Characterization of Volatile Compounds of Rosa roxburghii Tratt by Gas Chromatography-Olfactometry, Quantitative Measurements, Odor Activity Value, and Aroma Intensity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification and Quantitation of Compounds and OAV Analysis
2.2. Omission Tests
2.3. Odor Intensity of Binary Mixtures
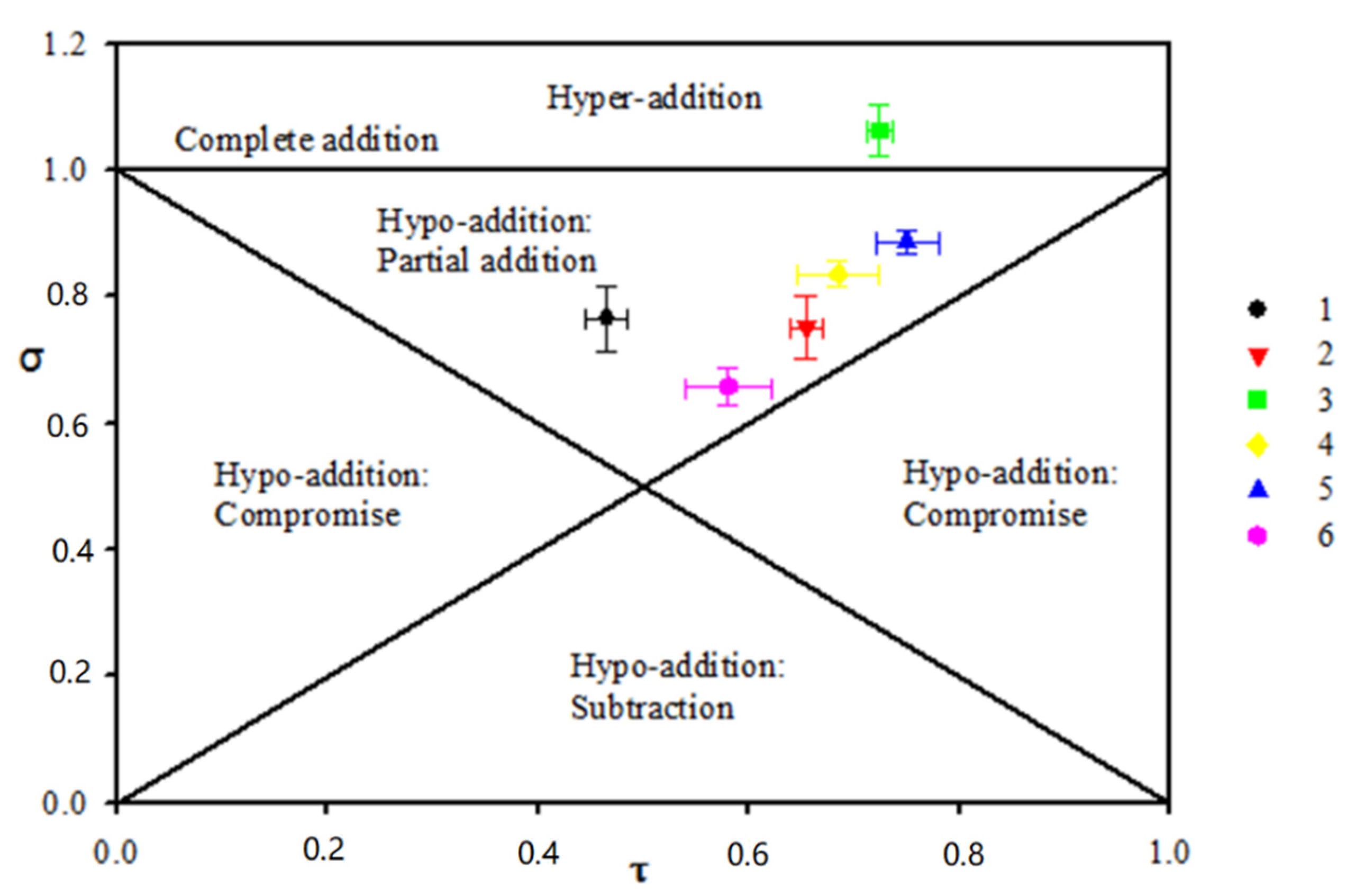
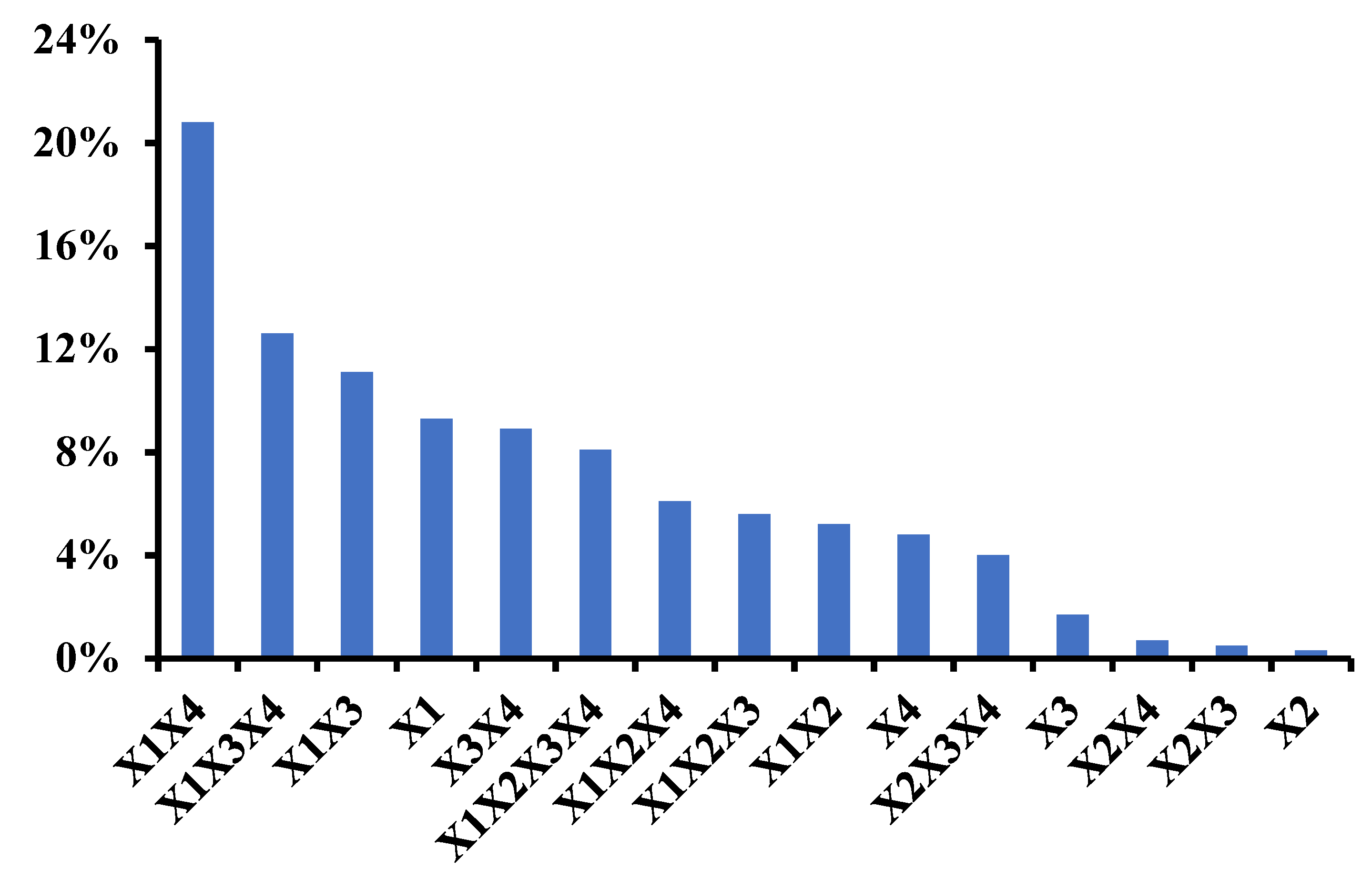
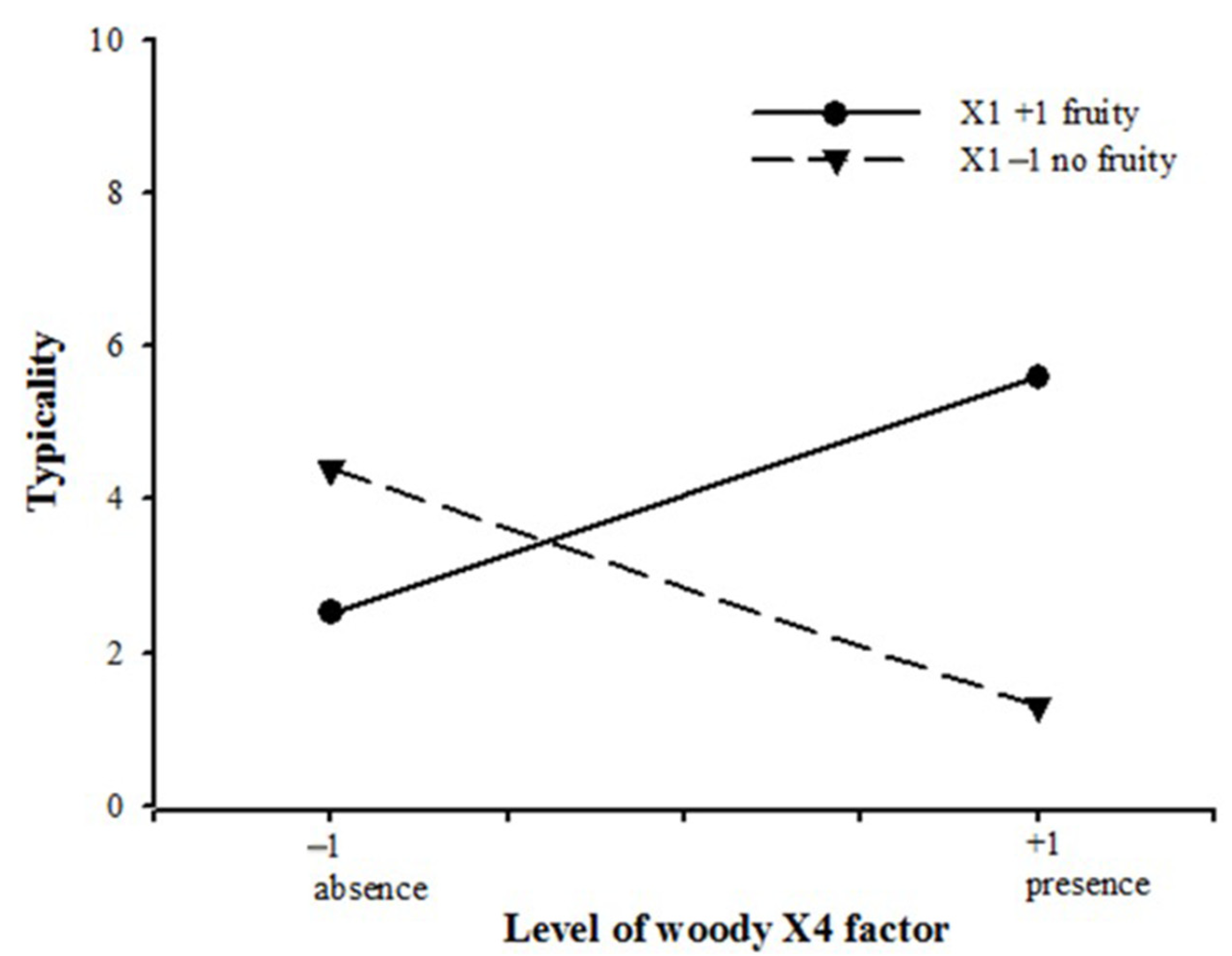
2.4. Factorial Design
3. Materials and Methods
3.1. Chemicals
3.2. Materials
3.3. Headspace-Solid-Phase Microextraction (HS-SPME) Absorption of Aroma Compounds
3.4. Gas Chromatography-Olfactometry (GC–O)
3.5. Gas Chromatography–Mass Spectrometry (GC–MS)
3.6. Odor Activity Values (OAV)
3.7. Sensory Analyses
3.7.1. General Conditions
3.7.2. Sensory Panel
3.7.3. Descriptive Sensory Analysis
3.7.4. Omission Experiments
3.7.5. Determination of Aroma Intensity of Binary Mixtures of the Notes
3.7.6. Factorial Design
3.8. Data Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef]
- Chen, G.; Kan, J. Characterization of a novel polysaccharide isolated from Rosa roxburghii Tratt fruit and assessment of its antioxidant in vitro and in vivo. Int. J. Biol. Macromol. 2017, 107 Pt A, 166–174. [Google Scholar] [CrossRef]
- He, J.Y.; Zhang, Y.H.; Ma, N.; Zhang, X.L.; Liu, M.H.; Fu, W.M. Comparative analysis of multiple ingredients in Rosa roxburghii and R. sterilis fruits and their antioxidant activities. J. Funct. Foods 2016, 27, 29–41. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, W.B.; Cai, X.H.; Lu, D.D.; He, X.Y.; Qiu, P.Y.; Wu, J. Flavonoids of Rosa roxburghii Tratt act as radioprotectors. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 8171–8175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, L.U.; Huaming, A.N.; Wang, D. Characterization of amino acid composition in fruits of three Rosa roxburghii genotypes. Hortic. Plant J. 2017, 3, 232–236. [Google Scholar]
- Chen, Y.; Liu, Z.J.; Liu, J.; Liu, L.K.; Zhang, E.S.; Li, W.L. Inhibition of metastasis and invasion of ovarian cancer cells by crude polysaccharides from Rosa roxburghii tratt in vitro. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 10351–10354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, G.H.; Steltenkamp, R.; Tisserand, G.A. The Gas Chromatograph with Human Sensor: Perfumer Model. Ann. N. Y. Acad. Sci. 1964, 116, 711–724. [Google Scholar] [CrossRef]
- Brattoli, M.; Cisternino, E.; Dambruoso, P.R.; Gennaro, G.D.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas Chromatography Analysis with Olfactometric Detection (GC-O) as a Useful Methodology for Chemical Characterization of Odorous Compounds. Sensors 2013, 13, 16759–16800. [Google Scholar] [CrossRef] [Green Version]
- Lytra, G.; Tempere, S.; de Revel, G.; Barbe, J.-C. Impact of Perceptive Interactions on Red Wine Fruity Aroma. J. Agric. Food Chem. 2012, 60, 12260–12269. [Google Scholar] [CrossRef]
- Cain, W.S.; Drexler, M. Scope and evaluation of odor counteraction and masking. Ann. N. Y. Acad. Sci. 1974, 237, 427–439. [Google Scholar] [CrossRef]
- Niu, Y.; Yao, Z.; Xiao, Z.; Zhu, G.; Zhu, J.; Chen, J. Sensory evaluation of the synergism among ester odorants in light aroma-type liquor by odor threshold, aroma intensity and flash GC electronic nose. Food Res. Int. 2018, 113, 102–114. [Google Scholar] [CrossRef]
- Xiao, Z.; Luo, J.; Niu, Y.; Wang, P.; Wang, R.; Sun, X. Olfactory impact of esters on rose essential oil floral alcohol aroma expression in model solution. Food Res. Int. 2018, 116, 211–222. [Google Scholar] [CrossRef]
- Hallier, A.; Courcoux, P.; Sérot, T.; Prost, C. New gas chromatography–olfactometric investigative method, and its application to cooked Silurus glanis (European catfish) odor characterization. J. Chromatogr. A 2004, 1056, 201–208. [Google Scholar] [CrossRef]
- Paravisini, L.; Septier, C.; Moretton, C.; Nigay, H.; Arvisenet, G.; Guichard, E.; Dacremont, C. Caramel odor: Contribution of volatile compounds according to their odor qualities to caramel typicality. Food Res. Int. 2014, 57, 79–88. [Google Scholar] [CrossRef]
- Elston, A.; Sims, C.; Mahattanatawee, K.; Rouseff, R. Determination of commercial orange juice quality factors using descriptive and GCO analyses. Dev. Food Sci. 2006, 43, 541–544. [Google Scholar]
- Aprea, E.; Corollaro, M.L.; Betta, E.; Endrizzi, I.; Demattè, M.L.; Biasioli, F.; Gasperi, F. Sensory and instrumental profiling of 18 apple cultivars to investigate the relation between perceived quality and odour and flavour. Food Res. Int. 2012, 49, 677–686. [Google Scholar] [CrossRef]
- Munafo, J.P.; Didzbalis, J.; Schnell, R.J.; Steinhaus, M. Insights into the key aroma compounds in mango (Mangifera indica L. ‘Haden’) fruits by stable isotope dilution quantitation and aroma simulation experiments. J. Agric. Food Chem. 2016, 64, 4312–4318. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, Q.; Quan, J.; Zheng, Q.; Xi, W. Determination of sugars, organic acids, aroma components, and carotenoids in grapefruit pulps. Food Chem. 2016, 205, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Guth, H. Quantification and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Rehman Qaisar, F.U.; Zhang, F.; Pant, R.R.; Wang, G.; Khan, S.; Zeng, C. Spatial variation, source identification, and quality assessment of surface water geochemical composition in the Indus River Basin, Pakistan. Environ. Sci. Pollut. Res. 2018, 25, 12749–12763. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Chen, H.; Wang, H.; Xiao, Z. Characterization of the Key Aroma Volatile Compounds in Cranberry (Vaccinium macrocarpon Ait.) Using Gas Chromatography-Olfactometry (GC-O) and Odor Activity Value (OAV). J. Agric. Food Chem. 2016, 64, 4990–4999. [Google Scholar] [CrossRef] [PubMed]
- Engel, E.; Nicklaus, S.; Salles, C.; Quéré, J.L.L. Relevance of omission tests to determine flavour-active compounds in food: Application to cheese taste. Food Qual. Prefer. 2002, 13, 505–513. [Google Scholar] [CrossRef]
- Scharbert, S.; Hofmann, T. Molecular Definition of Black Tea Taste by Means of Quantitative Studies, Taste Reconstitution, and Omission Experiments. J. Agric. Food Chem. 2005, 53, 5377–5384. [Google Scholar] [CrossRef]
- Sun, H.; Ni, H.; Yang, Y.; Chen, F.; Cai, H.; Xiao, A. Sensory evaluation and gas chromatography–mass spectrometry (GC-MS) analysis of the volatile extracts of pummelo (Citrus maxima) peel. Flavour Fragr. J. 2014, 29, 305–312. [Google Scholar] [CrossRef]
- Laing, D.G.; Panhuber, H.; Willcox, M.E.; Pittman, E.A. Quality and intensity of binary odor mixtures. Physiol. Behav. 1984, 33, 309–319. [Google Scholar] [CrossRef]
- Atanasova, B.; Thomas-Danguin, T.; Langlois, D.; Nicklaus, S.; Etievant, P. Perceptual interactions between fruity and woody notes of wine. Flavour Fragr. J. 2004, 19, 476–482. [Google Scholar] [CrossRef]
- Ferreira, V. Revisiting psychophysical work on the quantitative and qualitative odour properties of simple odour mixtures: A flavour chemistry view. Part 1: Intensity and detectability. A review. Flavour Fragr. J. 2012, 27, 124–140. [Google Scholar] [CrossRef]
- Fan, W.; Qian, M.C. Identification of aroma compounds in Chinese ‘Yanghe Daqu’ liquor by normal phase chromatography fractionation followed by gas chromatography[sol]olfactometry. Flavour Fragr. J. 2006, 21, 333–342. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, H.; Niu, Y.; Liu, Q.; Zhu, J.; Chen, H.; Ma, N. Characterization of aroma compositions in different Chinese congou black teas using GC–MS and GC–O combined with partial least squares regression. Flavour Fragr. J. 2017, 32, 265–276. [Google Scholar] [CrossRef]
- Cliff, M.; Stanich, K.; Trujillo, J.M.; Toivonen, P.; Forney, C.F. Determination and prediction of odor thresholds for odor active volatiles in an apple juice matrix. J. Food Qual. 2011, 34, 177–186. [Google Scholar] [CrossRef]
- Gemert, L.J.V. Compilations of Odour Threshold Values in Air, Water and Other Media; Boelens Aroma Chemical Information Service: Utrecht, The Nederlands, 2003. [Google Scholar]
- Martin, N.; Revel, G.D. Sensory evaluation: Scientific bases and oenological applications. J. Int. Des. Sci. Vigne Vin 1999, 33, 81–93. [Google Scholar]
- Fan, H.; Fan, W.; Xu, Y. Characterization of key odorants in Chinese chixiang aroma-type liquor by gas chromatography-olfactometry, quantitative measurements, aroma recombination, and omission studies. J. Agric. Food Chem. 2015, 63, 3660–3668. [Google Scholar] [CrossRef] [PubMed]
- Patte, F.; Laffort, P. An alternative model of olfactory quantitative interaction in binary mixtures. Chem. Senses 1979, 4, 267–274. [Google Scholar] [CrossRef]
| (a) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Code | Compound | RI a | RI b | Identification c | Aroma Intensity d | Odor Description | |||
| 1 | Ethyl acetate | 918 | 653 | AD, RI, Std | 3.1 | fruity | |||
| 2 | Ethyl 2-methylpropanoate | 992 | 788 | AD, RI, Std | 4.3 | fruity | |||
| 3 | Ethyl butanoate | 1062 | 829 | AD, RI, Std | 4.9 | fruity | |||
| 4 | Ethyl 2-methylbutyrate | 1073 | 877 | AD, RI, Std | 4.2 | fruity | |||
| 5 | 3-Methylbutyl acetate | 1142 | 901 | AD, RI, Std | 3.3 | fruity, banana | |||
| 6 | 2-Heptanone | 1201 | 916 | AD, RI, Std | 1.5 | fatty | |||
| 7 | 3-Methyl-1-butanol | 1233 | 765 | AD, RI, Std | 2.4 | fatty | |||
| 8 | E-2-hexenal | 1241 | 880 | AD, RI, Std | 3.9 | green, leaf | |||
| 9 | Ethyl hexanoate | 1254 | 1026 | AD, RI, Std | 4.6 | fruity | |||
| 10 | Ethyl tiglate | 1259 | 967 | AD, RI, Std | 1.6 | fruity | |||
| 11 | 3,7-Dimethyl-1,3,6-Octatriene | 1269 | 1077 | AD, RI, Std | 3.0 | green | |||
| 12 | Phenyl ethylene | 1281 | 917 | AD, RI, Std | 1.9 | floral | |||
| 13 | Ethyl 3-hexenoate | 1327 | 1034 | AD, RI, Std | 2.2 | fruity | |||
| 14 | 2-Heptanol | 1342 | 927 | AD, RI, Std | 3.0 | fatty | |||
| 15 | Ethyl heptanoate | 1356 | 1125 | AD, RI, Std | 2.4 | fruity | |||
| 16 | Ethyl E-2-hexenoate | 1370 | 1072 | AD, RI, Std | 3.3 | fruity, green | |||
| 17 | Hexanol | 1375 | 897 | AD, RI, Std | 2.5 | fatty | |||
| 18 | 2-Nonanone | 1412 | 1119 | AD, RI, Std | 2.4 | fatty | |||
| 19 | Nonanal | 1418 | 1133 | AD, RI, Std | 2.2 | fatty | |||
| 20 | Etheyl octanoat | 1459 | 1226 | AD, RI, Std | 3.8 | fruity | |||
| 21 | Acetic acid | 1479 | 641 | AD, RI, Std | 2.9 | sour | |||
| 22 | 2-Nonanol | 1542 | 1132 | AD, RI, Std | 2.6 | fatty | |||
| 23 | Benzaldehyde | 1561 | 987 | AD, RI, Std | 2.4 | nutty | |||
| 24 | 2,6,6,10-Tetramethyl-1-oxaspiro(4.5)dec-9-ene | 1576 | 1344 | AD, RI, Std | 2.6 | tea, woody | |||
| 25 | 2-Undecanone | 1629 | 1326 | AD, RI, Std | 1.9 | fruity, green | |||
| 26 | Caryophyllene | 1638 | 1472 | AD, RI, Std | 2.2 | woody | |||
| 27 | Butyric acid | 1659 | 818 | AD, RI, Std | 2.6 | sour | |||
| 28 | 3-Methylbutanoic acid | 1700 | 875 | AD, RI, Std | 3.2 | sour | |||
| 29 | Ethyl benzoate | 1707 | 1202 | AD, RI, Std | 2.7 | floral | |||
| 30 | Hexanoic acid | 1880 | 1035 | AD, RI, Std | 4.6 | sour | |||
| 31 | α-Ionone | 1899 | 1532 | AD, RI, Std | 1.8 | floral, woody | |||
| 32 | α-Iononol | 1940 | 1425 | AD, RI, Std | 1.7 | floral, woody | |||
| 33 | E-3-hexenoic acid | 1980 | 1053 | AD, RI, Std | 2.7 | sour, fruity | |||
| 34 | Heptanoic acid | 1989 | 1109 | AD, RI, Std | 3.0 | sour | |||
| 35 | Octanoic acid | 2110 | 1213 | AD, RI, Std | 1.8 | sour | |||
| 36 | Ethyl cinnamate | 2195 | 1508 | AD, RI, Std | 2.0 | floral | |||
| 37 | Eugenol | 2227 | 1397 | AD, RI, Std | 1.7 | woody, floral | |||
| (b) | |||||||||
| Code | Compound | Identification e | Standard Curves f | Range g | R2 | Concentration (mg/kg) | TH Literature (mg/kg) h | OAV | |
| 1 | Ethyl acetate | MS, RI, Std | y = 0.0047x + 0.0002 | 0.17–63.85 | 0.997 | 45.24 i ± 2.02 j | 3.3 | 14 | |
| 2 | Ethyl 2-methylpropanoate | MS, RI, Std | y = 0.0791x − 0.0001 | 0.00069–0.27 | 0.994 | 0.12 ± 0.01 | 0.0001 | 1167 | |
| 3 | Ethyl butanoate | MS, RI, Std | y = 0.1131x + 0.0004 | 0.0050–2.01 | 0.999 | 0.59 ± 0.00 | 0.00018 | 3279 | |
| 4 | Ethyl 2-methylbutyrate | MS, RI, Std | y = 0.2248x − 0.0011 | 0.0040–1.61 | 0.997 | 0.24 ± 0.00 | 0.0003 | 811 | |
| 5 | 3-Methyl butylacetate | MS, RI, Std | y = 0.2804x + 0.005 | 0.0044–1.77 | 0.999 | 0.19 ± 0.01 | 0.005 | 38 | |
| 6 | 2-Heptanone | MS, RI, Std | y = 0.2183x + 0.0056 | 0.0025–0.98 | 0.993 | 0.12 ± 0.00 | 0.14 | <1 | |
| 7 | 3-Methyl-1-butanol | MS, RI, Std | y = 0.0135x − 0.0001 | 0.0081–3.24 | 1.000 | 2.67 ± 0.14 | 1 | 3 | |
| 8 | E-2-hexenal | MS, RI, Std | y = 0.0517x + 0.0046 | 0.0073–2.90 | 0.991 | 1.78 ± 0.09 | 0.082 | 22 | |
| 9 | Ethyl hexanoate | MS, RI, Std | y = 0.3425x + 0.185 | 0.071–28.21 | 0.993 | 2.21 ± 0.10 | 0.001 | 2205 | |
| 10 | Ethyl tiglate | MS, RI, Std | y = 0.3065x + 0.0087 | 0.002–0.81 | 0.991 | 0.06 ± 0.00 | 0.065 | <1 | |
| 11 | 3,7-Dimethyl-1,3,6-octatriene | MS, RI, Std | y = 0.0535x − 0.001 | 0.0019–0.76 | 0.992 | 0.49 ± 0.01 | 0.034 | 14 | |
| 12 | Phenyl ethylene | MS, RI, Std | y = 0.3585x − 0.0023 | 0.0021–0.84 | 0.995 | 0.08 ± 0.00 | 0.065 | 1 | |
| 13 | Ethyl 3-hexenoate | MS, RI, Std | y = 0.5282x + 0.0055 | 0.0014–0.57 | 0.990 | 0.03 ± 0.00 | 0.25 | <1 | |
| 14 | 2-Heptanol | MS, RI, Std | y = 0.1362x + 0.0151 | 0.012–4.80 | 0.996 | 1.06 ± 0.08 | 0.081 | 13 | |
| 15 | Ethyl heptanoate | MS, RI, Std | y = 0.6947x − 0.0037 | 0.00098–0.40 | 0.995 | 0.02 ± 0.00 | 0.002 | 12 | |
| 16 | Ethyl E-2-hexenoate | MS, RI, Std | y = 0.6697x + 0.0035 | 0.0021–0.83 | 1.000 | 0.04 ± 0.00 | 0.00119 | 30 | |
| 17 | Hexanol | MS, RI, Std | y = 0.0157x + 0.001 | 0.027–10.6 | 0.995 | 2.20 ± 0.10 | 1.6 | 1 | |
| 18 | 2-Nonanone | MS, RI, Std | y = 0.7779x − 0.0003 | 0.0030–1.19 | 1.000 | 0.05 ± 0.00 | 0.082 | <1 | |
| 19 | Nonanal | MS, RI, Std | y = 0.2457x + 0.0063 | 0.00092–0.37 | 0.997 | 0.02 ± 0.00 | 0.04 | <1 | |
| 20 | Etheyl octanoat | MS, RI, Std | y = 0.5162x − 0.0779 | 0.015–6.09 | 0.993 | 0.54 ± 0.02 | 0.015 | 36 | |
| 21 | Acetic acid | MS, RI, Std | y = 0.0014x + 0.001 | 0.28–112.81 | 0.992 | 88.72 ± 3.03 | 26 | 3 | |
| 22 | 2-Nonanol | MS, RI, Std | y = 0.5774x + 0.0702 | 0.014–5.52 | 0.999 | 0.20 ± 0.01 | 0.082 | 2 | |
| 23 | Benzaldehyde | MS, RI, Std | y = 0.1418x + 0.0056 | 0.0036–1.45 | 0.994 | 0.30 ± 0.01 | 3.5 | <1 | |
| 24 | 2,6,6,10-Tetramethyl-1-oxaspiro(4.5)dec-9-ene | MS, RI, Std | y = 0.4911x − 0.0095 | 0.0032–1.28 | 0.997 | 0.11 ± 0.01 | 0.1 | 1 | |
| 25 | 2-Undecanone | MS, RI, Std | y = 0.8446x − 0.0066 | 0.0011–0.44 | 0.996 | 0.03 ± 0.00 | 0.082 | <1 | |
| 26 | Caryophyllene | MS, RI, Std | y = 0.0853x − 0.013 | 0.0031–1.25 | 0.991 | 0.64 ± 0.03 | 1.5 | <1 | |
| 27 | Butyric acid | MS, RI, Std | y = 0.1664x + 0.0143 | 0.0019–0.76 | 0.997 | 0.07 ± 0.00 | 1.4 | <1 | |
| 28 | 3-Methylbutanoic acid | MS, RI, Std | y = 0.0081x + 0.0005 | 0.053–21.26 | 0.999 | 8.69 ± 0.14 | 0.25 | 35 | |
| 29 | Ethyl benzoate | MS, RI, Std | y = 0.7402x + 0.0903 | 0.012–4.96 | 0.996 | 0.10 ± 0.01 | 0.06 | 2 | |
| 30 | Hexanoic acid | MS, RI, Std | y = 0.0267x + 0.0036 | 0.38–155.80 | 0.994 | 97.12 ± 4.32 | 1.8 | 54 | |
| 31 | α-Ionone | MS, RI, Std | y = 1.4384x − 0.0186 | 0.0015–0.59 | 0.993 | 0.03 ± 0.00 | 0.0027 | 10 | |
| 32 | α-Iononol | MS, RI, Std | y = 0.9664x − 0.0453 | 0.0067–2.69 | 0.995 | 0.14 ± 0.01 | Unknown | - | |
| 33 | E-3-hexenoic acid | MS, RI, Std | y = 0.1469x − 0.0025 | 0.0013–0.53 | 0.992 | 0.14 ± 0.01 | Unknown | - | |
| 34 | Heptanoic acid | MS, RI, Std | y = 0.1257x − 0.0374 | 0.0057–2.29 | 0.993 | 0.89 ± 0.02 | 0.91 | <1 | |
| 35 | Octanoic acid | MS, RI, Std | y = 0.2847x − 0.0094 | 0.00063–0.25 | 0.992 | 0.06 ± 0.00 | 1.9 | <1 | |
| 36 | Ethyl cinnamate | MS, RI, Std | y = 0.7075x − 0.0592 | 0.011–4.56 | 0.994 | 0.30 ± 0.02 | 0.04 | 7 | |
| 37 | Eugenol | MS, RI, Std | y = 0.2918x − 0.004 | 0.0013–0.52 | 0.996 | 0.07 ± 0.00 | 0.15 | <1 | |
| Fruity | Sour | Green | Floral | Woody | Fatty | N a | Difference Observed | |
|---|---|---|---|---|---|---|---|---|
| Complete TAR in RJMS | x | x | x | x | x | x | ||
| Test1 | - | x | x | x | x | x | 14 | *** |
| Test2 | x | - | x | x | x | x | 14 | *** |
| Test3 | x | x | - | x | x | x | 10 | ** |
| Test4 | x | x | x | - | x | x | 5 | = |
| Test5 | x | x | x | x | - | x | 11 | ** |
| Test6 | x | x | x | x | x | - | 7 | = |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Y.; Wang, R.; Xiao, Z.; Sun, X.; Wang, P.; Zhu, J.; Cao, X. Characterization of Volatile Compounds of Rosa roxburghii Tratt by Gas Chromatography-Olfactometry, Quantitative Measurements, Odor Activity Value, and Aroma Intensity. Molecules 2021, 26, 6202. https://doi.org/10.3390/molecules26206202
Niu Y, Wang R, Xiao Z, Sun X, Wang P, Zhu J, Cao X. Characterization of Volatile Compounds of Rosa roxburghii Tratt by Gas Chromatography-Olfactometry, Quantitative Measurements, Odor Activity Value, and Aroma Intensity. Molecules. 2021; 26(20):6202. https://doi.org/10.3390/molecules26206202
Chicago/Turabian StyleNiu, Yunwei, Ruolin Wang, Zuobing Xiao, Xiaoxin Sun, Pinpin Wang, Jiancai Zhu, and Xueying Cao. 2021. "Characterization of Volatile Compounds of Rosa roxburghii Tratt by Gas Chromatography-Olfactometry, Quantitative Measurements, Odor Activity Value, and Aroma Intensity" Molecules 26, no. 20: 6202. https://doi.org/10.3390/molecules26206202
APA StyleNiu, Y., Wang, R., Xiao, Z., Sun, X., Wang, P., Zhu, J., & Cao, X. (2021). Characterization of Volatile Compounds of Rosa roxburghii Tratt by Gas Chromatography-Olfactometry, Quantitative Measurements, Odor Activity Value, and Aroma Intensity. Molecules, 26(20), 6202. https://doi.org/10.3390/molecules26206202





