Androstenedione (a Natural Steroid and a Drug Supplement): A Comprehensive Review of Its Consumption, Metabolism, Health Effects, and Toxicity with Sex Differences
Abstract
1. Introduction and Occurrence
2. Methods
3. Distribution in Nature
4. Androstenedione Distribution and Metabolism
4.1. Absorption and Distribution
4.2. Metabolism
4.2.1. Metabolism in Fungi
4.2.2. Metabolism in Humans and Non-Human Primates
4.2.3. Metabolism in Animals and Rodents
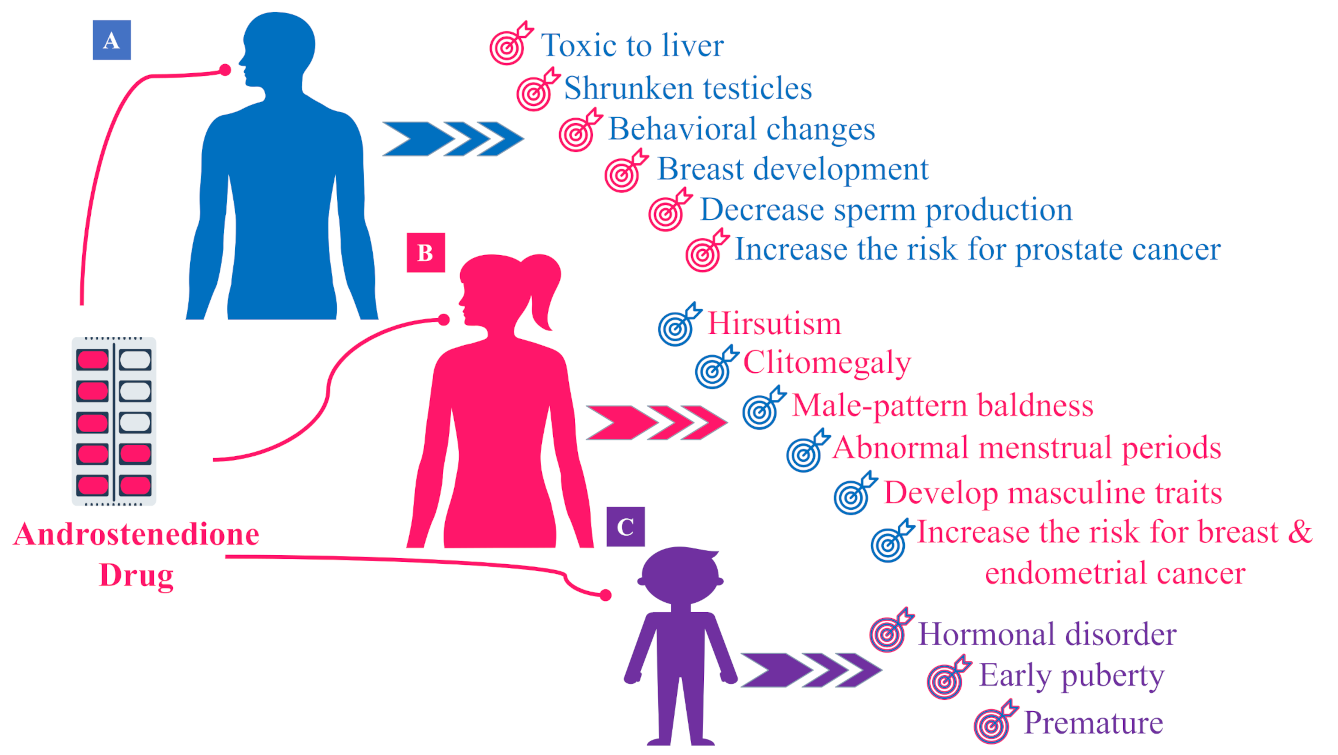
5. Hormonal Effects of Androstenedione AD (or 4A)
5.1. Hormonal Effects of AD in Males
5.2. Hormonal Effects of AD in Females
5.3. Hormonal Effects of AD in Children
5.4. Hormonal Effects in Female Rats
6. Receptors of Androstenedione
7. Androstenedione Doping
8. Androstenedione Toxicity
9. Conclusions
Funding
Conflicts of Interest
References
- Miller, D.L. Reproductive Biology and Phylogeny of Cetacea: Whales, Porpoises and Dolphins; CRC Press: Tifton, GA, USA, 2016. [Google Scholar]
- Malaviya, A.; Gomes, J. Androstenedione production by biotransformation of phytosterols. Bioresour. Technol. 2008, 99, 6725–6737. [Google Scholar] [CrossRef]
- Baker, H.W.G.; Burger, H.G.; De Kretser, D.M.; Hudson, B.; O’Connor, S.; Wang, C.; Mirovics, A.; Court, J.; Dunlop, M.; Rennie, G.C. Changes in The Pituitary-Testicular System with Age. Clin. Endocrinol. 1976, 5, 349–372. [Google Scholar] [CrossRef] [PubMed]
- Stepan, J.; Lachman, M.; Zvěřina, J.; Pacovský, V.; Baylink, D.J. Castrated Men Exhibit Bone Loss: Effect of Calcitonin Treatment on Biochemical Indices of Bone Remodeling. J. Clin. Endocrinol. Metab. 1989, 69, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.S.; Neer, R.M.; Greenspan, S.L.; Rosenthal, D.I.; Crowlev, W.F. Osteooorosis in men with idiouathic hvuogonadotropl’c hypogonadism. Ann. Intern. Med. 1987, 106, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Need, A.G.; Durbridge, T.C.; Nordin, B.E. Anabolic steroids in postmenopausal osteoporosis. Wien. Med. Wochenschr. 1993, 143, 392–395. [Google Scholar] [PubMed]
- Kasperk, C.H.; Wergedal, J.E.; Farley, J.R.; Linkhart, T.A.; Turner, R.T.B. ANDROGENS DIRECTLY STIMULATE PROLIFERATION OF BONE CELLS IN VITRO. Endocrinology 1989, 124, 1576–1578. [Google Scholar] [CrossRef]
- Liesegang, P.; Romalo, G.; Sudmann, M.; Wolf, L.; Schweikert, H.U.; Elger, W. Human Osteoblast—Like Cells Contain Specific, Saturable, High-Affinity Glucocorticoid, Androgen, Estrogen, and 1α,—Dihydroxycholecalciferol Receptors. J. Androl. 1994, 15, 194–199. [Google Scholar] [CrossRef][Green Version]
- Bruch, H.R.; Wolf, L.; Budde, R.; Romalo, G.; Schweikert, H.U. Androstenedione metabolism in cultured human osteoblast-like cells. J. Clin. Endocrinol. Metab. 1992, 75, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Leder, B.Z.; Longcope, C.; Catlin, D.H.; Ahrens, B.; Schoenfeld, D.A.; Finkelstein, J.S. Oral androstenedione administration and serum testosterone concentrations in young men. JAMA 2000, 283, 779–782. [Google Scholar] [CrossRef][Green Version]
- Quigley, C.A.; De Bellis, A.; Marschke, K.B.; El-Awady, M.K.; Wilson, E.M.; French, F.S. Androgen Receptor Defects: Historical, Clinical, and Molecular Perspectives*. Endocr. Rev. 1995, 16, 271–321. [Google Scholar] [CrossRef]
- Catlin, D.H.; Leder, B.Z.; Ahrens, B.D.; Hatton, C.K.; Finkelstein, J.S. Effects of androstenedione administration on epitestosterone metabolism in men. Steroids 2002, 67, 559–564. [Google Scholar] [CrossRef]
- Tokish, J.M.; Kocher, M.S.; Hawkins, R.J. Ergogenic aids: A review of basic science, performance, side effects, and status in sports. Am. J. Sports Med. 2004, 32, 1543–1553. [Google Scholar] [CrossRef]
- Farag, M.A.; Wessjohann, L.A. Metabolome classification of commercial Hypericum perforatum (St. John’s Wort) preparations via UPLC-qTOF-MS and chemometrics. Planta Med. 2012, 78, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Prakash, D.; Gupta, S. Nutraceuticals for athletes. Adv. Food Technol. Nutr. Sci. 2016, 2, 73–82. [Google Scholar] [CrossRef]
- Brown, G.A.; Vukovich, M.D.; Martini, E.R.; Kohut, M.L.; Franke, W.D.; Jackson, D.A.; King, D.S. Endocrine responses to chronic androstenedione intake in 30-to 56-year-old men. J. Clin. Endocrinol. Metab. 2000, 85, 4074–4080. [Google Scholar] [CrossRef]
- Ritchie, I.; Henne, K. Amateurism, scientific control, and crime: Historical fluctuations in anti-doping discourses in sport. J. Criminol. Res. Policy Pr. 2018, 4, 18–29. [Google Scholar] [CrossRef]
- Uralets, V.P.; Gillette, P.A. Over-the-Counter Anabolic Steroids 4-Androsten-3,17-dione; 4-Androsten-3,17 -diol; and 19-nor-4-Androsten-3,17-dione: Excretion Studies in Men. J. Anal. Toxicol. 1999, 23, 357–366. [Google Scholar] [CrossRef]
- Dehennin, L.; Matsumoto, A.M. Long-term administration of testosterone enanthate to normal men: Alterations of the urinary profile of androgen metabolites potentially useful for detection of testosterone misuse in sport. J. Steroid Biochem. Mol. Biol. 1993, 44, 179–189. [Google Scholar] [CrossRef]
- Klein, H.; Bressel, M.; Kastendieck, H.; Voigt, K.D. Androgens, Adrenal Androgen Precursors, and Their Metabolism in Untreated Primary Tumors and Lymph Node Metastases of Human Prostatic Cancer. Am. J. Clin. Oncol. 1988, 11, S30–S36. [Google Scholar] [CrossRef]
- Eberling, P.; Koivisto, V. Physiological importance of dehydroepiandrosterone. Lancet 1994, 343, 1479–1481. [Google Scholar] [CrossRef]
- Shackleton, C.H.; Roitman, E.; Phillips, A.; Chang, T. Androstanediol and 5-androstenediol profiling for detecting exogenously administered dihydrotestosterone, epitestosterone, and dehydroepiandrosterone: Potential use in gas chromatography isotope ratio mass spectrometry. Steroids 1997, 62, 665–673. [Google Scholar] [CrossRef]
- Orentreich, N.; Brind, J.L.; Rizer, R.L.; Vogelman, J.H. Age Changes and Sex Differences in Serum Dehydroepiandrosterone Sulfate Concentrations throughout Adulthood. J. Clin. Endocrinol. Metab. 1984, 59, 551–555. [Google Scholar] [CrossRef]
- Swartz, C.H.; Reddy, S.; Benotti, M.J.; Yin, H.; Barber, L.B.; Brownawell, B.J.; Rudel, R.A. Steroid Estrogens, Nonylphenol Ethoxylate Metabolites, and Other Wastewater Contaminants in Groundwater Affected by a Residential Septic System on Cape Cod, MA. Environ. Sci. Technol. 2006, 40, 4894–4902. [Google Scholar] [CrossRef]
- Phillips, P.J.; Chalmers, A.T.; Gray, J.L.; Kolpin, D.W.; Foreman, W.T.; Wall, G.R. Combined sewer overflows: An environmental source of hormones and wastewater micropollutants. Environ. Sci. Technol. 2012, 46, 5336–5343. [Google Scholar] [CrossRef]
- Mansell, D.S.; Bryson, R.J.; Harter, T.; Webster, J.P.; Kolodziej, E.P.; Sedlak, D.L. Fate of Endogenous Steroid Hormones in Steer Feedlots Under Simulated Rainfall-Induced Runoff. Environ. Sci. Technol. 2011, 45, 8811–8818. [Google Scholar] [CrossRef]
- Lee, L.S.; Carmosini, N.; Sassman, S.A.; Dion, H.M.; Sepúlveda, M.S. Agricultural Contributions of Antimicrobials and Hormones on Soil and Water Quality. Adv. Agron. 2007, 93, 1–68. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Gray, J.L.; Furlong, E.T.; Davis, J.G.; Revello, R.C.; Borch, T. Steroid Hormone Runoff from Agricultural Test Plots Applied with Municipal Biosolids. Environ. Sci. Technol. 2012, 46, 2746–2754. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Borch, T.; Young, R.B.; Goodridge, L.D.; Davis, J.G. Degradation kinetics of testosterone by manure-borne bacteria: Influence of temperature, pH, glucose amendments, and dissolved oxygen. J. Environ. Qual. 2010, 39, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Donova, M.V.; Egorova, O. Microbial steroid transformations: Current state and prospects. Appl. Microbiol. Biotechnol. 2012, 94, 1423–1447. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, H.N.; Khera, R.A. Biological transformations of steroidal compounds: A review. Steroids 2012, 77, 1267–1290. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Fan, S.; Wang, F.; Wei, D. A New Steroid-Transforming Strain of Mycobacterium neoaurum and Cloning of 3-Ketosteroid 9α-Hydroxylase in NwIB-01. Appl. Biochem. Biotechnol. 2010, 162, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.L.; Wilson, E.M.; Angus, R.A.; Howell, W.M.; Kirk, M. Androstenedione and Progesterone in the Sediment of a River Receiving Paper Mill Effluent. Toxicol. Sci. 2003, 73, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.; Angus, R.A.; McNatt, H.; Howell, W.M.; Kemppainen, J.A.; Kirk, M.; Wilson, E.M. Identification of androstenedione in a river containing paper mill effluent. Environ. Toxicol. Chem. Int. J. 2001, 20, 1325–1331. [Google Scholar] [CrossRef]
- Chang, H.; Wan, Y.; Wu, S.; Fan, Z.; Hu, J. Occurrence of androgens and progestogens in wastewater treatment plants and receiving river waters: Comparison to estrogens. Water Res. 2011, 45, 732–740. [Google Scholar] [CrossRef]
- Liu, S.; Ying, G.-G.; Zhao, J.-L.; Zhou, L.-J.; Yang, B.; Chen, Z.-F.; Lai, H.-J. Occurrence and fate of androgens, estrogens, glucocorticoids and progestagens in two different types of municipal wastewater treatment plants. J. Environ. Monit. 2012, 14, 482–491. [Google Scholar] [CrossRef]
- Chang, H.; Wu, S.; Hu, J.; Asami, M.; Kunikane, S. Trace analysis of androgens and progestogens in environmental waters by ultra-performance liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. A 2008, 1195, 44–51. [Google Scholar] [CrossRef]
- Ellis, R.J.; Heuvel, M.R.; Bandelj, E.; Smith, M.A.; McCarthy, L.H.; Stuthridge, T.R.; Dietrich, D.R. In vivo and in vitro assessment of the androgenic potential of a pulp and paper mill effluent. Environ. Toxicol. Chem. Int. J. 2003, 22, 1448–1456. [Google Scholar] [CrossRef]
- Parks, L.G.; Lambright, C.S.; Orlando, E.F.; Guillette, L.J., Jr.; Ankley, G.T.; Gray, L.E., Jr. Masculinization of female mosquitofish in kraft mill effluent-contaminated Fenholloway River water is associated with androgen receptor agonist activity. Toxicol. Sci. 2001, 62, 257–267. [Google Scholar] [CrossRef]
- Hou, L.-P.; Shu, H.; Lin, L.-L.; Xu, S.-Y.; Wu, Y.-X.; Rong, X.-J.; Hu, J.-J.; Song, L.-Y.; Liang, Y.-Q.; Chen, H.-X.; et al. Modulation of transcription of genes related to the hypothalamic–pituitary–gonadal and the hypothalamic–pituitary–adrenal axes in zebrafish (Danio rerio) embryos/larvae by androstenedione. Ecotoxicol. Environ. Saf. 2018, 156, 403–408. [Google Scholar] [CrossRef]
- Nerusu, A.; Reddy, P.S.; Ramachary, D.B.; Subramanyam, R. Unraveling the Stability of Plasma Proteins upon Interaction of Synthesized Androstenedione and Its Derivatives. A Biophysical and Computational Approach. ACS Omega 2017, 2, 6514–6524. [Google Scholar] [CrossRef]
- Peters, T., Jr. All about Albumin: Biochemistry, Genetics and Medical Applications; Academic Press: New York, NY, USA, 1995. [Google Scholar]
- Bahrke, M.S.; Yesalis, C.E. Abuse of anabolic androgenic steroids and related substances in sport and exercise. Curr. Opin. Pharmacol. 2004, 4, 614–620. [Google Scholar] [CrossRef]
- Brzezowska, E.; Dmochowska-Gładysz, J.; Kołek, T. Biotransformation XXXIX. Metabolism of testosterone, androstenedione, progesterone and testosterone derivatives in Absidia coerulea culture. J. Steroid Biochem. Mol. Biol. 1996, 57, 357–362. [Google Scholar] [CrossRef]
- Rege, J.; Garber, S.; Conley, A.J.; Elsey, R.M.; Turcu, A.F.; Auchus, R.J.; Rainey, W.E. Circulating 11-oxygenated androgens across species. J. Steroid Biochem. Mol. Biol. 2019, 190, 242–249. [Google Scholar] [CrossRef]
- Hobkirk, R. Hydroxylated C18 and C19 Steroids; Their Significance and Factors Related to Their Biosynthesis; CRC Press: Boca Raton, FL, USA, 2018; pp. 133–179. [Google Scholar] [CrossRef]
- Handelsman, D.J. Androgen Physiology, Pharmacology, Use and Misuse. In Endotext [Internet]. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK279000/ (accessed on 13 September 2021).
- Audi, L.; Carrascosa, A.; Ballabriga, A. Androgen Metabolism by Human Fetal Epiphyseal Cartilage and Its Chondrocytes in Primary Culture*. J. Clin. Endocrinol. Metab. 1984, 58, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Schweikert, H.U.; Wilson, J.D. Regulation of human hair growth by steroid hormones. II. Androstenedione metabolism in isolated hairs. J. Clin. Endocrinol. Metab. 1974, 39, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Luu-The, V. Analysis and characteristics of multiple types of human 17β-hydroxysteroid dehydrogenase. J. Steroid Biochem. Mol. Biol. 2001, 76, 143–151. [Google Scholar] [CrossRef]
- Liu, L.; Kang, J.; Ding, X.; Chen, D.; Zhou, Y.; Ma, H. Dehydroepiandrosterone-regulated testosterone biosynthesis via activation of the ERK1/2 signaling pathway in primary rat leydig cells. Cell. Physiol. Biochem. 2015, 36, 1778–1792. [Google Scholar] [CrossRef]
- Davis, D.L.; Telang, N.T.; Osborne, M.P.; Bradlow, H.L. Medical hypothesis: Bifunctional genetic-hormonal pathways to breast cancer. Environ. Health Perspect. 1997, 105, 571–576. [Google Scholar] [CrossRef]
- Henderson, B.E.; Feigelson, H.S. Hormonal carcinogenesis. Carcinogenesis 2000, 21, 427–433. [Google Scholar] [CrossRef]
- Stanczyk, F.Z. Diagnosis of hyperandrogenism: Biochemical criteria. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 177–191. [Google Scholar] [CrossRef]
- Petrov, R.V.; Kuzina, I.N.; Kilikovsky, V.V.; Smirnova, O.V. Age-dependent changes in the concentration of active sex steroids, precursors, metabolites, and regulatory agents in blood serum of male subjects. Russ. J. Dev. Biol. 2009, 40, 373–381. [Google Scholar] [CrossRef]
- Beamer, W.G.; Shultz, K.L.; Tennent, B.J. Induction of ovarian granulosa cell tumors in SWXJ-9 mice with dehydroepiandrosterone. Cancer Res. 1988, 48, 2788–2792. [Google Scholar]
- Mostl, E.; Choi, H.S.; Bamberg, E. Rapid conversion of androstenedione into epitestosterone in bovine blood invitro. IRCS Med. Sci. Biochem. 1980, 8, 440. [Google Scholar] [CrossRef]
- Tsang, C.P.W. Metabolism of [4-14C]estrone by sheep erythrocytes around the time of parturition. Can. J. Biochem. 1976, 54, 666–669. [Google Scholar] [CrossRef]
- Kochakian, C.D. 17α and 17β-oxidoreductases of adult female hamster liver and kidney. J. Steroid Biochem. 1982, 17, 541–546. [Google Scholar] [CrossRef]
- De Prada, P.; Setchell, K.D.; Hylemon, P.B. Purification and characterization of a novel 17 alpha-hydroxysteroid dehydrogenase from an intestinal Eubacterium sp. VPI 12708. J. Lipid Res. 1994, 35, 922–929. [Google Scholar] [CrossRef]
- Valdés, E.; Orellana, M.; Del Villar, E.; Vargas, M. Androstenedione metabolism in streptozotocin diabetes and fasting male rats: Similarities and differences. Comp. Biochem. Physiol. Part. A Physiol. 1994, 107, 261–267. [Google Scholar] [CrossRef]
- Verslycke, T.; De Wasch, K.; De Brabander, H.F.; Janssen, C. Testosterone Metabolism in the Estuarine Mysid Neomysis integer (Crustacea; Mysidacea): Identification of Testosterone Metabolites and Endogenous Vertebrate-Type Steroids. Gen. Comp. Endocrinol. 2002, 126, 190–199. [Google Scholar] [CrossRef]
- Mindnich, R.; Möller, G.; Adamski, J. The role of 17 beta-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 2004, 218, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Janer, G.; Leblanc, G.A.; Porte, C. A comparative study on androgen metabolism in three invertebrate species. Gen. Comp. Endocrinol. 2005, 143, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Janer, G.; Bachmann, J.; Oehlmann, J.; Schulte-Oehlmann, U.; Porte, C. The effect of organotin compounds on gender specific androstenedione metabolism in the freshwater ramshorn snail Marisa cornuarietis. J. Steroid Biochem. Mol. Biol. 2006, 99, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Devlin, T. Textbook of Biochemistry with Clinical Correlations, 7th ed.; John Wiley Sons: Hoboken, NY, USA, 2010. [Google Scholar]
- Ballantyne, C.S.; Phillips, S.M.; Macdonald, J.R.; Tarnopolsky, M.A.; MacDougall, J.D. The Acute Effects of Androstenedione Supplementation in Healthy Young Males. Can. J. Appl. Physiol. 2000, 25, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.B.; Lim, J.; Cutler, A.; Bucci, L. Effects of dehydroepiandrosterone vs. androstenedione supplementation in men. Med. Sci. Sports Exerc. 1999, 31, 1788. [Google Scholar] [CrossRef] [PubMed]
- Judge, L.W.; Bellar, D.M.; Hoover, D.L.; Biggs, D.; Leitzelar, B.N.; Craig, B.W. Effects of acute androstenedione supplementation on testosterone levels in older men. Aging Male 2016, 19, 161–167. [Google Scholar] [CrossRef]
- Leder, B.Z.; Leblanc, K.M.; Longcope, C.; Lee, H.; Catlin, D.H.; Finkelstein, J.S. Effects of oral androstenedione administration on serum testosterone and estradiol levels in postmenopausal women. J. Clin. Endocrinol. Metab. 2002, 87, 5449–5454. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kicman, A.T.; Bassindale, T.; Cowan, D.A.; Dale, S.; Hutt, A.J.; Leeds, A.R. Effect of Androstenedione Ingestion on Plasma Testosterone in Young Women; a Dietary Supplement with Potential Health Risks. Clin. Chem. 2003, 49, 167–169. [Google Scholar] [CrossRef][Green Version]
- Brown, G.A.; Dewey, J.C.; Brunkhorst, J.A.; Vukovich, M.D.; King, D.S. Changes in Serum Testosterone and Estradiol Concentrations Following Acute Androstenedione Ingestion in Young Women. Horm. Metab. Res. 2004, 36, 62–66. [Google Scholar] [CrossRef]
- L’Allemand, D.; Schmidt, S.; Rousson, V.; Brabant, G.; Gasser, T.; Grüters, A. Associations between body mass, leptin, IGF-I and circulating adrenal androgens in children with obesity and premature adrenarche. Eur. J. Endocrinol. 2002, 146, 537–543. [Google Scholar] [CrossRef]
- Kwon, J.H.; Lee, H.A.; Kim, Y.J.; Lee, H.; Park, E.A.; Cho, S.J.; Gwak, H.S.; Ha, E.; Park, H.; Kim, H.S. Effects of Adrenal Androgen Levels on Bone Age Advancement in Prepubertal Children: Using the Ewha Birth and Growth Cohort Study. J. Korean Med. Sci. 2017, 32, 968–973. [Google Scholar] [CrossRef]
- Vanderschueren, D.; Vandenput, L.; Boonen, S.; Lindberg, M.K.; Bouillon, R.; Ohlsson, C. Androgens and bone. Endocr. Rev. 2004, 25, 389–425. [Google Scholar] [CrossRef]
- Weinstein, R.S.; Jilka, R.L.; Parfitt, A.M.; Manolagas, S.C. The effects of androgen deficiency on murine bone remodeling and bone mineral density are mediated via cells of the osteoblastic lineage. Endocrinology 1997, 138, 4013–4021. [Google Scholar] [CrossRef]
- Müller, S.T.; Pählig, S.; Merabet, A.; Abdelsamie, A.S.; van Koppen, C.J.; Marchais-Oberwinkler, S.; Hartmann, R.W.; Zierau, O.; Vollmer, G. Effects of 17β-HSD2 inhibition in bones on osteoporosis based on an animal rat model. J. Steroid Biochem. Mol. Biol. 2019, 192, 105405. [Google Scholar] [CrossRef]
- Flynn, T.J.; Sapienza, P.P.; Wiesenfeld, P.W.; Ross, I.A.; Sahu, S.; Kim, C.S.; O’Donnell, M.W., Jr.; Collinsa, T.F.X.; Sprando, R.L. Effects of oral androstenedione on steroid metabolism in liver of pregnant and non-pregnant female rats. Food Chem. Toxicol. 2005, 43, 537–542. [Google Scholar] [CrossRef]
- Thum, T.; Borlak, J. Testosterone, cytochrome P450, and cardiac hypertrophy. FASEB J. 2002, 16, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.E. Steroid receptor phylogeny and vertebrate origins. Mol. Cell. Endocrinol. 1997, 135, 101–107. [Google Scholar] [CrossRef]
- Baker, M.E. Evolution of adrenal and sex steroid action in vertebrates: A ligand-based mechanism for complexity. BioEssays 2003, 25, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, G.K.; Jurutka, P.; Haussler, C.A.; Haussler, M.R. Steroid hormone receptors: Evolution, ligands, and molecular basis of biologic function. J. Cell. Biochem. 1999, 75, 110–122. [Google Scholar] [CrossRef]
- Bryan, M.B.; Scott, A.P.; Li, W. The Sea Lamprey (Petromyzon marinus) Has a Receptor for Androstenedione1. Biol. Reprod. 2007, 77, 688–696. [Google Scholar] [CrossRef]
- Zucker, T.P.; Higashiura, K.; Mathur, R.S.; Halushka, P.V. Androstenedione increases thromboxane A2 receptors in human erythroleukemia cells. Life Sci. 1996, 58, 683–690. [Google Scholar] [CrossRef]
- Papayannopoulou, T.M.; Brice, T.; Yokochi, P.S.; Rabinovitch, D.; Stamatoyannopoulos, L.; Stamatoyannopoulos, G. Anti-HEL cell monoclonal antibodies recognize determinants that are also present in hemopoietic progenitors. Blood 1984, 63, 326–334. [Google Scholar] [CrossRef]
- Basaria, S. Androgen Abuse in Athletes: Detection and Consequences. J. Clin. Endocrinol. Metab. 2010, 95, 1533–1543. [Google Scholar] [CrossRef]
- Food And Drug Administration. “FDA Warns Manufacturers To Stop Distributing Products Containing ‘Andro’”. ScienceDaily. Available online: www.sciencedaily.com/releases/2004/03/040323075302.htm (accessed on 25 September 2021).
- IOC Medical Code and Explanatory Documents. Available online: https://stillmed.olympic.org/media/Document%20Library/OlympicOrg/IOC/Who-We-Are/Commissions/Medical-and-Scientific-Commission/Olympic-Movement-Medical-Code-31-03-2016.pdf (accessed on 8 June 2021).
- Venken, K.; De Gendt, K.; Boonen, S.; Ophoff, J.; Bouillon, R.; Swinnen, J.V.; Verhoeven, G.; Vanderschueren, D. Relative Impact of Androgen and Estrogen Receptor Activation in the Effects of Androgens on Trabecular and Cortical Bone in Growing Male Mice: A Study in the Androgen Receptor Knockout Mouse Model. J. Bone Miner. Res. 2006, 21, 576–585. [Google Scholar] [CrossRef]
- Ayotte, C.; Goudreault, D.; Charlebois, A. Testing for natural and synthetic anabolic agents in human urine. J. Chromatogr. B: Biomed. Sci. Appl. 1996, 687, 3–25. [Google Scholar] [CrossRef]
- Hacker, R.; Mattern, C. Arzneimittel zur Erhöhung des Testosteronspiegels. German patent DE 42 14953 A1, 6 May 1992. [Google Scholar]
- The 2012 Prohibited List Code. Available online: https://www.coni.it/images/documenti/antidoping/normativa/WADA2012.pdf (accessed on 8 June 2021).
- Lévesque, J.F.; Gaudreault, M.; Houle, R.; Chauret, N. Evaluation of human hepatocyte incubation as a new tool for metabolism study of androstenedione and norandrostenedione in a doping control perspective. J. Chromatogr. B 2002, 780, 145–153. [Google Scholar] [CrossRef]
- Blystone, C.R.; Elmore, S.A.; Witt, K.L.; Malarkey, D.E.; Foster, P.M. Toxicity and carcinogenicity of androstenedione in F344/N rats and B6C3F1 mice. Food Chem. Toxicol. 2011, 49, 2116–2124. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, F. Cocarcinogenic Activity of Cholesterol Oxidation Products and Sesame Oil2. J. Natl. Cancer Inst. 1957, 19. [Google Scholar] [CrossRef]
- Dauvois, S.; Labrie, F. Androstenedione and androst-5-ene-3β, 17β-diol stimulate DMBA-induced rat mammary tumors—role of aromatase. Breast Cancer Res. Treat. 1989, 13, 61–69. [Google Scholar] [CrossRef]
- Orner, G.A.; Mathews, C.; Hendricks, J.D.; Carpenter, H.M.; Bailey, G.S.; Williams, D.E. Dehydroepiandrosterone is a complete hepatocarcinogen and potent tumor promoter in the absence of peroxisome proliferation in rainbow trout. Carcinogenesis 1995, 16, 2893–2898. [Google Scholar] [CrossRef]
- Rao, M.S.; Subbarao, V.; Yeldandi, A.V.; Reddy, J.K. Hepatocarcinogenicity of dehydroepiandrosterone in the rat. Cancer Res. 1992, 52, 2977–2979. [Google Scholar]
- Sahu, S.C.; Sapienza, P.P.; Sprando, R.L.; Collins, T.F.; Ross, I.A.; Flynn, T.J.; Wiesenfeld, P.L.; O’Donnell, M.W.; Kim, C.S. Hepatotoxicity of androstenedione in pregnant rats. Food Chem. Toxicol. 2005, 43, 341–344. [Google Scholar] [CrossRef]
- Wieczerzak, M.; Kudłak, B.; Namieśnik, J. Study of the effect of residues of pharmaceuticals on the environment on the example of bioassay Microtox. Mon. Für Chem. Chem. Mon. 2016, 147, 1455–1460. [Google Scholar] [CrossRef]
- Sprando, R.; Collins, T.; Black, T.; Olejnik, N.; Sapienza, P.; Ramos-Valle, M.; Ruggles, D. Maternal exposure to androstenedione does not induce developmental toxicity in the rat. Food Chem. Toxicol. 2005, 43, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Sprando, R.L.; Thomas, F.X.; Collins, T.N.B.; Nicholas, O.; Erich, G.; Dennis, I.R. Effects of androstenedione on in utero development in rats. Food Chem. Toxicol. 2004, 42, 917–924. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badawy, M.T.; Sobeh, M.; Xiao, J.; Farag, M.A. Androstenedione (a Natural Steroid and a Drug Supplement): A Comprehensive Review of Its Consumption, Metabolism, Health Effects, and Toxicity with Sex Differences. Molecules 2021, 26, 6210. https://doi.org/10.3390/molecules26206210
Badawy MT, Sobeh M, Xiao J, Farag MA. Androstenedione (a Natural Steroid and a Drug Supplement): A Comprehensive Review of Its Consumption, Metabolism, Health Effects, and Toxicity with Sex Differences. Molecules. 2021; 26(20):6210. https://doi.org/10.3390/molecules26206210
Chicago/Turabian StyleBadawy, Marwa T., Mansour Sobeh, Jianbo Xiao, and Mohamed A. Farag. 2021. "Androstenedione (a Natural Steroid and a Drug Supplement): A Comprehensive Review of Its Consumption, Metabolism, Health Effects, and Toxicity with Sex Differences" Molecules 26, no. 20: 6210. https://doi.org/10.3390/molecules26206210
APA StyleBadawy, M. T., Sobeh, M., Xiao, J., & Farag, M. A. (2021). Androstenedione (a Natural Steroid and a Drug Supplement): A Comprehensive Review of Its Consumption, Metabolism, Health Effects, and Toxicity with Sex Differences. Molecules, 26(20), 6210. https://doi.org/10.3390/molecules26206210







