In Vivo Antimalarial Activity of Leaf Extracts and a Major Compound Isolated from Ranunculus multifidus Forsk
Abstract
:1. Introduction
2. Results and Discussion
2.1. Acute Oral Toxicity
2.2. Antimalarial Activity of Extracts
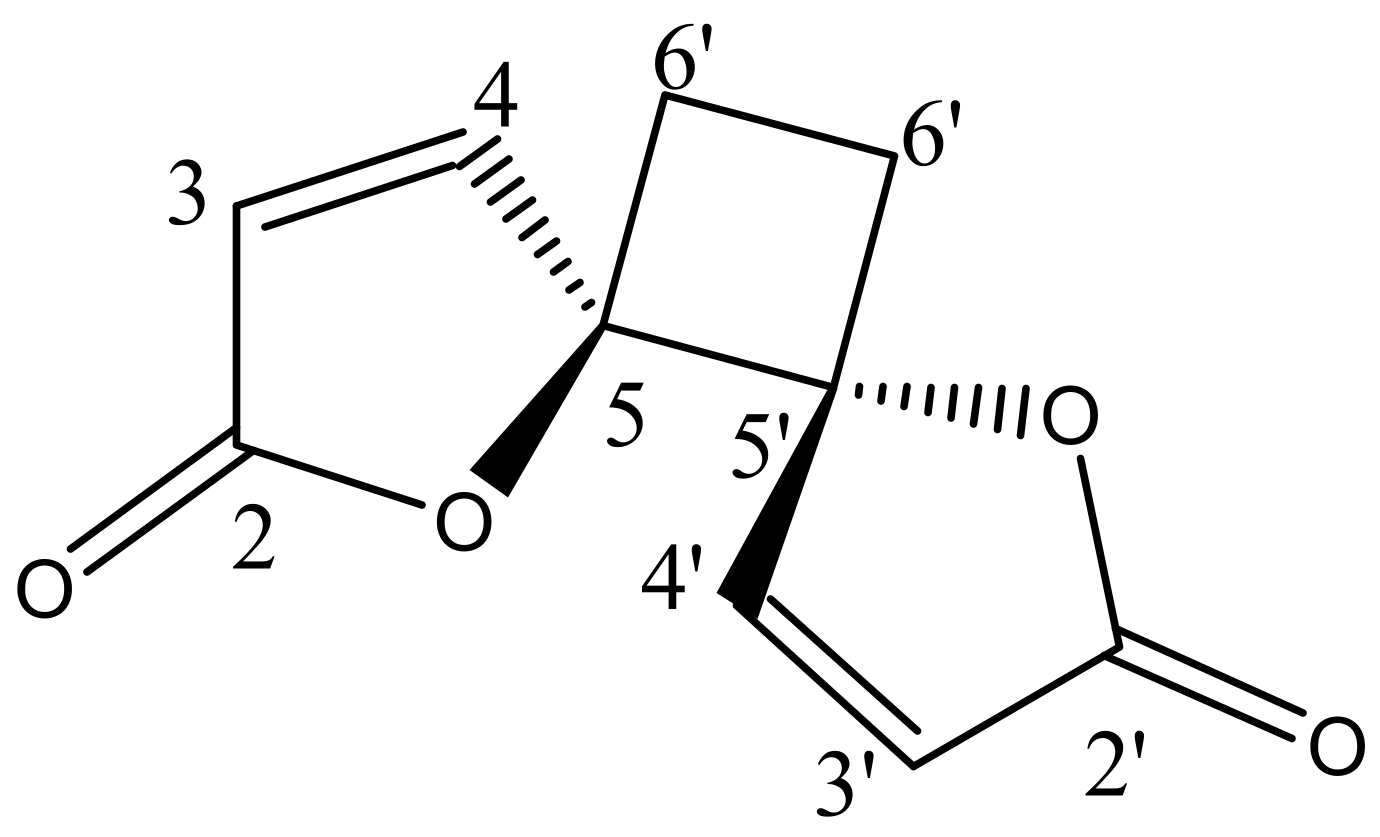
2.3. Structural Elucidation of the Isolated Compound
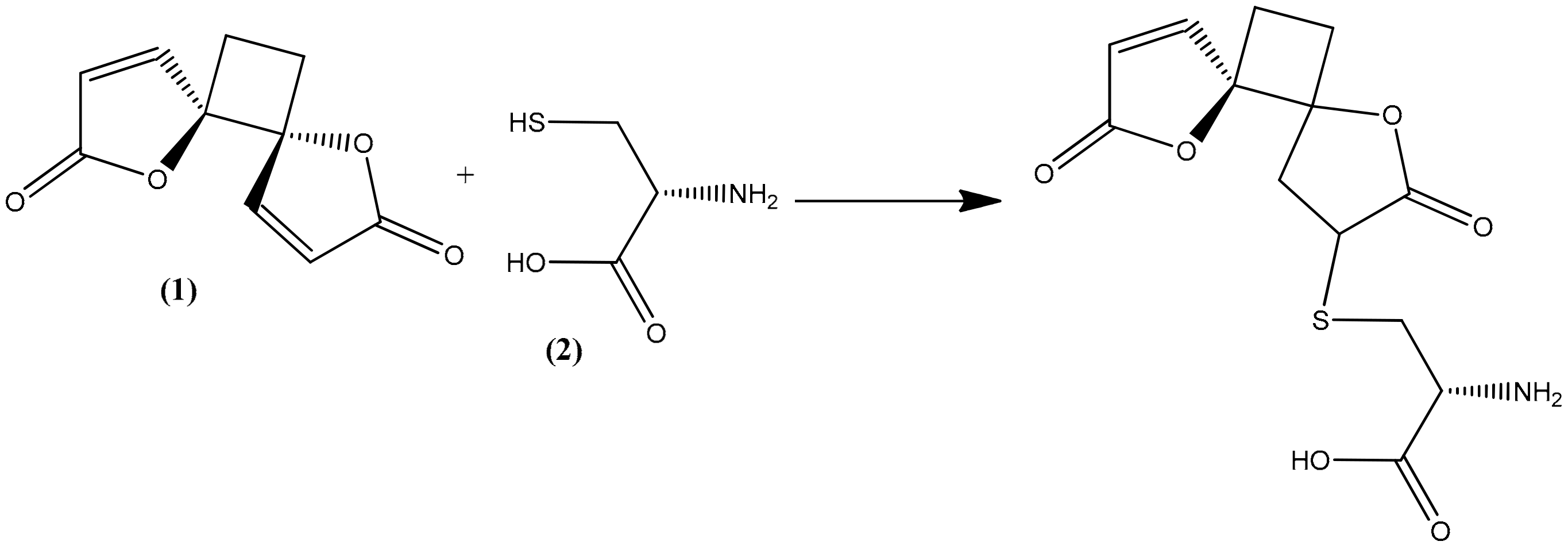
2.4. Structural Elucidation of the Hydrolysis Product of Anemonin
2.5. Acute Oral Toxicity of Anemonin
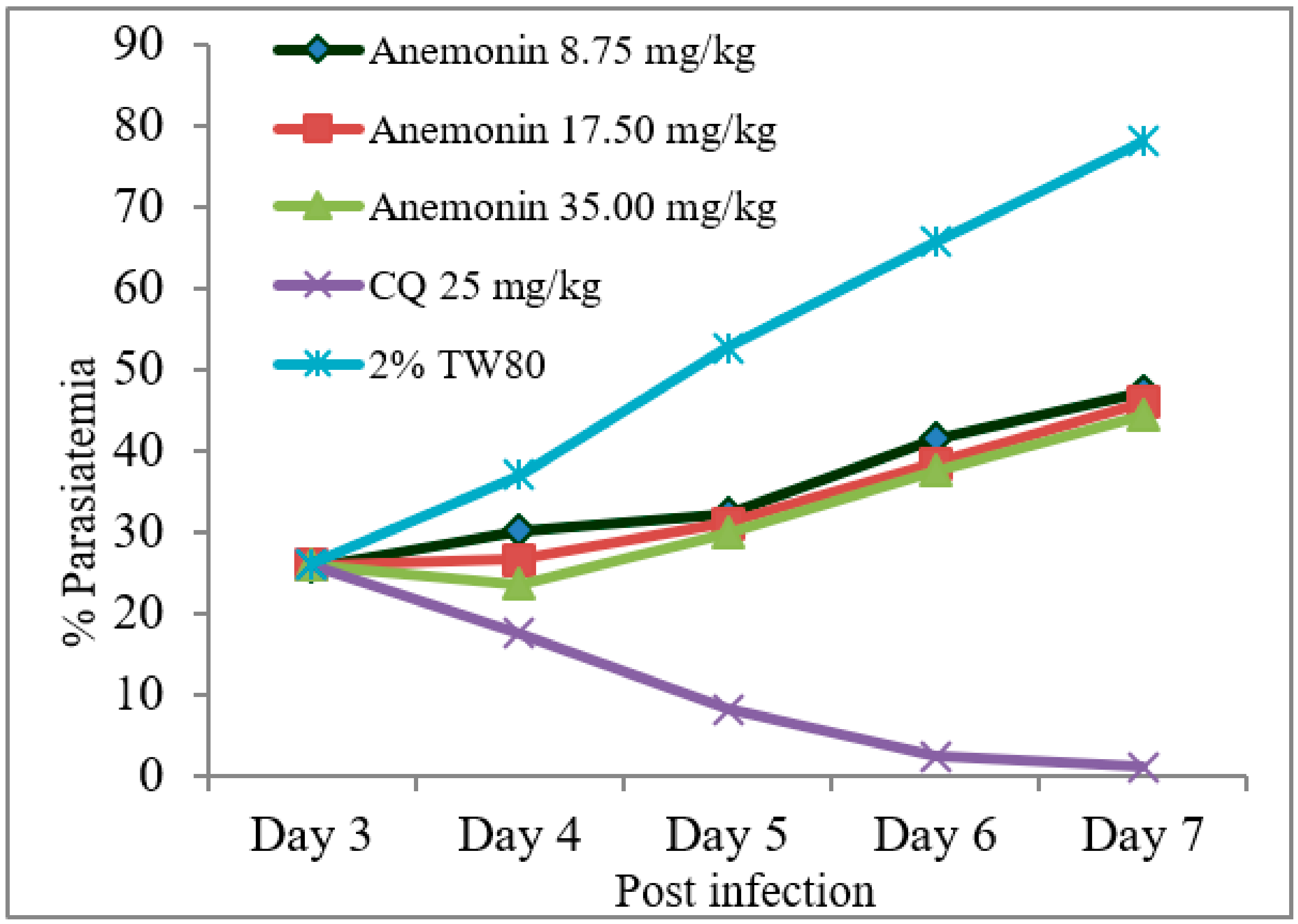
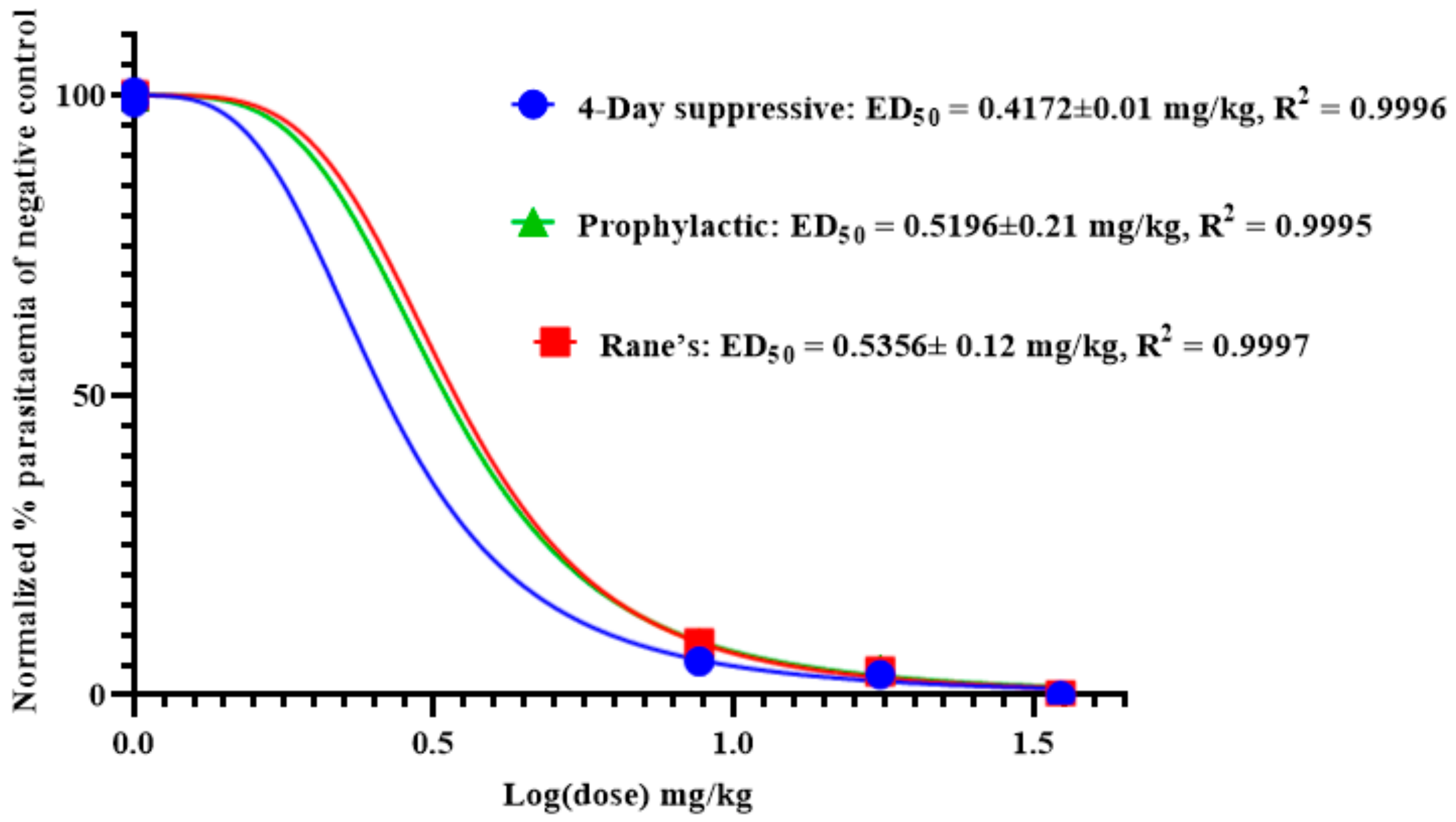
2.6. Antimalarial Activity of Anemonin
2.6.1. Four-Day Suppressive Test
2.6.2. Rane’s Test
2.6.3. Prophylactic Test
2.7. Antimycobacterial Activity of Anemonin
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Ranunculus multifidus Leaf Extracts
3.3. Chemicals
3.4. Preparative Thin Layer Chromatography
3.5. Mass Spectrometry
3.6. NMR
3.7. Experimental Animals and Parasite
3.8. Acute Oral Toxicity
3.9. In Vivo Antimalarial Assay
3.9.1. Inoculation of Mice
3.9.2. 4-Day Suppressive Test (Peter’s Test)
3.9.3. Rane’s Test
3.9.4. Prophylactic Test
3.10. Determination of Parasitemia and Survival Time
3.11. Determination of Packed Cell Volume, Rectal Temperature, and Body Weight
3.12. In Vitro Antimycobacterial Assay
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- World Health Organization. World malaria report 2020: 20 years of global progress and challenges. In World Malaria Report 2020: 20 Years of Global Progress and Challenges; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Afrane, Y.A.; Zhou, G.; Githeko, A.K.; Yan, G. Clinical malaria case definition and malaria attributable fraction in the highlands of western Kenya. Malar. J. 2014, 13, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, S.I.; Guerra, C.A.; Tatem, A.J.; Noor, A.M.; Snow, R.W. The global distribution and population at risk of malaria: Past, present, and future. Lancet Infect. 2004, 4, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Girum, T.; Shumbej, T.; Shewangizaw, M. Burden of malaria in Ethiopia, 2000–2016: Findings from the Global Health Estimates 2016. Trop. Dis. Travel Med. Vaccines 2019, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Kendie, F.A.; W/kiros, T.H.; Nibret Semegn, E.; Ferede, M.W. Prevalence of malaria among adults in Ethiopia: A systematic review and meta-analysis. J. Trop. Med. 2021, 2021, 8863002. [Google Scholar] [CrossRef] [PubMed]
- Abebe, W. An overview of Ethiopian traditional medicinal plants used for cancer treatment. Eur. J. Med. Plants 2016, 14, 1–16. [Google Scholar] [CrossRef]
- Kasali, F.M.; Mahano, A.O.; Kadima, N.J.; Mpiana, P.T.; Ngbolua, K.N.; Tshibangu, T.S.D. Ethnopharmacological survey of medicinal plants used against malaria in Butembo City (DR Congo). J. Adv. Bot. Zool. 2014, 1, 1–11. [Google Scholar]
- Aschale, Y.; Wubetu, M.; Reta, H. Ethnobotany of medicinal plants used to treat leishmaniasis in Ethiopia: A systematic review. J. Tradit. Med. Clin. Naturopat. 2018, 7, 2–7. [Google Scholar] [CrossRef]
- Birhan, Y.S.; Kitaw, S.L.; Alemayehu, Y.A.; Mengesha, N.M. Ethnobotanical study of medicinal plants used to treat human diseases in Enarj Enawga district, East Gojjam zone, Amhara region, Ethiopia. SM J. Med. Plant Stud. 2017, 1, 1–9. [Google Scholar] [CrossRef]
- Wolde-Mariam, M.; Limenih, Y.; Umer, S. Ethnobotanical study on traditional medicinal plants in Dega Damot woreda, Amhara Region, North Ethiopia. Int. J. Res. Pharm. Chem. 2015, 5, 258–273. [Google Scholar]
- De Wet, H.; Ngubane, S.C. Traditional herbal remedies used by women in a rural community in northern Maputaland (South Africa) for the treatment of gynecology and obstetrics complaints. S. Afr. J. Bot. 2014, 94, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Naidoo, D.; van Vuuren, S.F.; van Zyl, R.L.; De Wet, H. Plants traditionally used individually and in combination to treat sexually transmitted infections in northern Maputaland, South Africa: Antimicrobial activity and cytotoxicity. J. Ethnopharmacol. 2013, 149, 656–667. [Google Scholar] [CrossRef]
- Southwell, I.A.; Tucker, D.J. Protoanemonin in Australian Clematis. Phytochemistry 1993, 33, 1099–1102. [Google Scholar] [CrossRef]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, P.J.; Folb, P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef]
- Basir, R.; Rahiman, S.F.; Hasballah, K.; Chong, W.C.; Talib, H.; Yam, M.F.; Jabbarzare, M.; Tie, T.H.; Othman, F.; Moklas, M.A.M.; et al. Plasmodium berghei ANKA infection in ICR mice as a model of cerebral malaria. Iran. J. Parasitol. 2012, 7, 62–66. [Google Scholar]
- Krettli, A.U.; Adebayo, J.O.; Krettli, L.G. Testing of natural products and synthetic molecules aiming at new antimalarials. Curr. Drug Targets 2009, 10, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Khatoon, S.; Amber, F.; Abbas, Q.; Ismail, M.; Engel, N.; Ahmad, V.U. Isolation of anemonin from Pulsatilla wallichiana and its biological activities. J. Chem. Soc. Pak. 2019, 41, 325. [Google Scholar]
- Asahina, Y.; Fujita, A. Synthesis and constitution of anemonin. J. Pharm. Soc. Jpn. 1920, 455, 1–4. [Google Scholar]
- Nono, R.N.; Nguelefack-Mbuyo, E.P.; Nzowa, L.K.; Ponou, B.K.; Teponno, R.B.; Nguelefack, T.B.; Barboni, L.; Tapondjou, L.A.; Park, H.J. Antioxidant C-glycosylflavones of Drymaria cordata (Linn.) Willd. Arch. Pharmacal Res. 2016, 39, 43–50. [Google Scholar] [CrossRef]
- Saidi, R.; Ghrab, F.; Kallel, R.; El Feki, A.; Boudawara, T.; Chesné, C.; Ammar, E.; Jarraya, R.M. Tunisian Clematis flammula essential oil enhances wound healing: GC-MS analysis, biochemical and histological assessment. J. Oleo Sci. 2018, 67, 1483–1499. [Google Scholar] [CrossRef]
- Gertsch, J. How scientific is the science in ethnopharmacology? Historical perspectives and epistemological problems. J. Ethnopharmacol. 2009, 122, 177–183. [Google Scholar] [CrossRef]
- Becker, K.; Rahlfs, S.; Nickel, C.; Schirmer, R.H. Glutathione–functions and metabolism in the malarial parasite Plasmodium falciparum. J. Biol. Chem. 2003, 384, 551–566. [Google Scholar] [CrossRef]
- Zhao, R.; Lind, J.; Merényi, G.; Eriksen, T.E. Significance of the intramolecular transformation of glutathione thiyl radicals to α-aminoalkyl radicals. Thermochemical and biological implications. J. Chem. Soc. Perkin Trans. 1997, 2, 569–574. [Google Scholar] [CrossRef]
- Zhang, Y.A.; Hempelmann, E.; Schirmer, R.H. Glutathione reductase inhibitors as potential antimalarial drugs: Effects of nitrosoureas on Plasmodium falciparum in vitro. Biochem. Pharmacol. 1988, 37, 855–860. [Google Scholar]
- Davioud-Charvet, E.; Becker, K.; Landry, V.; Gromer, S.; Logé, C.; Sergheraert, C. Synthesis of 5,5′-dithiobis (2-nitrobenzamides) as alternative substrates for trypanothione reductase and thioredoxin reductase: A microtiter colorimetric assay for inhibitor screening. Anal. Biochem. 1999, 268, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Färber, P.M.; Arscott, L.D.; Williams, C.H., Jr.; Becker, K.; Schirmer, R.H. Recombinant Plasmodium falciparum glutathione reductase is inhibited by the antimalarial dye methylene blue. FEBS Lett. 1998, 422, 311–314. [Google Scholar] [CrossRef] [Green Version]
- Vennerstrom, J.L.; Makler, M.T.; Angerhofer, C.K.; Williams, J.A. Antimalarial dyes revisited: Xanthenes, azines, oxazines, and thiazines. Antimicrob. Agents Chemother. 1995, 39, 2671–2677. [Google Scholar] [CrossRef] [Green Version]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Wang, S.T.; Chen, H.W.; Sheen, L.Y.; Lii, C.K. Methionine and cysteine affect glutathione level, glutathione-related enzyme activities and the expression of glutathione S-transferase isozymes in rat hepatocytes. J. Nutr. 1997, 127, 2135–2141. [Google Scholar] [CrossRef]
- Mares, D. Antimicrobial activity of protoanemonin, a lactone from ranunculaceous plants. Mycopathologia 1987, 98, 133–140. [Google Scholar] [CrossRef]
- Hall, I.H.; Lee, K.H.; Mar, E.C.; Starnes, C.O.; Waddell, T.G. Antitumor agents. 21. A proposed mechanism for inhibition of cancer growth by tenulin and helenalin and related cyclopentenones. J. Med. Chem. 1977, 20, 333–337. [Google Scholar] [CrossRef]
- Cavallito, C.J.; Haskell, T.H. The mechanism of action of antibiotics. The reaction of unsaturated lactones with cysteine and related compounds. J. Am. Chem. Soc. 1945, 67, 1991–1994. [Google Scholar] [CrossRef]
- Canepa, G.E.; Bouvier, L.A.; Miranda, M.R.; Uttaro, A.D.; Pereira, C.A. Characterization of Trypanosoma cruzi L-cysteine transport mechanisms and their adaptive regulation. FEMS Microbiol. Lett. 2009, 292, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, A.; Strauch, A.; Chao, J.; Ko, M.; Av-Gay, Y. Screening of preselected libraries targeting Mycobacterium abscessus for drug discovery. Antimicrob. Agents Chemother. 2018, 62, e00828-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute for Laboratory Animal Research (ILAR). Guide for the Care and Use of Laboratory Animals; The National Academy Press: Washington, DC, USA, 1996. [Google Scholar]
- Organization for Economic Co-operation and Development (OECD). Guideline for the Testing of Chemicals: Acute Oral Toxicity-Up-and-Down Procedure; Organization for Economic Co-operation and Development (OECD): Paris, France, 2008. [Google Scholar]
- Tang, Y.; Dong, Y.; Wittlin, S.; Charman, S.A.; Chollet, J.; Chiu, F.C.; Charman, W.N.; Matile, H.; Urwyler, H.; Dorn, A.; et al. Weak base dispiro-1,2,4-trioxolanes: Potent antimalarial ozonides. Bioorg. Med. Chem. Lett. 2007, 17, 1260–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fidock, D.A.; Rosenthal, P.J.; Croft, S.L.; Brun, R.; Nwaka, S. Antimalarial drug discovery: Efficacy models for compound screening. Nat. Rev. Drug Discov. 2004, 3, 509–520. [Google Scholar] [CrossRef]
- Hilou, A.; Nacoulma, O.G.; Guiguemde, T.R. In vivo antimalarial activities of extracts from Amaranthus spinosus L. and Boerhaavia erecta L. in mice. J. Ethnopharmacol. 2006, 103, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Peters, W. Antimalarial drugs and their actions. Postgrad. Med. J. 1973, 49, 573–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waako, P.J.; Gumede, B.; Smith, P.; Folb, P.I. The in vitro and in vivo antimalarial activity of Cardiospermum halicacabum L. and Momordica foetida Schumch. Et Thonn. J. Ethnopharmacol. 2005, 99, 137–143. [Google Scholar] [CrossRef]
- Mergia, E.; Shibeshi, W.; Terefe, G.; Teklehaymanot, T. Antitrypanosomal activity of Verbascum sinaiticum Benth. (Scrophulariaceae) against Trypanosoma congolense isolates. BMC Complement. Altern. Med. 2016, 16, 362. [Google Scholar] [CrossRef] [Green Version]
- Tamiru, W.; Engidawork, E.; Asres, K. Evaluation of the effects of 80% methanolic leaf extract of Caylusea abyssinica (fresen.) fisch. & Mey. on glucose handling in normal, glucose loaded and diabetic rodents. BMC Complement. Altern. Med. 2012, 12, 151. [Google Scholar]
- Misganaw, D.; Engidawork, E.; Nedi, T. Evaluation of the antimalarial activity of crude extract and solvent fractions of the leaves of Olea europaea (Oleaceae) in mice. BMC Complement. Altern. Med. 2019, 19, 171. [Google Scholar] [CrossRef]
- Ancelin, M.L.; Calas, M.; Bonhoure, A.; Herbute, S.; Vial, H.J. In vivo antimalarial activities of mono- and bis-quaternary ammonium salts interfering with Plasmodium phospholipid metabolism. Antimicrob. Agents Chemother. 2003, 47, 2598–2605. [Google Scholar] [CrossRef] [Green Version]
- Fentahun, S.; Makonnen, E.; Awas, T.; Giday, M. In vivo antimalarial activity of crude extracts and solvent fractions of leaves of Strychnos mitis in Plasmodium berghei infected mice. BMC Complement. Altern. Med. 2017, 17, 13. [Google Scholar] [CrossRef] [Green Version]
- Ryley, J.F.; Peters, W. The antimalarial activity of some quinolone esters. Ann. Trop. Med. Parasitol. 1970, 64, 209–222. [Google Scholar] [CrossRef]
- Mulaw, T.; Wubetu, M.; Dessie, B.; Demeke, G.; Molla, Y. Evaluation of antimalarial activity of the 80% methanolic stem bark extract of Combretum molle against Plasmodium berghei in mice. J. Evid.-Based Integr. Med. 2019, 24, 2515690X19890866. [Google Scholar] [CrossRef] [PubMed]
- Nureye, D.; Assefa, S.; Nedi, T.; Engidawork, E. In vivo antimalarial activity of the 80% methanolic root bark extract and solvent fractions of Gardenia ternifolia Schumach. and Thonn. (Rubiaceae) against Plasmodium berghei. Evid.-Based Complement. Altern. Med. 2018, 2018, 9217835. [Google Scholar] [CrossRef] [Green Version]
- Bantie, L.; Assefa, S.; Teklehaimanot, T.; Engidawork, E. In vivo antimalarial activity of the crude leaf extract and solvent fractions of Croton macrostachyus Hocsht. (Euphorbiaceae) against Plasmodium berghei in mice. BMC Complement. Altern. Med. 2014, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Mekuria, A.; Geta, M.; Birru, E.; Gelayee, A. Antimalarial activity of seed extracts of Schinus molle against Plasmodium berghei in mice. J. Evid.-Based Integr. Med. 2021, 26, 2515690X20984287. [Google Scholar] [CrossRef] [PubMed]
- Peters, W. Drug resistance in Plasmodium berghei Vincke and Lips, 1948. III. Multiple drug resistance. Exp. Parasitol. 1965, 17, 97–102. [Google Scholar] [CrossRef]
- Peters, W.; Robinson, B.L. The chemotherapy of rodent malaria. Studies on pyronaridine and other Mannich base antimalarials. Ann. Trop. Med. Parasitol. 1992, 86, 455–465. [Google Scholar] [CrossRef] [PubMed]
| Test Substances (mg/kg/day) | % Parasitemia | % Suppression | Mean Survival Time (in Days) |
|---|---|---|---|
| 2% TW80 | 26.6 ± 0.7 | - | 9.1 ± 0.3 |
| CQ 25 | 0.2 ± 0.2 a3 | 99.2 | 28 ± 0.0 a2 |
| RM-M 100 | 15.8 ± 1.0 a2,b2,c2,d1,e2,f2,g2 | 39.2 | 12.4 ± 0.4 a2,b2,g1 |
| RM-M 200 | 12.6 ± 0.2 a2,b2,e2,f2,g2 | 48.4 | 12.8 ± 0.5 a2,b2,g1 |
| RM-M 400 | 9.5 ± 0.2 a2,b2,g1,g2 | 53.1 | 13.1 ± 0.2 a2,b2 |
| RM-H 8.75 | 10.9 ± 0.5 a2,b2,g2 | 58.8 | 13.8 ± 0.8 a2,b2 |
| RM-H 17.50 | 9.6 ± 0.4 a2,b2,g1 | 63.6 | 14.0 ± 0.8 a2,b2 |
| RM-H 35.00 | 15.8 ± 1.0 a2,b2, | 70.6 | 14.4 ± 0.5 a2,b2 |
| Test Substances (mg/kg/day) | Body Weight (g) | Rectal Temperature (°C) | Packed Cell Volume (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 4 | % Change | Day 0 | Day 4 | % Change | Day 0 | Day 4 | % Change | |
| 2% TW80 | 26.0 ± 0.2 | 22.1 ± 0.6 | −14.7 | 36.5 ± 0.0 | 33.0 ± 0.2 | −9.6 | 59.2 ± 0.2 | 48.6 ± 0.9 | −17.9 |
| CQ 25 | 26.3 ± 0.5 | 29.2 ± 0.6 | 11.3 a3 | 36.6 ± 0.1 | 36.5 ± 0.1 | −0.0 a3 | 57.2 ± 0.5 | 58.8 ± 0.7 | 2.7 a3 |
| RM-M 100 | 26.6 ± 0.9 | 26.8 ± 0.9 | 0.7 a3,b3,c1,d3,e3,f3,g3 | 36.4 ± 0.2 | 35.1 ± 0.2 | −3.6 a3,b3,d1,e1,f3,g3 | 56.4 ± 0.7 | 50.2 ± 0.5 | −10.9 a1,b3 |
| RM-M 200 | 26.8 ± 0.9 | 27.9 ± 1.0 | 3.9 a3,b3,d3,e3,f3,g3 | 36.4 ± 0.1 | 35.5 ± 0.1 | −2.3 a3,b3,d1,g2 | 58.0 ± 0.7 | 52.2 ± 0.8 | −10.0 a3,b3 |
| RM-M 400 | 26.5 ± 1.0 | 28.3 ± 1.1 | 6.6 a3,b3,e1,f3,g3 | 36.6 ± 0.0 | 36.2 ± 0.2 | −1.0 a3,b1,e1,f1,g3 | 57.4 ± 0.9 | 52.2 ± 0.5 | −9.0 a3,b3 |
| RM-H 8.75 | 25.3 ± 0.9 | 26.6 ± 0.9 | 5.0 a3,b3,e1,g3 | 36.2 ± 0.0 | 35.1 ± 0.2 | −2.0 a3,b3,g2 | 57.2 ± 0.8 | 51.2 ± 0.7 | −10.4 a3,b3 |
| RM-H 17.50 | 24.8 ± 0.5 | 26.8 ± 0.9 | 7.8 a3,b1,g2 | 36.5 ± 0.1 | 35.8 ± 0.1 | −1.9 a3,b3,g2 | 55.6 ± 0.5 | 50.0 ± 0.5 | −10.0 a3,b3 |
| RM-H 35.00 | 26.1 ± 0.6 | 28.7 ± 1.0 | 10.0 a3 | 36.6 ± 0.1 | 36.4 ± 0.1 | −0.7 a3 | 57.6 ± 0.5 | 52.4 ± 0.6 | −9.0 a3,b3 |
| No. | 1H NMR Chemical Shift (δ, ppm) | 13C NMR Chemical Shift (δ, ppm) | ||
|---|---|---|---|---|
| Anemonin [17] | Anemonin [17] | |||
| 2 − 2′ | - | - | 171.8 | 170.8 |
| 3 − 3′ | 6.32, d (5.7Hz) | 6.30, d (5.6Hz) | 121.1 | 121.1 |
| 4 − 4′ | 8.28, d (5.7Hz) | 8.26, d (5.6Hz) | 156.5 | 153.2 |
| 5 − 5′ | - | - | 90.44 | 90.3 |
| 6 − 6′ | 6a − 6a′ = 2.64 6b − 6b′ = 2.25 | 6a − 6a′ = 2.61, m | 23.27 | 23.8 |
| 6b − 6b′ = 2.24, m | ||||
| Test Substance (mg/kg/day) | % Parasitemia | % Suppression | Mean Survival Time (in Days) |
|---|---|---|---|
| 2% TW80 | 26.6 ± 0.8 | - | 9.2 ± 0.4 |
| Chloroquine | 0.1 ± 0.3 a1 | 99.5 | 28.0 ± 0.0 a1 |
| Anemonin 8.75 | 5.7 ± 0.1 a1,b1 | 78.3 | 14.0 ± 0.3 a1,b1 |
| Anemonin 17.50 | 5.2 ± 0.1 a1,b1 | 80.1 | 14.4 ± 0.2 a1,b1 |
| Anemonin 35.00 | 4.5 ± 0.0 a1,b1 | 83.0 | 14.8 ± 0.4 a1,b1 |
| Test Substances (mg/kg/day) | Body Weight (g) | Rectal Temperature (℃) | Packed Cell Volume (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 4 | % Change | Day 0 | Day 4 | % Change | Day 0 | Day 4 | % Change | |
| 2% TW80 | 25.0 ± 0.4 | 21.2 ± 0.5 | −15.1 | 36.6 ± 0.0 | 33.0 ± 0.2 | −9.9 | 59.6 ± 0.2 | 49.2 ± 0.9 | −17.4 |
| CQ 25 | 26.2 ± 0.5 | 30.5 ± 0.8 | 16.4 a3 | 36.4 ± 0.3 | 36.4 ± 0.1 | 0.1 a3 | 58.4 ± 0.3 | 58.8 ± 0.5 | 0.6 a3 |
| Anemonin 8.75 | 24.8 ± 0.0 | 26.8 ± 0.9 | 7.8 a3 | 36.5 ± 0.1 | 35.8 ± 0.1 | −1.6 a3 | 57.2 ± 0.8 | 51.2 ± 0.7 | −10.4 a3,b2 |
| Anemonin 17.50 | 26.1 ± 0.6 | 28.7 ± 1.0 | 10.0 a3 | 36.7 ± 0.1 | 36.4 ± 0.1 | −0.7 a3 | 56.8 ± 0.8 | 52.4 ± 0.6 | −7.7 a3,b3 |
| Anemonin 35.00 | 26.8 ± 0.9 | 30.3 ± 0.6 | 13.0 a3 | 36.4 ±0.1 | 36.4 ± 0.1 | 0.0 a3 | 56.4 ± 0.8 | 53.6 ± 0.5 | −4.9 a3,b1 |
| Test Substances (mg/kg/day) | % Parasitemia | % Suppression | Mean survival Time (in Days) |
|---|---|---|---|
| 2% TW80 | 78.0 ± 0.2 | - | 8.6 ± 0.1 |
| CQ 25 | 1.1 ± 0.2 a1 | 98.4 | 28.0 ± 0.0 a1 |
| Anemonin 8.75 | 47.2 ± 0.4 a1,b1 | 38.8 | 11.6 ± 0.2 a1,b1 |
| Anemonin 17.50 | 45.9 ± 0.8 a1,b1 | 40.5 | 12.0 ± 0.3 a1,b1 |
| Anemonin 35.00 | 44.3 ± 0.7 a1,b1 | 42.6 | 13.0 ± 0.3 a1,b1 |
| Test Substances (mg/kg/day) | Body Weight (g) | Rectal Temperature (℃) | Packed Cell Volume (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 3 | Day 7 | % Change | Day 3 | Day 7 | % Change | Day 3 | Day 7 | % Change | |
| 2% TW80 | 26.6 ± 0.4 | 20.5 ± 0.8 | −22.8 | 36.6 ± 0.2 | 32.2 ± 0.3 | −11.8 | 58.2 ± 0.3 | 46.2 ± 0.4 | −20.6 |
| CQ 25 | 26.3 ± 0.7 | 27.3 ± 0.7 | 3.8 a1 | 36.8 ± 0.0 | 36.3 ± 0.1 | −1.3 a1 | 58.0 ± 0.7 | 53.0 ± 0.7 | −8.6 a1 |
| Anemonin 8.75 | 26.4 ± 0.8 | 23.6 ± 0.7 | −10.7 b1 | 36.7 ± 0.1 | 35.6 ± 0.2 | −2.7 a1 | 57.8 ± 0.5 | 51.4 ± 0.7 | −10.6 a1 |
| Anemonin 17.50 | 28.5 ± 0.3 | 27.2 ± 1.1 | −3.9 a1,b1 | 36.7 ± 0.1 | 36.2 ± 0.0 | −1.3 a1 | 56.0 ± 0.7 | 50.2 ± 0.8 | −10.3 a1 |
| Anemonin 35.00 | 26.3 ± 0.7 | 25.3 ± 0.7 | −3.8 a1, b1 | 36.8 ± 0.0 | 36.3 ± 0.3 | −1.3 a1 | 58.1 ± 0.7 | 53.0 ± 0.7 | −8.7 a1 |
| Test Substances (mg/kg/day) | % Parasitemia | % Suppression | Mean Survival Time (in Days) |
|---|---|---|---|
| 2% TW80 | 33.8 ± 0.9 | - | 8.4 ± 0.5 |
| CQ 25 | 3.8 ± 0.7 a1 | 88.6 | 27.4 ± 0.4 a1 |
| Anemonin 8.75 | 13.1 ± 0.4 a1,b1 | 60.7 | 14.0 ± 0.3 a1,b1 |
| Anemonin 17.50 | 12.1 ± 0.5 a1,b1 | 63.7 | 14.0 ± 0.3 a1,b1 |
| Anemonin 35.00 | 11.1 ± 0.3 a1,b1 | 66.7 | 14.4 ± 0.2 a1,b1 |
| Test Substances (mg/kg/day) | Body Weight (g) | Rectal Temperature (℃) | Packed Cell Volume (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 3 | Day 7 | % Change | Day 3 | Day 7 | % Change | Day 3 | Day 7 | % Change | |
| 2% TW80 | 26.7 ± 0.5 | 21.6 ± 0.0 | −19.0 | 36.6 ± 0.0 | 32.5 ± 0.2 | −11.2 | 58.9 ± 0.4 | 47.8± 0.6 | −18.8 |
| CQ 25 | 26.1 ± 0.6 | 27.9 ± 0.5 | 6.8 a2 | 36.7 ± 0.1 | 36.5 ± 0.1 | −0.3 a2 | 57.8 ± 0.5 | 53.4 ± 0.8 | −7.6 a2 |
| Anemonin 8.75 | 26.4 ± 0.8 | 23.6 ± 0.7 | −10.7 a2,b2 | 36.7 ± 0.1 | 35.6 ± 0.2 | −2.7 a2 | 58.4 ± 0.6 | 51.0 ± 0.4 | −12.6 a2,b1 |
| Anemonin 17.50 | 27.4 ± 0.5 | 27.5 ± 0.4 | 0.3 a2,b2 | 36.5 ± 0.1 | 36.1 ± 0.2 | −1.0 a2 | 58.0 ± 0.5 | 50.8 ± 0.3 | −12.4 a2,b1 |
| Anemonin 35.00 | 26.9 ± 0.3 | 27.2 ± 0.5 | 1.3 a2,b2 | 36.5 ± 0.2 | 36.2 ± 0.0 | −0.7 a2 | 58.6 ± 0.5 | 53.4 ± 0.8 | −8.8 a2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirak, B.; Mann, L.; Richter, A.; Asres, K.; Imming, P. In Vivo Antimalarial Activity of Leaf Extracts and a Major Compound Isolated from Ranunculus multifidus Forsk. Molecules 2021, 26, 6179. https://doi.org/10.3390/molecules26206179
Sirak B, Mann L, Richter A, Asres K, Imming P. In Vivo Antimalarial Activity of Leaf Extracts and a Major Compound Isolated from Ranunculus multifidus Forsk. Molecules. 2021; 26(20):6179. https://doi.org/10.3390/molecules26206179
Chicago/Turabian StyleSirak, Betelhem, Lea Mann, Adrian Richter, Kaleab Asres, and Peter Imming. 2021. "In Vivo Antimalarial Activity of Leaf Extracts and a Major Compound Isolated from Ranunculus multifidus Forsk" Molecules 26, no. 20: 6179. https://doi.org/10.3390/molecules26206179
APA StyleSirak, B., Mann, L., Richter, A., Asres, K., & Imming, P. (2021). In Vivo Antimalarial Activity of Leaf Extracts and a Major Compound Isolated from Ranunculus multifidus Forsk. Molecules, 26(20), 6179. https://doi.org/10.3390/molecules26206179







