Standardized Extract of Asparagus officinalis Stem Attenuates SARS-CoV-2 Spike Protein-Induced IL-6 and IL-1β Production by Suppressing p44/42 MAPK and Akt Phosphorylation in Murine Primary Macrophages
Abstract
:1. Introduction
2. Results
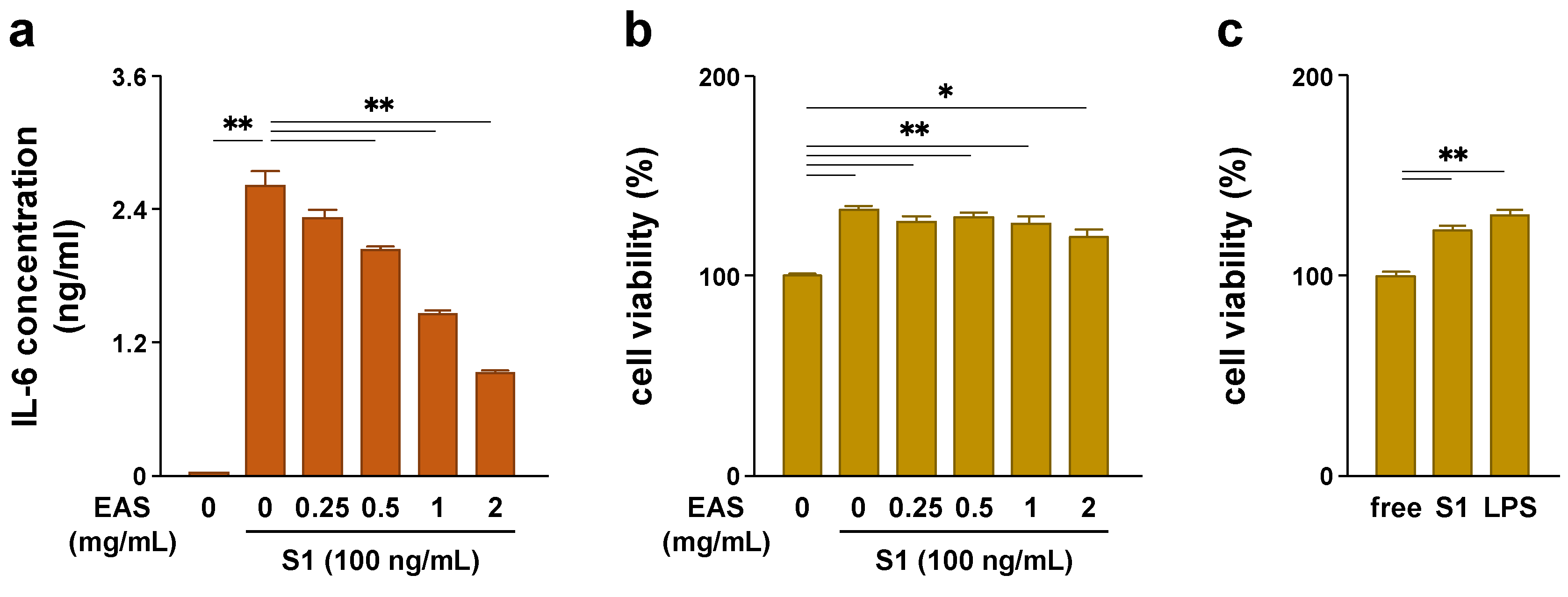
2.1. EAS Attenuated S1-Induced IL-6 Secretion in a Concentration-Dependent Manner without Reducing the Cell Viability of Macrophages
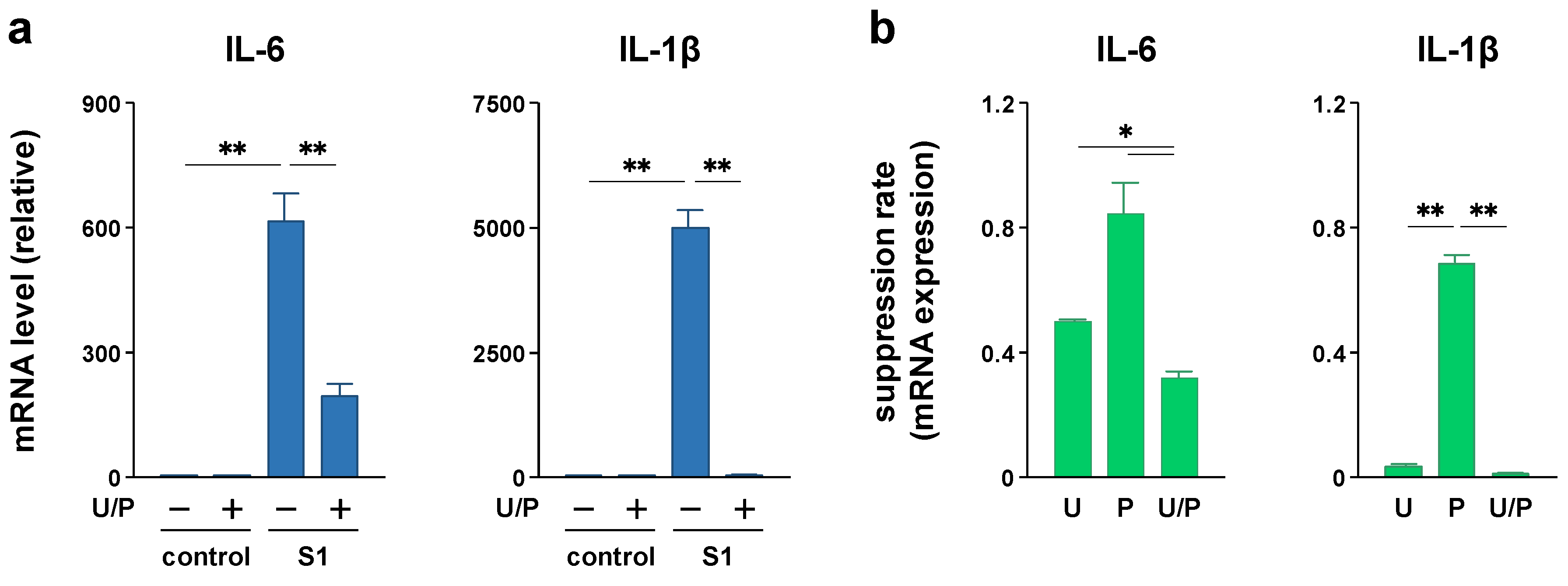
2.2. EAS Repressed S1-Induced IL-6 and IL-1β Transcription in Macrophages
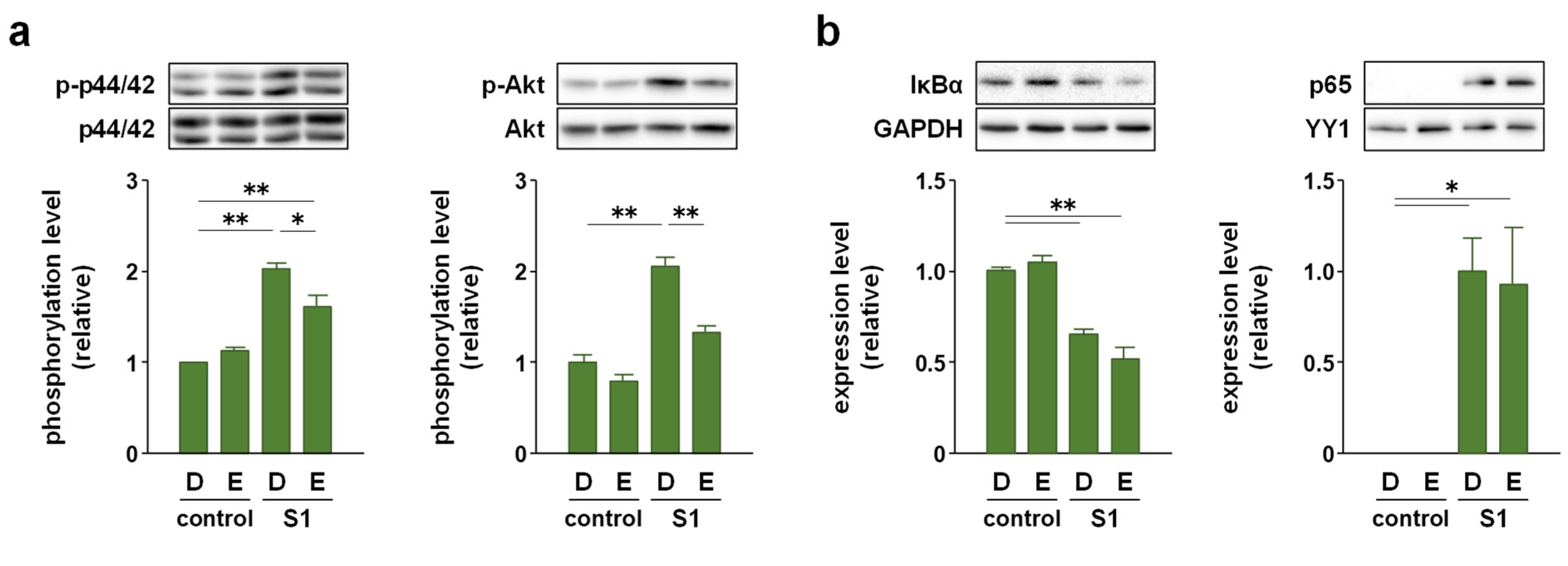
2.3. EAS Suppressed S1-Induced P44/42 MAPK and Akt Phosphorylation without Affecting NF-κB Nuclear Translocation and JNK Phosphorylation in Macrophages
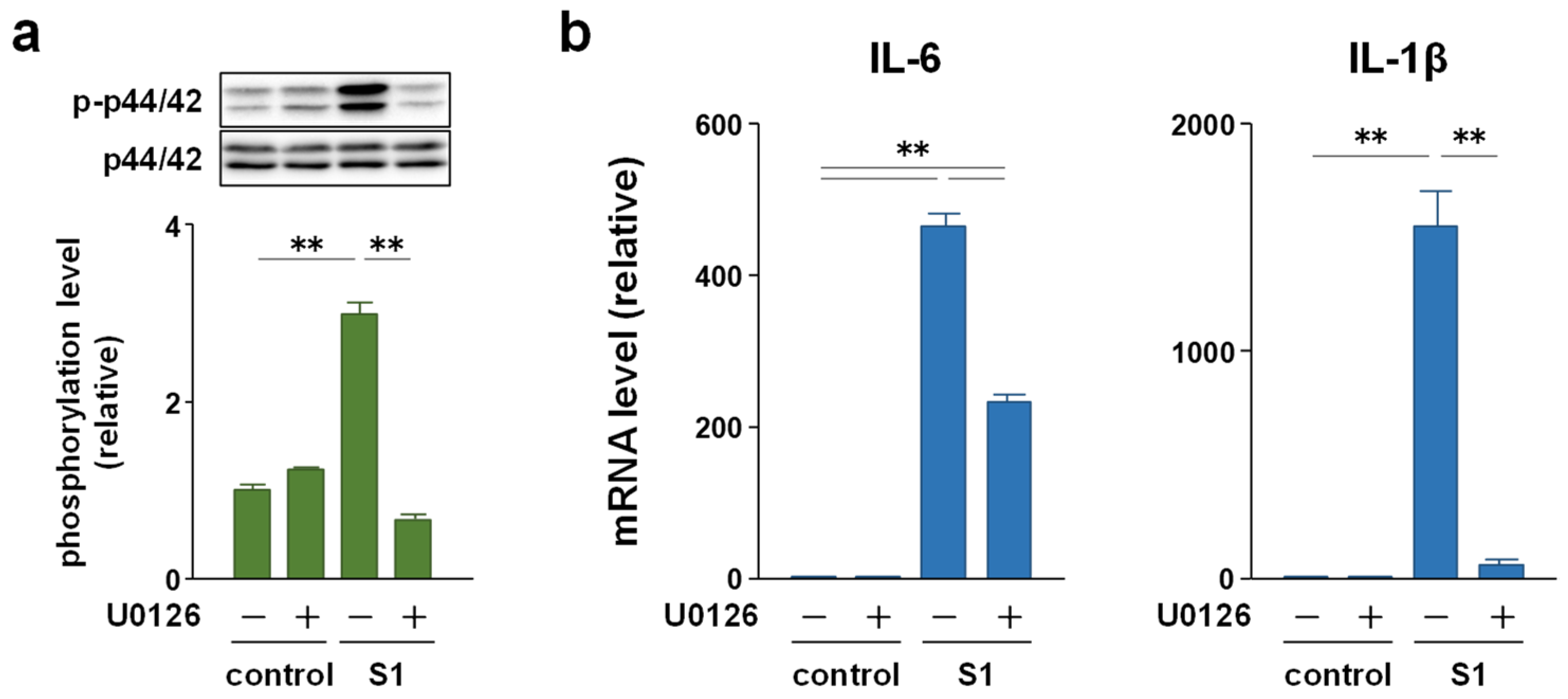
2.4. p44/42 MAPK Signaling Is More Involved in S1-Induced IL-6 and IL-1β Transcription in Macrophages Than Akt Signaling
3. Discussion
4. Materials and Methods
4.1. Preparation of EAS
4.2. Animal Care and Use
4.3. Preparation and Culture of Peritoneal Exudate Macrophages
4.4. Agents and Treatment
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Cell Viability Assay
4.7. Reverse Transcription and Real-Time Polymerase Chain Reaction (PCR)
4.8. Preparation of Nuclear Extracts
4.9. Western Blotting
4.10. Fluorescence Immunomicroscopy
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)–China, 2020. China CDC Wkly. 2020, 2, 113–122. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. J. Am. Med. Assoc. 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Ho, F.K.; Petermann-Rocha, F.; Gray, S.R.; Jani, B.D.; Katikireddi, S.V.; Niedzwiedz, C.L.; Foster, H.; Hastie, C.E.; Mackay, D.F.; Gill, J.M.R.; et al. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS ONE 2020, 15, e0241824. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, M.; Ribeiro Dos Santos, G.; Wang, L.; Cummings, D.A.T.; Azman, A.S.; Paireau, J.; Fontanet, A.; Cauchemez, S.; Salje, H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 2021, 590, 140–145. [Google Scholar] [CrossRef]
- Anderson, M.R.; Geleris, J.; Anderson, D.R.; Zucker, J.; Nobel, Y.R.; Freedberg, D.; Small-Saunders, J.; Rajagopalan, K.N.; Greendyk, R.; Chae, S.R.; et al. Body mass index and risk for intubation or death in SARS-CoV-2 infection: A retrospective cohort study. Ann. Intern. Med. 2020, 173, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Kompaniyets, L.; Goodman, A.B.; Belay, B.; Freedman, D.S.; Sucosky, M.S.; Lange, S.J.; Gundlapalli, A.V.; Boehmer, T.K.; Blanck, H.M. Body mass index and risk for COVID-19-related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death–United States, March–December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 355–361. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Qian, L.; Hong, V.; Wei, R.; Nadjafi, R.F.; Fischer, H.; Li, Z.; Shaw, S.F.; Caparosa, S.L.; Nau, C.L.; et al. Obesity and mortality among patients diagnosed with COVID-19: Results from an integrated health care organization. Ann. Intern. Med. 2020, 173, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Barron, E.; Bakhai, C.; Kar, P.; Weaver, A.; Bradley, D.; Ismail, H.; Knighton, P.; Holman, N.; Khunti, K.; Sattar, N.; et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: A whole-population study. Lancet Diabetes Endocrinol. 2020, 8, 813–822. [Google Scholar] [CrossRef]
- Dennis, J.M.; Mateen, B.A.; Sonabend, R.; Thomas, N.J.; Patel, K.A.; Hattersley, A.T.; Denaxas, S.; McGovern, A.P.; Vollmer, S.J. Type 2 diabetes and COVID-19-related mortality in the critical care setting: A national cohort study in England, March–July 2020. Diabetes Care 2021, 44, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Holman, N.; Knighton, P.; Kar, P.; O’Keefe, J.; Curley, M.; Weaver, A.; Barron, E.; Bakhai, C.; Khunti, K.; Wareham, N.J.; et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: A population-based cohort study. Lancet Diabetes Endocrinol. 2020, 8, 823–833. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. HLH across speciality collaboration, COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirato, K.; Kizaki, T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via Toll-like receptor 4 signaling in murine and human macrophages. Heliyon 2021, 7, e06187. [Google Scholar] [CrossRef]
- Della-Torre, E.; Lanzillotta, M.; Campochiaro, C.; Cavalli, G.; De Luca, G.; Tomelleri, A.; Boffini, N.; De Lorenzo, R.; Ruggeri, A.; Rovere-Querini, P.; et al. Respiratory impairment predicts response to IL-1 and IL-6 blockade in COVID-19 patients with severe pneumonia and hyper-inflammation. Front. Immunol. 2021, 12, 675678. [Google Scholar] [CrossRef]
- REMAP-CAP Investigators; Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; van Bentum-Puijk, W.; et al. Interleukin-6 receptor antagonists in critically ill patients with covid-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef]
- Ito, T.; Goto, K.; Takanari, J.; Miura, T.; Wakame, K.; Nishioka, H.; Tanaka, A.; Nishihira, J. Effects of enzyme-treated asparagus extract on heat shock protein 70, stress indices, and sleep in healthy adult men. J. Nutr. Sci. Vitaminol. 2014, 60, 283–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, T.; Maeda, T.; Goto, K.; Miura, T.; Wakame, K.; Nishioka, H.; Sato, A. Enzyme-treated asparagus extract promotes expression of heat shock protein and exerts antistress effects. J. Food Sci. 2014, 79, H413–H419. [Google Scholar] [CrossRef] [PubMed]
- Takanari, J.; Nakahigashi, J.; Sato, A.; Waki, H.; Miyazaki, S.; Uebaba, K.; Hisajima, T. Effect of enzyme-treated asparagus extract (ETAS) on psychological stress in healthy individuals. J. Nutr. Sci. Vitaminol. 2016, 62, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Bedi, S.; Mann, V.; Sundaresan, A.; Homma, K.; Gaskey, G.; Kowada, M.; Umar, S.; Kulkarni, A.D.; Eltzschig, H.K.; et al. Neuroprotective effects of Asparagus officinalis stem extract in transgenic mice overexpressing amyloid precursor protein. J. Immunol. Res. 2021, 2021, 8121407. [Google Scholar] [CrossRef]
- Shirato, K.; Takanari, J.; Koda, T.; Sakurai, T.; Ogasawara, J.; Ohno, H.; Kizaki, T. A standardized extract of Asparagus officinalis stem prevents reduction in heat shock protein 70 expression in ultraviolet-B-irradiated normal human dermal fibroblasts: An in vitro study. Environ. Health Prev. Med. 2018, 23, 40. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sato, A.; Ono, T.; Goto, K.; Maeda, T.; Takanari, J.; Nishioka, H.; Komatsu, K.; Matsuura, H. Isolation, structural elucidation, and biological evaluation of a 5-hydroxymethyl-2-furfural derivative, asfural, from enzyme-treated asparagus extract. J. Agric. Food Chem. 2013, 61, 9155–9159. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Takanari, J.; Abe, K.; Nagayama, A.; Ikeya, Y.; Kohda, N. Isolation and structure determination of a heat shock protein inducer, asparagus-derived proline-containing 3-alkyldiketopiperazines (Asparaprolines), from a standardized extract of Asparagus officinalis stem. Nat. Prod. Commun. 2020, 15, 1–7. [Google Scholar] [CrossRef]
- Shirato, K.; Takanari, J.; Ogasawara, J.; Sakurai, T.; Imaizumi, K.; Ohno, H.; Kizaki, T. Enzyme-treated asparagus extract attenuates hydrogen peroxide-induced matrix metalloproteinase-9 expression in murine skin fibroblast L929 cells. Nat. Prod. Commun. 2016, 11, 677–680. [Google Scholar] [CrossRef] [Green Version]
- Shirato, K.; Takanari, J.; Sakurai, T.; Ogasawara, J.; Imaizumi, K.; Ohno, H.; Kizaki, T. Enzyme-treated asparagus extract prevents hydrogen peroxide-induced pro-inflammatory responses by suppressing p65 nuclear translocation in skin L929 fibroblasts. Nat. Prod. Commun. 2016, 11, 1883–1888. [Google Scholar] [CrossRef] [Green Version]
- Shirato, K.; Koda, T.; Takanari, J.; Sakurai, T.; Ogasawara, J.; Imaizumi, K.; Ohno, H.; Kizaki, T. Anti-inflammatory effect of ETAS®50 by inhibiting nuclear factor-κB p65 nuclear import in ultraviolet-B-irradiated normal human dermal fibroblasts. Evid. Based Complement. Alternat. Med. 2018, 2018, 5072986. [Google Scholar] [CrossRef] [Green Version]
- Shirato, K.; Koda, T.; Takanari, J.; Ogasawara, J.; Sakurai, T.; Ohno, H.; Kizaki, T. ETAS®50 attenuates ultraviolet-B-induced interleukin-6 expression by suppressing Akt phosphorylation in normal human dermal fibroblasts. Evid. Based Complement. Alternat. Med. 2018, 2018, 1547120. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 2016, 167, 457–470.e13. [Google Scholar] [CrossRef] [Green Version]
- Pozzolini, M.; Scarfi, S.; Benatti, U.; Giovine, M. Interference in MTT cell viability assay in activated macrophage cell line. Anal. Biochem. 2003, 313, 338–341. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [Green Version]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Chang, Y.S.; Ko, B.H.; Ju, J.C.; Chang, H.H.; Huang, S.H.; Lin, C.W. SARS unique domain (SUD) of severe acute respiratory syndrome coronavirus induces NLRP3 inflammasome-dependent CXCL10-mediated pulmonary inflammation. Int. J. Mol. Sci. 2020, 21, 3179. [Google Scholar] [CrossRef]
- Lara, P.C.; Macías-Verde, D.; Burgos-Burgos, J. Age-induced NLRP3 inflammasome over-activation increases lethality of SARS-CoV-2 pneumonia in elderly patients. Aging Dis. 2020, 11, 756–762. [Google Scholar] [CrossRef]
- Theobald, S.J.; Simonis, A.; Georgomanolis, T.; Kreer, C.; Zehner, M.; Eisfeld, H.S.; Albert, M.C.; Chhen, J.; Motameny, S.; Erger, F.; et al. Long-lived macrophage reprogramming drives spike protein-mediated inflammasome activation in COVID-19. EMBO Mol. Med. 2021, 13, e14150. [Google Scholar] [CrossRef]
- Fan, Z.; Cai, L.; Wang, Y.; Zhu, Q.; Wang, S.; Chen, B. The acidic fraction of Isatidis Radix regulates inflammatory response in LPS-stimulated RAW264.7 macrophages through MAPKs and NF-κB Pathway. Evid. Based Complement. Alternat. Med. 2021, 2021, 8879862. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Kim, K.S.; Lee, Y.C.; Moon, H.I.; Lee, J.H. Oleifolioside A, a new active compound, attenuates LPS-stimulated iNOS and COX-2 expression through the downregulation of NF-κB and MAPK activities in RAW 264.7 macrophages. Evid. Based Complement. Alternat. Med. 2012, 2021, 637512. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.R.; Lee, S.J.; Kim, D.H.; Lim, D.W.; Kim, H.; Park, W.H.; Kim, J.E. Literature-based drug repurposing in traditional Chinese medicine: Reduced inflammatory M1 macrophage polarization by Jisil Haebaek Gyeji-Tang alleviates cardiovascular disease in vitro and ex vivo. Evid. Based Complement. Alternat. Med. 2020, 2020, 8881683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, Q.; Liang, Z.; Wang, M.; Wang, B.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Wang, J.; Ma, C.; Kang, W. Anti-inflammatory and antioxidant effects of Chaetoglobosin Vb in LPS-induced RAW264.7 cells: Achieved via the MAPK and NF-κB signaling pathways. Food Chem. Toxicol. 2021, 147, 111915. [Google Scholar] [CrossRef]
- Guo, J.; Tang, S.; Miao, Y.; Ge, L.; Xu, J.; Zeng, X. The anti-inflammatory effects of lignan glycosides from Cistanche tubulosa stems on LPS/IFN-γ-induced RAW264.7 macrophage cells via PI3K/AKT pathway. Curr. Pharm. Biotechnol. 2021, 22, 1380–1391. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Jantan, I.; Harikrishnan, H.; Ahmad, W. Standardized ethanol extract of Tinospora crispa upregulates pro-inflammatory mediators release in LPS-primed U937 human macrophages through stimulation of MAPK, NF-κB and PI3K-Akt signaling networks. BMC Complement. Med. Ther. 2020, 20, 245. [Google Scholar] [CrossRef]
- Najjar, R.S.; Akhavan, N.S.; Pourafshar, S.; Arjmandi, B.H.; Feresin, R.G. Cornus officinalis var. koreana Kitam polyphenol extract decreases pro-inflammatory markers in lipopolysaccharide (LPS)-induced RAW 264.7 macrophages by reducing Akt phosphorylation. J. Ethnopharmacol. 2021, 270, 113734. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, S.; Takeda, K. ERK signalling as a regulator of cell motility. J. Biochem. 2017, 162, 145–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.S.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, T.; Ono, T.; Sato, A.; Goto, K.; Miura, T.; Wakame, K.; Nishioka, H.; Maeda, T. Toxicological assessment of enzyme-treated asparagus extract in rat acute and subchronic oral toxicity studies and genotoxicity tests. Regul. Toxicol. Pharmacol. 2014, 68, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Colunga Biancatelli, R.M.L.; Solopov, P.A.; Sharlow, E.R.; Lazo, J.S.; Marik, P.E.; Catravas, J.D. The SARS-CoV-2 spike protein subunit S1 induces COVID-19-like acute lung injury in Κ18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L477–L484. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, J.; Ito, T.; Wakame, K.; Kitadate, K.; Sakurai, T.; Sato, S.; Ishibashi, Y.; Izawa, T.; Takahashi, K.; Ishida, H.; et al. ETAS, an enzyme-treated asparagus extract, attenuates amyloid beta-induced cellular disorder in PC12 cells. Nat. Prod. Commun. 2014, 9, 561–564. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, T.; Ito, T.; Wakame, K.; Kitadate, K.; Arai, T.; Ogasawara, J.; Kizaki, T.; Sato, S.; Ishibashi, Y.; Fujiwara, T.; et al. Enzyme-treated Asparagus officinalis extract shows neuroprotective effects and attenuates cognitive impairment in senescence-accelerated mice. Nat. Prod. Commun. 2014, 9, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.C.; Wu, C.S.; Wu, T.C.; Lin, Y.H.; Chang, S.J. A standardized extract of Asparagus officinalis stem (ETAS®) ameliorates cognitive impairment, inhibits amyloid β deposition via BACE-1 and normalizes circadian rhythm signaling via MT1 and MT2. Nutrients 2019, 11, 1631. [Google Scholar] [CrossRef] [Green Version]
- Alasmari, F.; Alshammari, M.A.; Alasmari, A.F.; Alanazi, W.A.; Alhazzani, K. Neuroinflammatory cytokines induce amyloid beta neurotoxicity through modulating amyloid precursor protein levels/metabolism. Biomed. Res. Int. 2018, 2018, 3087475. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, B.; Wei, M.; Li, Y.; Liu, J.; Ma, L.; Shang, S.; Huo, K.; Wang, J.; Li, R.; et al. Activation of inflammation is associated with amyloid-β accumulation induced by chronic sleep restriction in rats. J. Alzheimers Dis. 2020, 74, 759–773. [Google Scholar] [CrossRef]
- Shirato, K.; Imaizumi, K.; Sakurai, T.; Ogasawara, J.; Ohno, H.; Kizaki, T. Regular voluntary exercise potentiates interleukin-1β and interleukin-18 secretion by increasing caspase-1 expression in murine macrophages. Mediators Inflamm. 2017, 2017, 9290416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, S.; Shirato, K.; Imaizumi, K.; Toyota, H.; Mizuguchi, J.; Odawara, M.; Che, X.F.; Akiyama, S.; Abe, A.; Tomoda, A. Anticancer effects of phenoxazine derivatives combined with tumor necrosis factor-related apoptosis-inducing ligand on pancreatic cancer cell lines, KLM-1 and MIA-PaCa-2. Oncol. Rep. 2006, 15, 843–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Species | Gene | Probe | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|---|---|
| Mouse | Il6 | #6 (Roche) | 5’-GAT GGA TGC TAC CAA ACT GGA-3’ | 5’-CCA GGT AGC TAT GGT ACT CCA GAA-3’ |
| Mouse | Il1b | #78 (Roche) | 5’-TGT AAT GAA AGA CGG CAC ACC-3’ | 5’-TCT TCT TTG GGT ATT GCT TGG-3’ |
| Mouse | Rn18s | #55 (Roche) | 5’-GGA GAA AAT CTG GCA CCA CAC CTT-3’ | 5’-CCT TAA TGT CAC GCA CGA TTT CCC-3’ |
| Protein | m.w. | Cat. No. | Manufacturer | Dilution | Protein | m.w. | Cat. No. | Manufacturer | Dilution |
|---|---|---|---|---|---|---|---|---|---|
| IκBα | 39 kDa | 4814S | CST | 1/1000 | GAPDH | 37 kDa | 5174S | CST | 1/2000 |
| p65 | 65 kDa | 8242S | CST | 1/1000 | YY1 | 68 kDa | ab109237 | Abcam | 1/2000 |
| p-JNK | 54/46 kDa | 4668S | CST | 1/1000 | JNK | 54/46 kDa | 9258S | CST | 1/1000 |
| p-p44/42 | 44/42 kDa | 4370P | CST | 1/1000 | p44/42 | 44/42 kDa | 4695P | CST | 1/1000 |
| p-Akt | 60 kDa | 4060S | CST | 1/1000 | Akt | 60 kDa | 4691S | CST | 1/1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirato, K.; Takanari, J.; Kizaki, T. Standardized Extract of Asparagus officinalis Stem Attenuates SARS-CoV-2 Spike Protein-Induced IL-6 and IL-1β Production by Suppressing p44/42 MAPK and Akt Phosphorylation in Murine Primary Macrophages. Molecules 2021, 26, 6189. https://doi.org/10.3390/molecules26206189
Shirato K, Takanari J, Kizaki T. Standardized Extract of Asparagus officinalis Stem Attenuates SARS-CoV-2 Spike Protein-Induced IL-6 and IL-1β Production by Suppressing p44/42 MAPK and Akt Phosphorylation in Murine Primary Macrophages. Molecules. 2021; 26(20):6189. https://doi.org/10.3390/molecules26206189
Chicago/Turabian StyleShirato, Ken, Jun Takanari, and Takako Kizaki. 2021. "Standardized Extract of Asparagus officinalis Stem Attenuates SARS-CoV-2 Spike Protein-Induced IL-6 and IL-1β Production by Suppressing p44/42 MAPK and Akt Phosphorylation in Murine Primary Macrophages" Molecules 26, no. 20: 6189. https://doi.org/10.3390/molecules26206189
APA StyleShirato, K., Takanari, J., & Kizaki, T. (2021). Standardized Extract of Asparagus officinalis Stem Attenuates SARS-CoV-2 Spike Protein-Induced IL-6 and IL-1β Production by Suppressing p44/42 MAPK and Akt Phosphorylation in Murine Primary Macrophages. Molecules, 26(20), 6189. https://doi.org/10.3390/molecules26206189







