Food Addit. Contam. Part. A 2015, 32, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.S.; Yan, X.Z.; Wong, J.H.; Chan, M.; Watson, C.H. In Situ Derivatization and Quantification of Seven Carbonyls in Cigarette Mainstream Smoke. Chem. Res. Toxicol. 2016, 29, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Tarr, M.A. Aldehyde and Ketone Photoproducts from Solar-Irradiated Crude Oil-Seawater Systems Determined by Electrospray Ionization-Tandem Mass Spectrometry. Environ. Sci. Technol. 2017, 51, 11858–11866. [Google Scholar] [CrossRef]
- Chen, J.L.; Huang, Y.J.; Pan, C.H.; Hu, C.W.; Chao, M.R. Determination of urinary malondialdehyde by isotope dilution LC-MS/MS with automated solid-phase extraction: A cautionary note on derivatization optimization. Free Radic. Biol. Med. 2011, 51, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lin, K.; Sequria, C.; Yang, J.C.; Borchers, C.H. Quantitation of low molecular weight sugars by chemical derivatization-liquid chromatography/multiple reaction monitoring/mass spectrometry. Electrophoresis 2016, 37, 1851–1860. [Google Scholar] [CrossRef]
- Han, J.; Gagnon, S.; Eckle, T.; Borchers, C.H. Metabolomic analysis of key central carbon metabolism carboxylic acids as their 3-nitrophenylhydrazones by UPLC/ESI-MS. Electrophoresis 2013, 34, 2891–2900. [Google Scholar] [CrossRef]
- Hofstetter, C.K.; Dunkel, A.; Hofmann, T. Unified Flavor Quantitation: Toward High-Throughput Analysis of Key Food Odorants and Tastants by Means of Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry. J. Agric. Food. Chem. 2019, 67, 8599–8608. [Google Scholar] [CrossRef]
- Tie, C.; Hu, T.; Jia, Z.X.; Zhang, J.L. Derivatization Strategy for the Comprehensive Characterization of Endogenous Fatty Aldehydes Using HPLC-Multiple Reaction Monitoring. Anal. Chem. 2016, 88, 7762–7768. [Google Scholar] [CrossRef]
- Tie, C.; Hu, T.; Zhang, X.X.; Zhou, J.; Zhang, J.L. HPLC-MRM relative quantification analysis of fatty acids based on a novel derivatization strategy. Analyst 2014, 139, 6154–6159. [Google Scholar]
- Magda, B.; Dobi, Z.; Meszaros, K.; Szabo, E.; Marta, Z.; Imre, T.; Szabo, P.T. Charged derivatization and on-line solid phase extraction to measure extremely low cortisol and cortisone levels in human saliva with liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2017, 140, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Nishio, T.; Hayashi, N.; Shimada, K. Alternative procedure for charged derivatization to enhance detection responses of steroids in electrospray ionization-MS. Chem. Pharm. Bull. 2007, 55, 662–665. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sosvorova, L.; Vitku, J.; Chlupacova, T.; Mohapl, M.; Hampl, R. Determination of seven selected neuro- and immunomodulatory steroids in human cerebrospinal fluid and plasma using LC-MS/MS. Steroids 2015, 98, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, L.; De Andres, F.; Capi, M.; Curto, M.; Sabato, D.; Simmaco, M.; Bossu, P.; Sacchinelli, E.; Orfei, M.D.; Piras, F.; et al. LC-MS/MS simultaneous analysis of allopregnanolone, epiallopregnanolone, pregnanolone, dehydroepiandrosterone and dehydroepiandrosterone 3-sulfate in human plasma. Bioanalysis 2017, 9, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, N.; Skadberg, O.; Adaway, J.; Brede, C. Multiplexed analysis of steroid hormones in saliva by LC-MS/MS with 2-hydrazinopyridine derivatization. Clin. Mass Spectrom. 2017, 4–5, 1–10. [Google Scholar] [CrossRef]
- Dury, A.Y.; Ke, Y.Y.; Labrie, F. Precise and accurate assay of pregnenolone and five other neurosteroids in monkey brain tissue by LC-MS/MS. Steroids 2016, 113, 64–70. [Google Scholar] [CrossRef]
- Johnson, D.W. A modified Girard derivatizing reagent for universal profiling and trace analysis of aldehydes and ketones by electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2926–2932. [Google Scholar] [CrossRef]
- Frey, A.J.; Wang, Q.; Busch, C.; Feldman, D.; Bottalico, L.; Mesaros, C.A.; Blair, I.A.; Vachani, A.; Snyder, N.W. Validation of highly sensitive simultaneous targeted and untargeted analysis of keto-steroids by Girard P derivatization and stable isotope dilution-liquid chromatography-high resolution mass spectrometry. Steroids 2016, 116, 60–66. [Google Scholar] [CrossRef]
- Crick, P.J.; Aponte, J.; Bentley, T.W.; Matthews, I.; Wang, Y.Q.; Griffiths, W.J. Evaluation of novel derivatisation-reagents for the analysis of oxysterols. Biochem. Biophys. Res. Commun. 2014, 446, 756–761. [Google Scholar] [CrossRef]
- Jiang, H.-P.; Liu, T.; Guo, N.; Yu, L.; Yuan, B.-F.; Feng, Y.-Q. Determination of formylated DNA and RNA by chemical labeling combined with mass spectrometry analysis. Anal. Chim. Acta 2017, 981, 1–10. [Google Scholar] [CrossRef]
- Guo, N.; Peng, C.Y.; Zhu, Q.F.; Yuan, B.F.; Feng, Y.Q. Profiling of carbonyl compounds in serum by stable isotope labeling Double precursor ion scan—Mass spectrometry analysis. Anal. Chim. Acta 2017, 967, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Schafer, J.; Kuhn, K.; Kienle, S.; Schwarz, J.; Schmidt, G.; Neumann, T.; Hamon, C. Tandem mass tags: A novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal. Chem. 2003, 75, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Rigdova, K.; Wang, Y.; Ward, M.; Griffiths, W.J. A new derivative for oxosteroid analysis by mass spectrometry. Biochem. Biophys. Res. Commun. 2014, 446, 762–767. [Google Scholar] [CrossRef][Green Version]
- Milic, I.; Fedorova, M. Derivatization and Detection of Small Aliphatic and Lipid-Bound Carbonylated Lipid Peroxidation Products by ESI-MS. In Advanced Protocols in Oxidative Stress III; Armstrong, D., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2015; Volume 1208, pp. 3–20. [Google Scholar]
- Brkljacic, L.; Jeric, I. Glutamic acid-related hydrazine reagent for the derivatization of carbonyl compounds. J. Mass Spectrom. 2018, 53, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.; Meinema, A.C.; Permentier, H.; Hopfgartner, G.; Bischoff, R. Integrated Quantification and Identification of Aldehydes and Ketones in Biological Samples. Anal. Chem. 2014, 86, 5089–5100. [Google Scholar] [CrossRef]
- Tomono, S.; Miyoshi, N.; Ohshima, H. Comprehensive analysis of the lipophilic reactive carbonyls present in biological specimens by LC/ESI-MS/MS. J. Chromatogr. B 2015, 988, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chi, Q.; Fan, R.T.; Tian, H.D.; Wang, X. Quantitative-Profiling Method of Serum Steroid Hormones by Hydroxylamine-Derivatization HPLC-MS. Nat. Prod. Bioprospect. 2019, 9, 201–208. [Google Scholar] [CrossRef]
- Chen, D.; Ding, J.; Wu, M.K.; Zhang, T.Y.; Qi, C.B.; Feng, Y.Q. A liquid chromatography-mass spectrometry method based on post column derivatization for automated analysis of urinary hexanal and heptanal. J. Chromatogr. A 2017, 1493, 57–63. [Google Scholar] [CrossRef]
- Ma, R.N.; Chen, X.Y.; Xiao, Z.Y.; Natarajan, M.; Lu, C.X.; Jiang, X.J.; Zhong, W.; Liu, X.M. Beckmann rearrangement of ketoximes promoted by cyanuric chloride and dimethyl sulfoxide under a mild condition. Tetrahedron Lett. 2021, 63, 1–6. [Google Scholar] [CrossRef]
- Qin, Q.; Feng, D.; Hu, C.; Wang, B.; Chang, M.; Liu, X.; Yin, P.; Shi, X.; Xu, G. Parallel derivatization strategy coupled with liquid chromatography-mass spectrometry for broad coverage of steroid hormones. J. Chromatogr. A 2020, 1614, 1–7. [Google Scholar] [CrossRef]
- Di Donna, L.; Benabdelkamel, H.; Taverna, D.; Indelicato, S.; Aiello, D.; Napoli, A.; Sindona, G.; Mazzotti, F. Determination of ketosteroid hormones in meat by liquid chromatography tandem mass spectrometry and derivatization chemistry. Anal. Bioanal. Chem. 2015, 407, 5835–5842. [Google Scholar] [CrossRef] [PubMed]
- Star-Weinstock, M.; Williamson, B.L.; Dey, S.; Pillai, S.; Purkayastha, S. LC-ESI-MS/MS Analysis of Testosterone at Sub-Picogram Levels Using a Novel Derivatization Reagent. Anal. Chem. 2012, 84, 9310–9317. [Google Scholar] [CrossRef]
- Bilkova, Z.; Adamkova, M.; Albrecht, T.; Simek, Z. Determination of testosterone and corticosterone in feathers using liquid chromatography-mass spectrometry. J. Chromatogr. A 2019, 1590, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, S.J.; Xu, T.; Nantz, M.H.; Higashi, R.M.; Fan, T.W.M. A carbonyl capture approach for profiling oxidized metabolites in cell extracts. Metabolomics 2012, 8, 989–996. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deng, P.; Higashi, R.M.; Lane, A.N.; Bruntz, R.C.; Sun, R.C.; Raju, M.V.R.; Nantz, M.H.; Qi, Z.; Fan, T.W.M. Quantitative profiling of carbonyl metabolites directly in crude biological extracts using chemoselective tagging and nanoESI-FTMS. Analyst 2018, 143, 311–322. [Google Scholar] [CrossRef]
- Jouanin, I.; Chevolleau, S.; Canlet, C.; Lorber, C.; Pierre, F.; Gueraud, F.; Debrauwer, L. Facile Oxime Ether Synthesis: Free Carbonyl Compound Derivatization by a Brominated O-Benzylhydroxylamine. Synth. Commun. 2015, 45, 1585–1591. [Google Scholar] [CrossRef]
- Yuan, W.; Li, S.; Edwards, J.L. Extraction and Quantitation of Ketones and Aldehydes from Mammalian Cells Using Fluorous Tagging and Capillary LC-MS. Anal. Chem. 2015, 87, 7660–7666. [Google Scholar] [CrossRef]
- Conway, L.P.; Garg, N.; Lin, W.; Vujasinovic, M.; Lohr, J.M.; Globisch, D. Chemoselective probe for detailed analysis of ketones and aldehydes produced by gut microbiota in human samples. Chem. Commun. 2019, 55, 9080–9083. [Google Scholar] [CrossRef]
- Lin, W.; Conway, L.P.; Block, A.; Sommi, G.; Vujasinovic, M.; Lohr, J.M.; Globisch, D. Sensitive mass spectrometric analysis of carbonyl metabolites in human urine and fecal samples using chemoselective modification. Analyst 2020, 145, 3822–3831. [Google Scholar] [CrossRef]
- Guan, Z.B.; Ding, M.M.; Sun, Y.; Yu, S.S.; Zhang, A.; Xia, S.G.; Hu, X.S.; Lin, Y.W. The synthesis of two long-chain N-hydroxy amino coumarin compounds and their applications in the analysis of aldehydes. RSC Adv. 2017, 7, 19707–19716. [Google Scholar] [CrossRef]
- Zhang, A.; Guan, Z.B.; Cai, H.W.; Huang, Y.Y.; Lin, Y.W.; Hu, X.S. Nitrone formation: A new strategy for the derivatization of aldehydes and its application on the determination of furfurals in foods by high performance liquid chromatography with fluorescence detection. Talanta 2018, 178, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Borch, R.F.; Bernstein, M.D.; Durst, H.D. The cyanohydridoborate anion as a selective reducing agent. J. Am. Chem. Soc. 1971, 93, 2897–2904. [Google Scholar] [CrossRef]
- Vilkhovoy, M.; Dai, D.; Vadhin, S.; Adhikari, A.; Varner, J.D. Absolute Quantification of Cell-Free Protein Synthesis Metabolism by Reversed-Phase Liquid Chromatography-Mass Spectrometry. J. Vis. Exp. 2019, 152, 1–9. [Google Scholar] [CrossRef]
- Bawazeer, S.; Ali, A.M.; Alhawiti, A.; Khalaf, A.; Gibson, C.; Tusiimire, J.; Watson, D.G. A method for the analysis of sugars in biological systems using reductive amination in combination with hydrophilic interaction chromatography and high resolution mass spectrometry. Talanta 2017, 166, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.C.; Sedlak, M.; Regnier, F.E.; Mosier, N.; Ho, N.; Adamec, J. Simultaneous Quantification of Metabolites Involved in Central Carbon and Energy Metabolism Using Reversed-Phase Liquid Chromatography-Mass Spectrometry and in Vitro C-13 Labeling. Anal. Chem. 2008, 80, 9508–9516. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Tschernutter, V.; Yang, J.C.; Eckle, T.; Borchers, C.H. Analysis of Selected Sugars and Sugar Phosphates in Mouse Heart Tissue by Reductive Amination and Liquid Chromatography-Electrospray Ionization Mass Spectrometry. Anal. Chem. 2013, 85, 5965–5973. [Google Scholar] [CrossRef]
- Qin, Q.; Wang, B.H.; Chang, M.M.; Zhou, Z.H.; Shi, X.Z.; Xu, G.W. Highly efficient solid-phase derivatization of sugar phosphates with titanium-immobilized hydrophilic polydopamine-coated silica. J. Chromatogr. A 2016, 1457, 125–133. [Google Scholar] [CrossRef]
- Eggink, M.; Wijtmans, M.; Ekkebus, R.; Lingeman, H.; de Esch, I.J.P.; Kool, J.; Niessen, W.M.A.; Irth, H. Development of a Selective ESI-MS Derivatization Reagent: Synthesis and Optimization for the Analysis of Aldehydes in Biological Mixtures. Anal. Chem. 2008, 80, 9042–9051. [Google Scholar] [CrossRef]
- Yu, L.; Liu, P.; Wang, Y.L.; Yu, Q.W.; Yuan, B.F.; Feng, Y.Q. Profiling of aldehyde-containing compounds by stable isotope labelling-assisted mass spectrometry analysis. Analyst 2015, 140, 5276–5286. [Google Scholar] [CrossRef]
- Eggink, M.; Wijtmans, M.; Kretschmer, A.; Kool, J.; Lingeman, H.; de Esch, I.J.P.; Niessen, W.M.A.; Irth, H. Targeted LC-MS derivatization for aldehydes and carboxylic acids with a new derivatization agent 4-APEBA. Anal. Bioanal. Chem. 2010, 397, 665–675. [Google Scholar] [CrossRef]
- Sun, Z.W.; Wang, X.X.; Cai, Y.P.; Fu, J.Q.; You, J.M. Development of a pair of differential H/D isotope-coded derivatization reagents d(0)/d(3)-4-(1-methyl-1H-phenanthro 9,10-d imidazol-2-yl) phenlamine and its application for determination of aldehydes in selected aquatic products by liquid chromatography-tandem mass spectrometry. Talanta 2014, 120, 84–93. [Google Scholar]
- Gensberger-Reigl, S.; Huppert, J.; Pischetsrieder, M. Quantification of reactive carbonyl compounds in icodextrin-based peritoneal dialysis fluids by combined UHPLC-DAD and -MS/MS detection. J. Pharm. Biomed. Anal. 2016, 118, 132–138. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Measurement of methylglyoxal by stable isotopic dilution analysis LC-MS/MS with corroborative prediction in physiological samples. Nat. Protoc. 2014, 9, 1969–1979. [Google Scholar] [CrossRef]
- Yamashita, K.; Masuda, A.; Hoshino, Y.; Komatsu, S.; Numazawa, M. Assay of labile estrogen o-quinones, potent carcinogenic molecular species, by high performance liquid chromatography-electrospray ionization tandem mass spectrometry with phenazine derivatization. J. Steroid Biochem. Mol. Biol. 2010, 119, 141–148. [Google Scholar] [CrossRef]
- Degen, J.; Hellwig, M.; Henle, T. 1,2-Dicarbonyl Compounds in Commonly Consumed Foods. J. Agric. Food. Chem. 2012, 60, 7071–7079. [Google Scholar] [CrossRef]
- Kold-Christensen, R.; Jensen, K.K.; Smedegard-Holmquist, E.; Sorensen, L.K.; Hansen, J.; Jorgensen, K.A.; Kristensen, P.; Johannsen, M. ReactELISA method for quantifying methylglyoxal levels in plasma and cell cultures. Redox Biol. 2019, 26, 8. [Google Scholar] [CrossRef]
- Hurtado-Sanchez, M.D.; Espinosa-Mansilla, A.; Duran-Meras, I. Influence of the presence of natural monosaccharides in the quantification of alpha-dicarbonyl compounds in high content sugar samples. A comparative study by ultra-high performance liquid chromatography-single quadrupole mass spectrometry using different derivatization reactions. J. Chromatogr. A 2015, 1422, 117–127. [Google Scholar] [PubMed]
- Oh, J.-A.; Shin, H.-S. Simple and sensitive determination of malondialdehyde in human urine and saliva using UHPLC-MS/MS after derivatization with 3,4-diaminobenzophenone. J. Sep. Sci. 2017, 40, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Shin, H.S. Simple derivatization of aldehydes with D-cysteine and their determination in beverages by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2011, 702, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Backe, W.J. A novel mass spectrometric method for formaldehyde in children’s personal-care products and water via derivatization with acetylacetone. Rapid Commun. Mass Spectrom. 2017, 31, 1047–1056. [Google Scholar] [CrossRef]
- Miralles, P.; Chisvert, A.; Jose Alonso, M.; Hernandorena, S.; Salvador, A. Determination of free formaldehyde in cosmetics containing formaldehyde-releasing preservatives by reversed-phase dispersive liquid-liquid microextraction and liquid chromatography with post-column derivatization. J. Chromatogr. A 2018, 1543, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Donegattii, T.A.; Lobato, A.; Goncalves, L.M.; Pereira, E.A. Cyclohexane-1,3-dione as a derivatizing agent for the analysis of aldehydes by micelar electrokinetic chromatography with diode array detection. Electrophoresis 2019, 40, 2929–2935. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.I.; Lovell, M.A.; Lynn, B.C. Analysis of derivatized biogenic aldehydes by LC tandem mass spectrometry. Anal. Chem. 2005, 77, 3383–3389. [Google Scholar] [CrossRef]
- Hu, Y.N.; Chen, D.; Zhang, T.Y.; Ding, J.; Feng, Y.Q. Use of ammonium sulfite as a post-column derivatization reagent for rapid detection and quantification of aldehydes by LC-MS. Talanta 2020, 206, 8. [Google Scholar] [CrossRef] [PubMed]
- El-Maghrabey, M.; Kishikawa, N.; Kuroda, N. 9,10-Phenanthrenequinone as a mass-tagging reagent for ultra-sensitive liquid chromatography-tandem mass spectrometry assay of aliphatic aldehydes in human serum. J. Chromatogr. A 2016, 1462, 80–89. [Google Scholar] [CrossRef]
- Cao, Y.; Guan, Q.; Sun, T.; Qi, W.; Guo, Y. Charged tag founded in N-(1-chloroalkyl) pyridinium quaternization for quantification of fatty aldehydes. Anal. Chim. Acta 2016, 937, 80–86. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Zhang, L.; Zhang, J.; Leng, J.; Cai, T.; Guo, Y. Improvement and extension of the application scope for matrix-assisted laser desorption/ionization mass spectrometric analysis-oriented N-alkylpyridinium isotope quaternization. Anal. Chim. Acta 2011, 707, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.G.; Amicucci, M.J.; Cheng, Z.; Galermo, A.G.; Lebrilla, C.B. Revisiting monosaccharide analysis—Quantitation of a comprehensive set of monosaccharides using dynamic multiple reaction monitoring. Analyst 2018, 143, 200–207. [Google Scholar] [CrossRef]
- Higashi, T.; Aiba, N.; Tanaka, T.; Yoshizawa, K.; Ogawa, S. Methods for differential and quantitative analyses of brain neurosteroid levels by LC/MS/MS with ESI-enhancing and isotope-coded derivatization. J. Pharm. Biomed. Anal. 2016, 117, 155–162. [Google Scholar] [CrossRef]
- Guo, N.; Liu, P.; Ding, J.; Zheng, S.J.; Yuan, B.F.; Feng, Y.Q. Stable isotope labeling—Liquid chromatography/mass spectrometry for quantitative analysis of androgenic and progestagenic steroids. Anal. Chim. Acta 2016, 905, 106–114. [Google Scholar] [CrossRef]
- DeBarber, A.E.; Luo, J.; Star-Weinstock, M.; Purkayastha, S.; Geraghty, M.T.; Chiang, J.; Merkens, L.S.; Pappu, A.S.; Steiner, R.D. A blood test for cerebrotendinous xanthomatosis with potential for disease detection in newborns. J. Lipid Res. 2014, 55, 146–154. [Google Scholar] [CrossRef]
- Sobsey, C.A.; Han, J.; Lin, K.; Swardfager, W.; Levitt, A.; Borchers, C.H. Development and evaluation of a liquid chromatography-mass spectrometry method for rapid, accurate quantitation of malondialdehyde in human plasma. J. Chromatogr. B 2016, 1029, 205–212. [Google Scholar] [CrossRef] [PubMed]
- El-Maghrabey, M.; Kishikawa, N.; Kuroda, N. Novel Isotope-Coded Derivatization Method for Aldehydes Using N-14/N-15-Am monium Acetate and 9,10-Phenanthrenequinone. Anal. Chem. 2018, 90, 13867–13875. [Google Scholar] [CrossRef] [PubMed]
- Dator, R.; Carra, A.; Maertens, L.; Guidolin, V.; Villalta, P.W.; Balbo, S. A High Resolution/Accurate Mass (HRAM) Data-Dependent MS3 Neutral Loss Screening, Classification, and Relative Quantitation Methodology for Carbonyl Compounds in Saliva. J. Am. Soc. Mass. Spectrom. 2017, 28, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Akaishi, M.; Yokota, M.; Suzuki, T.; Ogawa, S.; Sugiura, Y.; Nishikawa, T.; Nishimoto, K.; Suematsu, M. A method for determination of aldosterone in adrenal tributary venous serum by derivatization using Girard P reagent isotopologues followed by LC/ESI-MS/MS. J. Chromatogr. B 2018, 1092, 106–113. [Google Scholar] [CrossRef]
- Yutuc, E.; Dickson, A.L.; Pacciarini, M.; Griffiths, L.; Baker, P.R.S.; Connell, L.; Öhman, A.; Forsgren, L.; Trupp, M.; Vilarinho, S.; et al. Deep mining of oxysterols and cholestenoic acids in human plasma and cerebrospinal fluid: Quantification using isotope dilution mass spectrometry. Anal. Chim. Acta 2021, 1154, 338259. [Google Scholar] [CrossRef]
- Crick, P.J.; Bentley, T.W.; Abdel-Khalik, J.; Matthews, I.; Clayton, P.T.; Morris, A.A.; Bigger, B.W.; Zerbinati, C.; Tritapepe, L.; Iuliano, L.; et al. Quantitative Charge-Tags for Sterol and Oxysterol Analysis. Clin. Chem. 2015, 61, 400–411. [Google Scholar] [CrossRef]
- Honda, A.; Yamashita, K.; Numazawa, M.; Ikegami, T.; Doy, M.; Matsuzaki, Y.; Miyazaki, H. Highly sensitive quantification of 7 alpha-hydroxy-4-cholesten-3-one in human serum by LC-ESI-MS/MS. J. Lipid Res. 2007, 48, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Bleyle, L.; Huidekoper, H.H.; Vaz, F.M.; Singh, R.; Steiner, R.D.; DeBarber, A.E. Update on newborn dried bloodspot testing for cerebrotendinous xanthomatosis: An available high-throughput liquid-chromatography tandem mass spectrometry method. Mol. Genet. Metab. Rep. 2016, 7, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.S.; Wang, Y.J.; Cao, Y.Q.; Cao, Y.J.; Guan, Q.; Sun, T.Q.; Zhang, L.; Guo, Y.L. Simultaneous Analysis of Fatty Alcohols, Fatty Aldehydes, and Sterols in Thyroid Tissues by Electrospray Ionization-Ion Mobility-Mass Spectrometry Based on Charge Derivatization. Anal. Chem. 2020, 92, 8644–8648. [Google Scholar] [CrossRef]
- Zhao, S.; Li, H.; Han, W.; Chan, W.; Li, L. Metabolomic Coverage of Chemical-Group-Submetabolome Analysis: Group Classification and Four-Channel Chemical Isotope Labeling LC-MS. Anal. Chem. 2019, 91, 12108–12115. [Google Scholar] [CrossRef] [PubMed]
- He, X.M.; Ding, J.; Yu, L.; Hussain, D.; Feng, Y.Q. Black phosphorus-assisted laser desorption ionization mass spectrometry for the determination of low-molecular-weight compounds in biofluids. Anal. Bioanal. Chem. 2016, 408, 6223–6233. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.J.; Wang, Y.L.; Liu, P.; Zhang, Z.; Yu, L.; Yuan, B.F.; Feng, Y.Q. Stable isotope labeling-solid phase extraction-mass spectrometry analysis for profiling of thiols and aldehydes in beer. Food Chem. 2017, 237, 399–407. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).