Role of Na-Montmorillonite on Microbially Induced Calcium Carbonate Precipitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Conditions of Bacteria
2.2. Na-MMT and Sandy Soils Used
2.3. Precipitation and Consolidation Experiments
2.4. Analytical Methods
3. Results
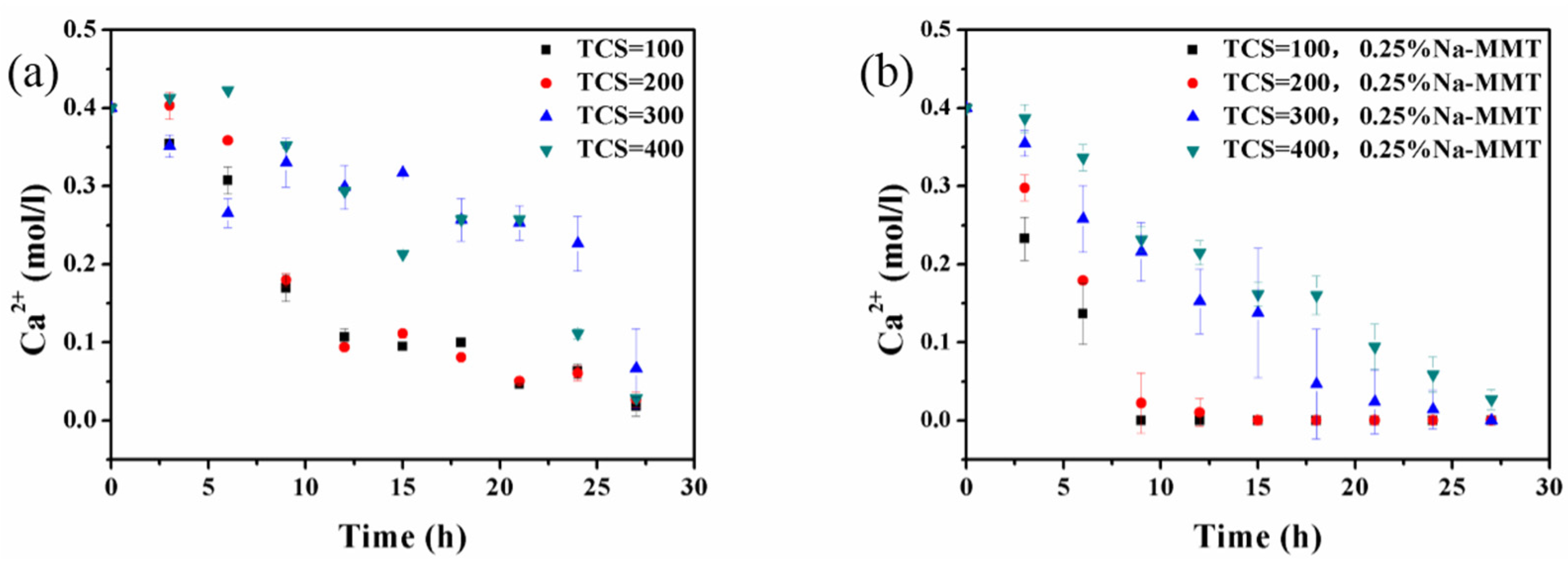
3.1. Mechanism of Na-MMT on CaCO3 Precipitates
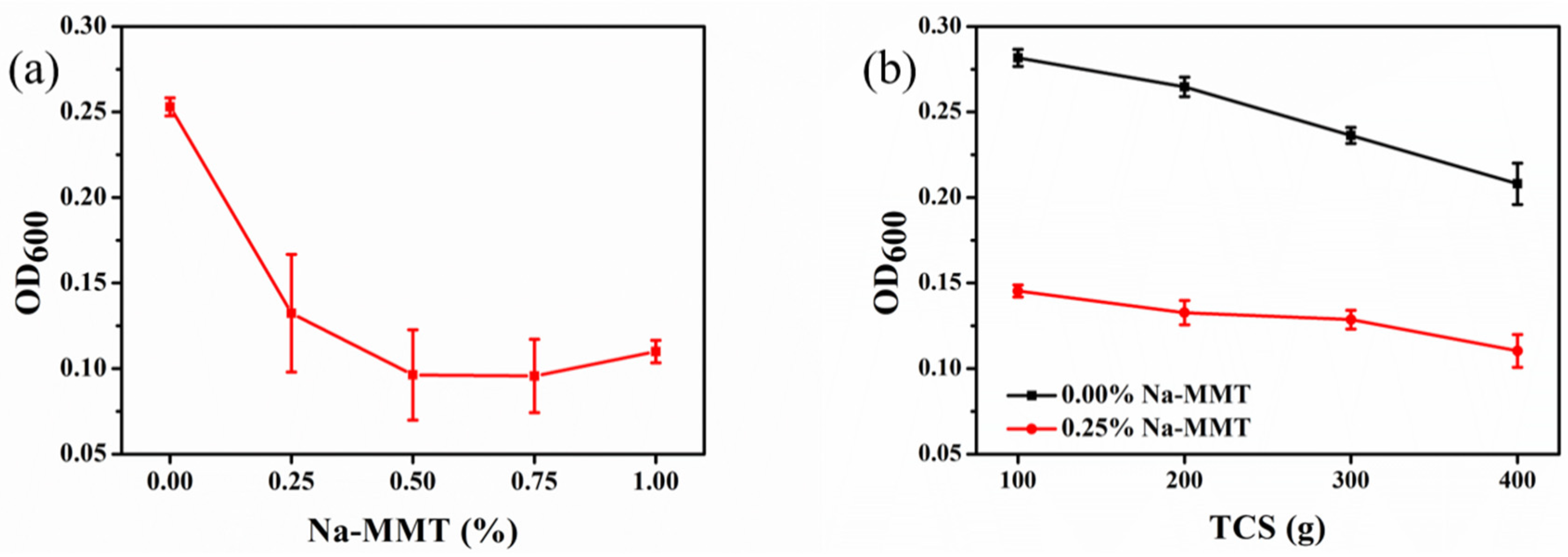
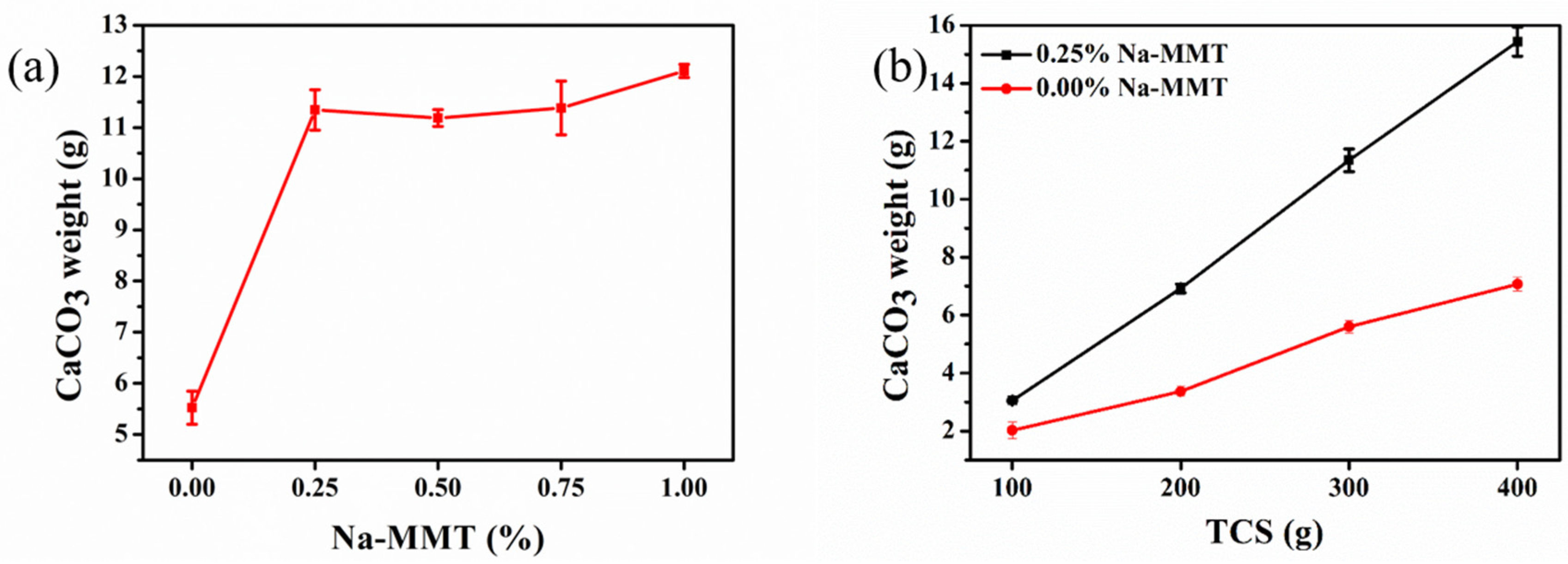
3.2. Influence of Na-MMT on CaCO3 Precipitates
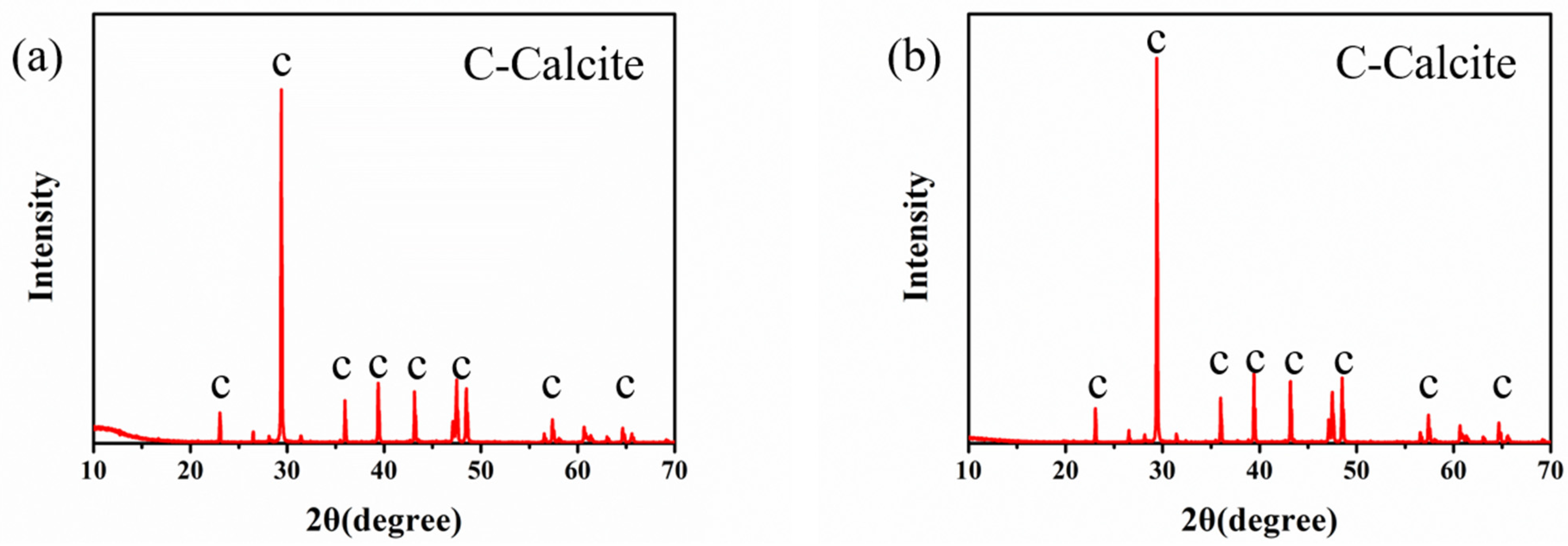
3.3. Influence of Na-MMT on the Polymorph and Morphology of CaCO3 Precipitates
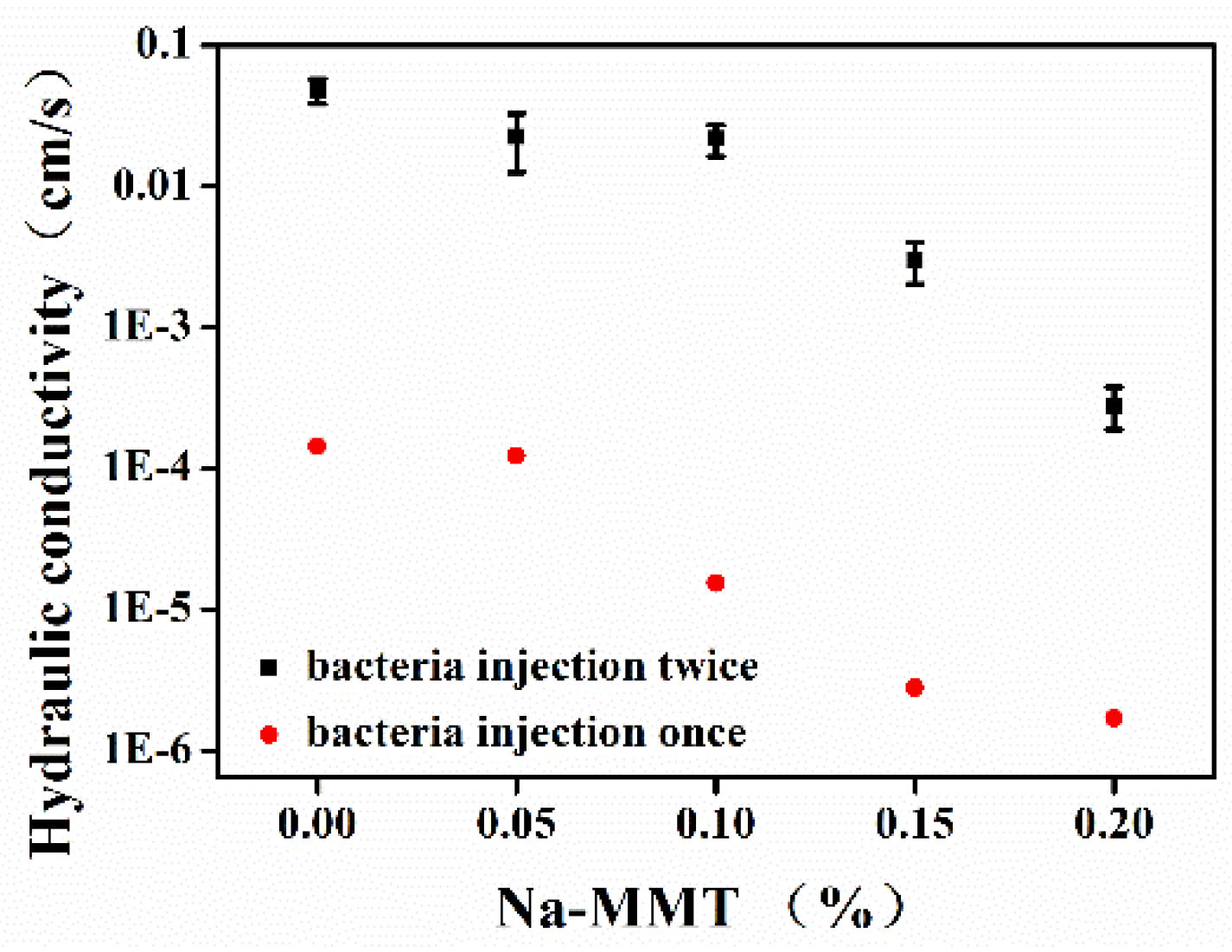
3.4. Influence of Na-MMT on Hydraulic Conductivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Approval
Sample Availability
References
- Qiu, J.; Ruan, S.; Unluer, C.; Yang, E.-H. Autogenous healing of fiber-reinforced reactive magnesia-based tensile strain-hardening composites. Cem. Concr. Res. 2019, 115, 401–413. [Google Scholar] [CrossRef]
- Chan, K.Y.; Zwieten, L.V.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Soil Res. 2008, 45, 629–634. [Google Scholar] [CrossRef]
- Ivanov, V.; Jian, C. Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev. Environ. Sci. Biotechnol. 2008, 7, 139–153. [Google Scholar] [CrossRef]
- Marouek, J.; Struneck, O.; Stehel, V. Biochar farming: Defining economically perspective applications. Clean Technol. Environ. Policy 2019, 21, 1389–1395. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Chu, J. Soil bio-cementation using a new one-phase low-pH injection method. Acta Geotech. 2018, 14, 615–626. [Google Scholar] [CrossRef] [Green Version]
- Paassen, L. Biogrout, Ground Improvement by Microbial Induced Carbonate Precipitation. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2009. [Google Scholar]
- Cardoso, R.; Pires, I.; Duarte, S.O.D.; Monteiro, G.A. Effects of clay’s chemical interactions on biocementation. Appl. Clay Sci. 2018, 156, 96–103. [Google Scholar] [CrossRef]
- Dejong, J.T.; Soga, K.; Kavazanjian, E.; Burns, S.E.; Van Paassen, L.A.; Al Qabany, A.; Aydilek, A.; Bang, S.S.; Burbank, M.; Caslake, L.F.; et al. Biogeochemical processes and geotechnical applications: Progress, opportunities and challenges. Geotechnique 2013, 63, 287–301. [Google Scholar] [CrossRef] [Green Version]
- Schwantes-Cezario, N.; Medeiros, L.P.; Nakazato, G.; Kobayashi, R.K.T.; Toralles, B.M. Bioprecipitation of calcium carbonate induced by Bacillus subtilis isolated in Brazil. Int. Biodeterior. Biodegrad. 2017, 123, 200–205. [Google Scholar] [CrossRef]
- Fujita, M.; Nakashima, K.; Achal, V.; Kawasaki, S. Whole-cell evaluation of urease activity of Pararhodobacter sp. isolated from peripheral beachrock. Biochem. Eng. J. 2017, 124, 1–5. [Google Scholar] [CrossRef]
- Khan, M.N.H.; Amarakoon, G.; Shimazaki, S.; Kawasaki, S. Coral Sand Solidification Test Based on Microbially Induced Carbonate Precipitation Using Ureolytic Bacteria. Mater. Trans. 2015, 56, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Nawarathna, T.H.K.; Nakashima, K.; Fujita, M.; Takatsu, M.; Kawasaki, S. Effects of Cationic Polypeptide on CaCO3 Crystallization and Sand Solidification by Microbial-Induced Carbonate Precipitation. ACS Sustain. Chem. Eng. 2018, 6, 10315–10322. [Google Scholar] [CrossRef]
- Li, M.D.; Li, L.; Ogbonnaya, U.; Wen, K.J.; Tian, A.G.; Amini, F. Influence of Fiber Addition on Mechanical Properties of MICP-Treated Sand. J. Mater. Civ. Eng. 2016, 28. [Google Scholar] [CrossRef]
- Soon, N.W.; Lee, L.M.; Khun, T.C.; Ling, H.S. Improvements in engineering properties of soils through microbial-induced calcite precipitation. KSCE J. Civ. Eng. 2013, 17, 718–728. [Google Scholar] [CrossRef]
- Ghosh, P.; Mandal, S.; Chattopadhyay, B.D.; Pal, S. Use of microorganism to improve the strength of cement mortar. Cem. Concr. Res. 2005, 35, 1980–1983. [Google Scholar] [CrossRef]
- Manzur, T.; Rahman, F.; Afroz, S.; Huq, R.S.; Efaz, I.H. Potential of a Microbiologically Induced Calcite Precipitation Process for Durability Enhancement of Masonry Aggregate Concrete. J. Mater. Civ. Eng. 2017, 29, 04016290. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.; Chen, W.; Yu, L.; Li, W.; Yu, H. Calcium carbonate precipitation and crystal morphology induced by microbial carbonic anhydrase and other biological factors. Process Biochem. 2010, 45, 1017–1021. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, B.; Ge, Q.; Yang, X. Calcium carbonate precipitation induced by calcifying bacteria in culture experiments: Influence of the medium on morphology and mineralogy. Int. Biodeterior. Biodegrad. 2018, 134, 83–92. [Google Scholar] [CrossRef]
- Daskalakis, M.I.; Rigas, F.; Bakolas, A.; Magoulas, A.; Kotoulas, G.; Katsikis, I.; Karageorgis, A.P.; Mavridou, A. Vaterite bio-precipitation induced by Bacillus pumilus isolated from a solutional cave in Paiania, Athens, Greece. Int. Biodeterior. Biodegrad. 2015, 99, 73–84. [Google Scholar] [CrossRef]
- Li, W.; Chen, W.S.; Zhou, P.P.; Cao, L.; Yu, L.J. Influence of initial pH on the precipitation and crystal morphology of calcium carbonate induced by microbial carbonic anhydrase. Colloids Surf. B Biointerfaces 2013, 102, 281–287. [Google Scholar] [CrossRef]
- Han, Z.; Meng, R.; Yan, H.; Zhao, H.; Han, M.; Zhao, Y.; Sun, B.; Sun, Y.; Wang, J.; Zhuang, D.; et al. Calcium carbonate precipitation by Synechocystis sp. PCC6803 at different Mg/Ca molar ratios under the laboratory condition. Carbonates Evaporites 2016, 32, 561–575. [Google Scholar] [CrossRef]
- Bao, R.T.; Li, J.H.; Li, L.; Cutright, T.J.; Chen, L.; Zhu, J.H.; Tao, J.L. Effect of Microbial-Induced Calcite Precipitation on Surface Erosion and Scour of Granular Soils Proof of Concept. Transp. Res. Rec. 2017, 2657, 10–18. [Google Scholar] [CrossRef]
- Maleki, M.; Ebrahimi, S.; Asadzadeh, F.; Tabrizi, M.E. Performance of microbial-induced carbonate precipitation on wind erosion control of sandy soil. Int. J. Environ. Sci. Technol. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Stabnikov, V.; Jian, C.; Myo, A.N.; Ivanov, V. Immobilization of Sand Dust and Associated Pollutants Using Bioaggregation. Water Air Soil Pollut. 2013, 224, 1–9. [Google Scholar] [CrossRef]
- Tian, K.L.; Wu, Y.Y.; Zhang, H.L.; Li, D.; Nie, K.Y.; Zhang, S.C. Increasing wind erosion resistance of aeolian sandy soil by microbially induced calcium carbonate precipitation. Land Degrad. Dev. 2018, 29, 4271–4281. [Google Scholar] [CrossRef]
- Qian, C.X.; Pan, Q.F.; Wang, R.X. Cementation of sand grains based on carbonate precipitation induced by microorganism. Sci. China 2010, 53, 2198–2206. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Zhang, D. Remediation of copper-contaminated soil by Kocuria flava CR1, based on microbially induced calcite precipitation. Ecol. Eng. 2011, 37, 1601–1605. [Google Scholar] [CrossRef]
- Kang, C.H.; Shin, Y.; Anbu, P.; Nam, I.H.; So, J.S. Biosequestration of copper by bacteria isolated from an abandoned mine by using microbially induced calcite precipitation. J. Gen. Appl. Microbiol. 2016, 62, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.G.; Wang, K.; Chu, J. Properties of biocemented, fiber reinforced sand. Constr. Build. Mater. 2016, 120, 623–629. [Google Scholar] [CrossRef]
- Heinemann, F.; Gummich, M.; Radmacher, M.; Fritz, M. Modification of CaCO3 precipitation rates by water-soluble nacre proteins. Mater. Sci. Eng. C 2011, 31, 99–105. [Google Scholar] [CrossRef]
- Skuce, R.L.; Tobler, D.J.; Maclaren, I.; Lee, M.R.; Phoenix, V.R. Immobilization of nanoparticles by occlusion into microbial calcite. Chem. Geol. 2017, 453, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Cheng, X.; Guo, H.; Yang, Z. Enhancement of the Yield and Strength of Microbially-induced Carbonate Precipitation by Optimum Cultivation and Grouting Measures for Civil Engineering Applications. J. Pure Appl. Microbiol. 2013, 7, 631–638. [Google Scholar]
- Yang, Y.A.; Jian, C.A.; Bc, A.; Hl, B.; Liang, C.C. Biocementation of soil using non-sterile enriched urease-producing bacteria from activated sludge. J. Clean. Prod. 2020, 262, 121315. [Google Scholar] [CrossRef]
- Pei, J.; Xing, X.; Xia, B.; Wang, Z.; Luo, Z. Study on the Adsorption Behavior between an Imidazolium Ionic Liquid and Na-Montmorillonite. Molecules 2019, 24, 1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandran, S.K.; Ramakrishnan, V.; Bang, S.S. Remediation of Concrete Using Microorganisms. ACI Mater. J. 2001, 98, 3–9. [Google Scholar]
- Zhao, Q.; Li, L.; Li, C.; Li, M.D.; Amini, F.; Zhang, H.Z. Factors Affecting Improvement of Engineering Properties of MICP-Treated Soil Catalyzed by Bacteria and Urease. J. Mater. Civ. Eng. 2014, 26, 04014094. [Google Scholar] [CrossRef]
- Bundeleva, I.A.; Shirokova, L.S.; Bénézeth, P.; Pokrovsky, O.S.; Kompantseva, E.I.; Balor, S. Zeta potential of anoxygenic phototrophic bacteria and Ca adsorption at the cell surface: Possible implications for cell protection from CaCO3 precipitation in alkaline solutions. J. Colloid Interface Sci. 2011, 360, 100–109. [Google Scholar] [CrossRef]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Takahashi, C.; Shirai, T.; Fuji, M. Study on intercalation of ionic liquid into montmorillonite and its property evaluation—Science Direct. Mater. Chem. Phys. 2012, 135, 681–686. [Google Scholar] [CrossRef]
- Kawano, M.; Hwang, J. Roles of microbial acidic polysaccharides in precipitation rate and polymorph of calcium carbonate minerals. Appl. Clay Sci. 2011, 51, 484–490. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, Q.; Chen, S.; Yu, Z. Adsorption of the insecticidal protein of Bacillus thuringiensis on montmorillonite, kaolinite, silica, goethite and Red soil. Appl. Clay Sci. 2005, 30, 87–93. [Google Scholar] [CrossRef]
- Bains, A.; Dhami, N.K.; Mukherjee, A.; Reddy, M.S. Influence of Exopolymeric Materials on Bacterially Induced Mineralization of Carbonates. Appl. Biochem. Biotechnol. 2015, 175, 3531–3541. [Google Scholar] [CrossRef]
- Cuthbert, M.O.; Riley, M.S.; Handleysidhu, S.; Renshaw, J.C.; Tobler, D.J.; Phoenix, V.R.; Mackay, R. Controls on the rate of ureolysis and the morphology of carbonate precipitated by S. Pasteurii biofilms and limits due to bacterial encapsulation. Ecol. Eng. 2012, 41, 32–40. [Google Scholar] [CrossRef]
- Gat, D.; Tsesarsky, M.; Shamir, D.; Ronen, Z. Accelerated microbial-induced CaCO3 precipitation in a defined co-culture of ureolytic and non-ureolytic bacteria. Biogeosci. Discuss. 2014, 10, 17249–17273. [Google Scholar]
- Yang, Y.; Wang, S.; Liu, J.; Xu, Y.; Zhou, X. Adsorption of Lysine on Na-Montmorillonite and Competition with Ca2+: A Combined XRD and ATR-FTIR Study. Langmuir 2016, 32, 4746–4754. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R. In situ soil cementation with ureolytic bacteria by surface percolation. Ecol. Eng. 2012, 42, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Harkes, M.P.; Paassen, L.A.V.; Booster, J.L.; Whiffin, V.S.; Loosdrecht, M.C.M.V. Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol. Eng. 2010, 36, 112–117. [Google Scholar] [CrossRef]
- Belbel, A.; Kharroubi, M.; Janot, J.M.; Abdessamad, M.; Haouzi, A.; Lefkaier, I.K.; Balme, S. Preparation and characterization of homoionic montmorillonite modified with ionic liquid: Application in dye adsorption. Colloid Surf. A 2018, 558, 219–227. [Google Scholar] [CrossRef]
- Mignon, P.; Ugliengo, P.; Sodupe, M. Theoretical Study of the Adsorption of RNA/DNA Bases on the External Surfaces of Na-Montmorillonite. J. Phys. Chem. C 2009, 113, 13741–13749. [Google Scholar] [CrossRef]
- Rong, X.; Huang, Q.; He, X.; Chen, H.; Cai, P.; Liang, W. Interaction of Pseudomonas putida with kaolinite and montmorillonite: A combination study by equilibrium adsorption, ITC, SEM and FTIR. Colloids Surf. B Biointerfaces 2008, 64, 49–55. [Google Scholar] [CrossRef]
- Bachmeier, K.L.; Williams, A.E.; Warmington, J.R.; Bang, S.S. Urease activity in microbiologically-induced calcite precipitation. J. Biotechnol. 2002, 93, 171–181. [Google Scholar] [CrossRef]
- Seifan, M.; Samani, A.K.; Berenjian, A. Induced calcium carbonate precipitation using Bacillus species. Appl. Microbiol. Biotechnol. 2016, 100, 9895. [Google Scholar] [CrossRef] [Green Version]
- Al Qabany, A.; Soga, K. Effect of chemical treatment used in MICP on engineering properties of cemented soils. Geotechnique 2013, 63, 331–339. [Google Scholar] [CrossRef]
- Whiffin, V.S.; Paassen, L.A.V.; Harkes, M.P. Microbial Carbonate Precipitation as a Soil Improvement Technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Vucak, M.; Pons, M.N.; Peric, J.; Vivier, H. Effect of precipitation conditions on the morphology of calcium carbonate: Quantification of crystal shapes using image analysis. Powder Technol. 1998, 97, 1–5. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, G.; Jia, C.; Wang, G.; Yu, P.; Zhang, H. Role of Na-Montmorillonite on Microbially Induced Calcium Carbonate Precipitation. Molecules 2021, 26, 6211. https://doi.org/10.3390/molecules26206211
Tang G, Jia C, Wang G, Yu P, Zhang H. Role of Na-Montmorillonite on Microbially Induced Calcium Carbonate Precipitation. Molecules. 2021; 26(20):6211. https://doi.org/10.3390/molecules26206211
Chicago/Turabian StyleTang, Guowang, Cangqin Jia, Guihe Wang, Peizhi Yu, and Haonan Zhang. 2021. "Role of Na-Montmorillonite on Microbially Induced Calcium Carbonate Precipitation" Molecules 26, no. 20: 6211. https://doi.org/10.3390/molecules26206211




