Choline Chloride-Based DES as Solvents/Catalysts/Chemical Donors in Pharmaceutical Synthesis
Abstract
:1. Introduction
2. Structure and Properties of Choline Chloride-Based DES
3. Choline-Based DES as Solvents/Catalysts in Pharmaceutical Synthesis
3.1. Choline-Based DES as Solvents
3.2. Choline-Based DES as Solvents/Catalysts
3.3. Choline-Based DES as Chemical Donors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joshi, D.R.; Adhikari, N. An overview on common organic solvents and their toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and sustainable solvents in chemical processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.T.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kollau, L.J.B.M.; Vis, M.; van den Bruinhorst, A.; Esteves, A.C.C.; Tuinier, R. Quantification of the liquid window of deep eutectic solvents. Chem. Commun. 2018, 54, 13351–13354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, G.; Atilhan, M.; Aparicio, S. An approach for the rationalization of melting temperature for deep eutectic solvents from DFT. Chem. Phys. Lett. 2015, 634, 151–155. [Google Scholar] [CrossRef]
- D’Agostino, C.; Harris, R.C.; Abbott, A.P.; Gladden, L.F.; Mantle, M.D. Molecular motion and ion diffusion in choline chloride based deep eutectic solvents studied by 1H pulsed field gradient NMR spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 21383–21391. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Gray, S. Design of improved Deep Eutectic Solvents using hole theory. ChemPhysChem 2006, 7, 803–806. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Abranches, D.O.; Martins, M.A.R.; Silva, L.P.; Schaeffer, N.; Pinho, S.P.; Coutinho, J.A.P. Phenolic hydrogen bond donors in the formation of non-ionic deep eutectic solvents: The quest for type V DES. Chem. Commun. 2019, 55, 10253–10256. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents − Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Espino, M.; de los Ángeles Fernández, M.; Gomez, F.J.V.; Silva, M.F. Natural designer solvents for greening analytical chemistry. Trends Analyt. Chem. 2016, 76, 126–136. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Romaní, A.; Rocchetti, G.; Lorenzo, J.M. Smart advanced solvents for bioactive compounds recovery from agri-food by-products: A review. Trends Food Sci. Technol. 2020, 101, 182–197. [Google Scholar] [CrossRef]
- Kovacs, A.; Neyts, E.C.; Cornet, I.; Wijnants, M.; Billen, P. Modeling the physicochemical properties of Natural Deep Eutectic Solvents. ChemSusChem 2020, 13, 3789–3804. [Google Scholar] [CrossRef] [PubMed]
- de Morais, P.; Gonçalves, F.; Coutinho, J.A.P.; Ventura, S.P.M. Ecotoxicity of cholinium-based Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2015, 3, 3398–3404. [Google Scholar] [CrossRef]
- Torregrosa-Crespo, J.; Marset, X.; Guillena, G.; Ramón, D.J.; Martínez-Espinosa, R.M. New guidelines for testing ‘‘Deep eutectic solvents” toxicity and their effects on the environment and living beings. Sci. Total Environ. 2020, 704, 135382. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep Eutectic Solvents: The organic reaction medium of the century. Eur. J. Org. Chem. 2016, 4, 612–632. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, M.S.; Zhang, Y. Sketching neoteric solvents for boosting drugs bioavailability. J. Control. Release 2019, 311–312, 225–232. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; McElroy, C.R.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process 2016, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Longo, L.S., Jr.; Craveiro, M.V. Deep Eutectic Solvents as unconventional media for multicomponent reactions. J. Braz. Chem. Soc. 2018, 29, 1999–2025. [Google Scholar] [CrossRef]
- Zeisel, S.H. A brief history of choline. Ann. Nutr. Metab. 2012, 61, 254–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radosevic, K.; Bubalo, M.C.; Srcek, V.G.; Grgas, D.; Dragicevic, T.L.; Redovnikovic, I.R. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.T.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Liu, X.; Fu, N.J.; Zhang, Q.G.; Cai, S.F.; Wang, Q.; Han, D.D.; Tang, B.K. Green tailoring with water of choline chloride deep eutectic solvents for the extraction of polyphenols from palm samples. J. Chromatogr. Sci. 2015, 57, 272–278. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-Transition-Temperature Mixtures (LTTMs): A new generation of designer solvents. Angew. Chem. Int. Ed. 2013, 52, 3074–3085. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Zhao, Y.; Wang, J.; Yu, Z. Insights into the hydrogen bond interactions in Deep Eutectic Solvents composed of choline chloride and polyols. ACS Sustain. Chem. Eng. 2019, 7, 7760–7767. [Google Scholar] [CrossRef]
- Naseem, Z.; Shehzad, R.A.; Ihsan, A.; Iqbal, J.; Zahid, M.; Pervaiz, A.; Sarwari, G. Theoretical investigation of supramolecular hydrogen-bonded choline chloride-based deep eutectic solvents using density functional theory. Chem. Phys. Lett. 2021, 769, 138427. [Google Scholar] [CrossRef]
- Maugeri, Z.; Domínguez deMaría, P. Novel choline chloride based deep eutectic solvents with renewable hydrogen bond donors: Levulinic acid and sugar-based polyols. RSC Adv. 2012, 2, 421–425. [Google Scholar] [CrossRef]
- Perkins, S.L.; Painter, P.; Colina, C.M. Experimental and computational studies of choline chloride-based deep eutectic solvents. J. Chem. Eng. Data 2014, 59, 3652–3662. [Google Scholar] [CrossRef]
- Biernacki, K.; Souza, H.K.S.; Almeida, C.M.R.; Magalhães, A.L.; Gonçalves, M.P. Physicochemical properties of choline chloride-based Deep Eutectic Solvents with polyols: An experimental and theoretical investigation. ACS Sustain. Chem. Eng. 2020, 8, 18712–18728. [Google Scholar] [CrossRef]
- Ünlü, A.E.; Arıkaya, A.; Takaç, S. Use of deep eutectic solvents as catalyst: A mini-review. Green Process Synth. 2019, 8, 355–372. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Wu, X.; Li, G. Theoretical study on the structures and properties of mixtures of urea and choline chloride. J. Mol. Model. 2013, 19, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, C.R.; Matthews, R.P.; Welton, T.; Hunt, P.A. Doubly ionic hydrogen bond interactions within the choline chloride-urea deep eutectic solvent. Phys. Chem. Chem. Phys. 2016, 18, 18145–18160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. Liquid structure of the choline chloride-urea deep eutectic solvent (reline) from neutron diffraction and atomistic modelling. Green Chem. 2016, 18, 2736–2744. [Google Scholar] [CrossRef] [Green Version]
- Suo, L.; Borodin, O.; Sun, W.; Fan, X.; Yang, C.; Wang, F.; Gao, T.; Ma, Z.; Schroeder, M.; von Cresce, A.; et al. Advanced highvoltage aqueous lithium-ion battery enabled by “water-in-bisalt” electrolyte. Angew. Chem. Int. Ed. 2016, 55, 7136–7141. [Google Scholar] [CrossRef] [PubMed]
- Triolo, A.; Lo Celso, F.; Brehm, M.; Di Lisio, V.; Russina, O. Liquid structure of a choline chloride-water natural deep eutectic solvent: A molecular dynamics characterization. J. Mol. Liq. 2021, 331, 115750. [Google Scholar] [CrossRef]
- Cicci, A.; Sed, G.; Bravi, M. Potential of choline chloride—based Natural Deep Eutectic Solvents (NaDES) in the extraction of microalgal metabolites. Chem. Eng. Trans. 2017, 57, 61–66. [Google Scholar]
- Mannu, A.; Cardano, F.; Fin, A.; Baldino, S.; Prandi, C. Choline chloride-based ternary deep band gap systems. J. Mol. Liq. 2021, 330, 115717. [Google Scholar] [CrossRef]
- Nirwan, S.; Chahal, V.; Kakkar, R. Thiazolidinones: Synthesis, reactivity, and their biological applications. J. Heterocycl. Chem. 2019, 56, 1239–1253. [Google Scholar] [CrossRef]
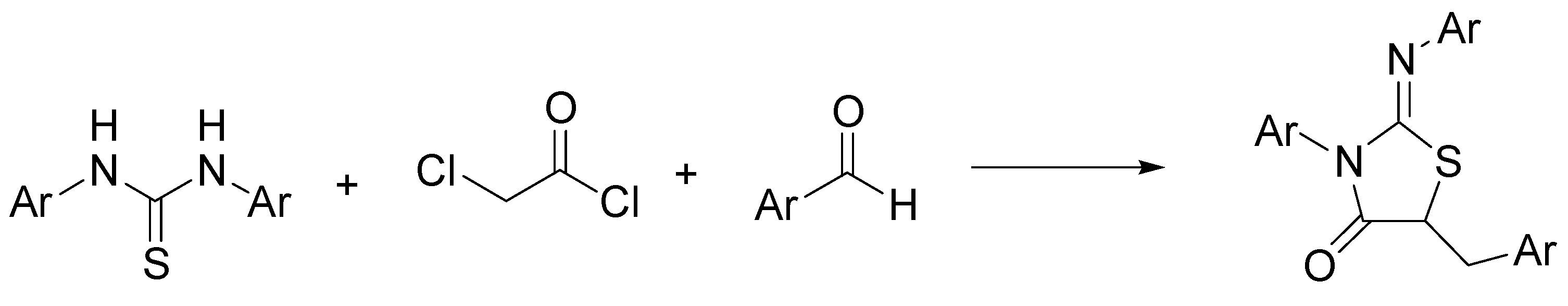
- Ashraf, S.; Saeed, A.; Moon, S.-H.; Flörke, U.; Kim, S.H.; Ashraf, Z.; Yaseen, M.; Muhammad, L. Design, synthesis and biological evaluation of 2-(naphthoyl) iminothiazolidin-4-ones as potential anticancer agents. ChemistrySelect 2020, 5, 3965–3970. [Google Scholar] [CrossRef]
- Mobinikhaledi, A.; Amiri, A.K. Natural eutectic salts catalyzed one-pot synthesis of 5-arylidene-2-imino-4-thiazolidinones. Res. Chem. Intermed. 2013, 39, 1491–1498. [Google Scholar] [CrossRef]
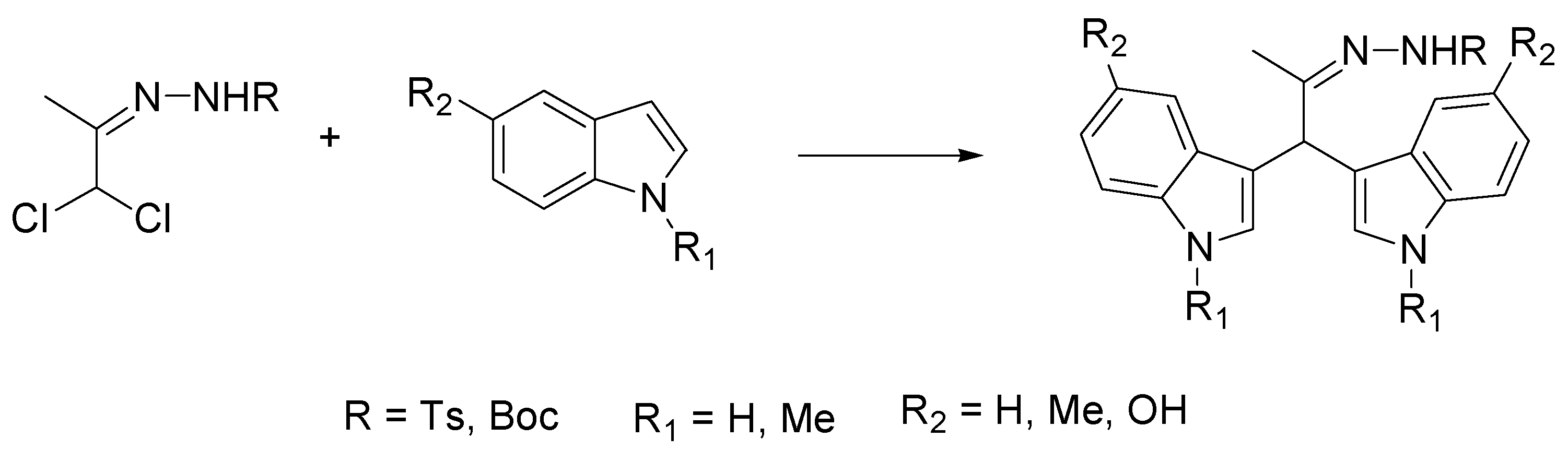
- Shiri, M.; Zolfigol, M.A.; Kruger, H.G.; Tanbakouchian, Z. Bis- and trisindolylmethanes (BIMs and TIMs). Chem. Rev. 2010, 110, 2250–2293. [Google Scholar] [CrossRef] [PubMed]
- Vanderlaag, K.; Su, Y.; Frankel, A.E.; Grage, H.; Smith, R., 3rd; Khan, S.; Safe, S. 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit proliferation of estrogen receptor-negative breast cancer cells by activation of multiple pathways. Breast Cancer Res. Treat. 2008, 109, 273–283. [Google Scholar] [CrossRef]
- Singh, A.; Kaur, G.; Bubun Banerjee, B. Recent developments on the synthesis of biologically significant bis/tris(indolyl)methanes under various reaction conditions: A review. Curr. Org. Chem. 2020, 24, 583–621. [Google Scholar] [CrossRef]
- Grosso, C.; Brigas, A.; de los Santos, J.M.; Palacios, F.; Lemos, A.; Pinho e Melo, T.M.V.D. Natural deep eutectic solvents in the hetero-Diels–Alder approach to bis(indolyl)methanes. Bioorg. Med. Chem. 2017, 25, 1122–1131. [Google Scholar] [CrossRef]
- Broggini, G.; Borsini, E.; Piarulli, U. Science of Synthesis, Cross Coupling and Heck-Type Reactions; Molander, G.A., Wolfe, J.P., Larhed, M., Eds.; Thieme: Stuttgart, Germany, 2013; Volume 3, pp. 521–583. [Google Scholar]
- Khose, V.N.; John, M.E.; Pandey, A.D.; Karnik, A.V. Chiral benzimidazoles and their applications in stereodiscrimination processes. Tetrahedron Asymmetry 2017, 28, 1233–1289. [Google Scholar] [CrossRef]
- Ñíguez, D.R.; Khazaeli, P.; Alonso, D.A.; Guillena, G. Deep eutectic mixtures as reaction media for the enantioselective organocatalyzed-amination of 1,3-dicarbonyl compounds. Catalysts 2018, 8, 217. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.-X.; Wang, H.; Li, X.-K.; Dong, S.-N.; Liu, W.-W.; Gong, Q.; Wang, T.-D.-Y.; Tang, Y.; Zhu, J.; Li, J.; et al. Discovery of novel propargylamine-modified 4-aminoalkyl imidazole substituted pyrimidinylthiourea derivatives as multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2018, 143, 33–47. [Google Scholar] [CrossRef]
- Yogev-Falach, M.; Amit, T.; Bar-Am, O.; Youdim, M.B.H. The importance of propargylamine moiety in the anti-Parkinson drug rasagiline and its derivatives in MAPK-dependent amyloid precursor protein processing. FASEB J. 2003, 17, 2325–2327. [Google Scholar] [CrossRef] [Green Version]
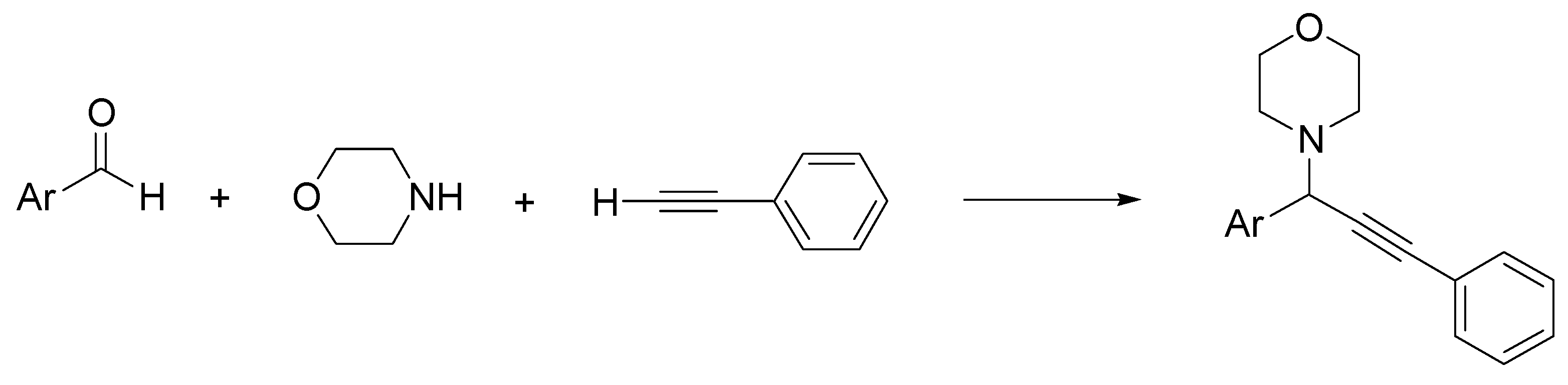
- Lauder, K.; Toscani, A.; Scalacci, N.; Castagnolo, D. Synthesis and reactivity of propargylamines in organic chemistry. Chem. Rev. 2017, 117, 14091–14200. [Google Scholar] [CrossRef] [Green Version]
- Abtahi, B.; Tavakol, H. Choline chloride-urea deep eutectic solvent as an efficient media for the synthesis of propargylamines via organocuprate intermediate. Appl. Organomet. Chem. 2020, 34, e5895. [Google Scholar] [CrossRef]
- Matralis, A.N.; Katselou, M.G.; Nikitakis, A.; Kourounakis, A.P. Novel benzoxazine and benzothiazine derivatives as multifunctional antihyperlipidemic agents. J. Med. Chem. 2011, 54, 5583–5591. [Google Scholar] [CrossRef] [PubMed]
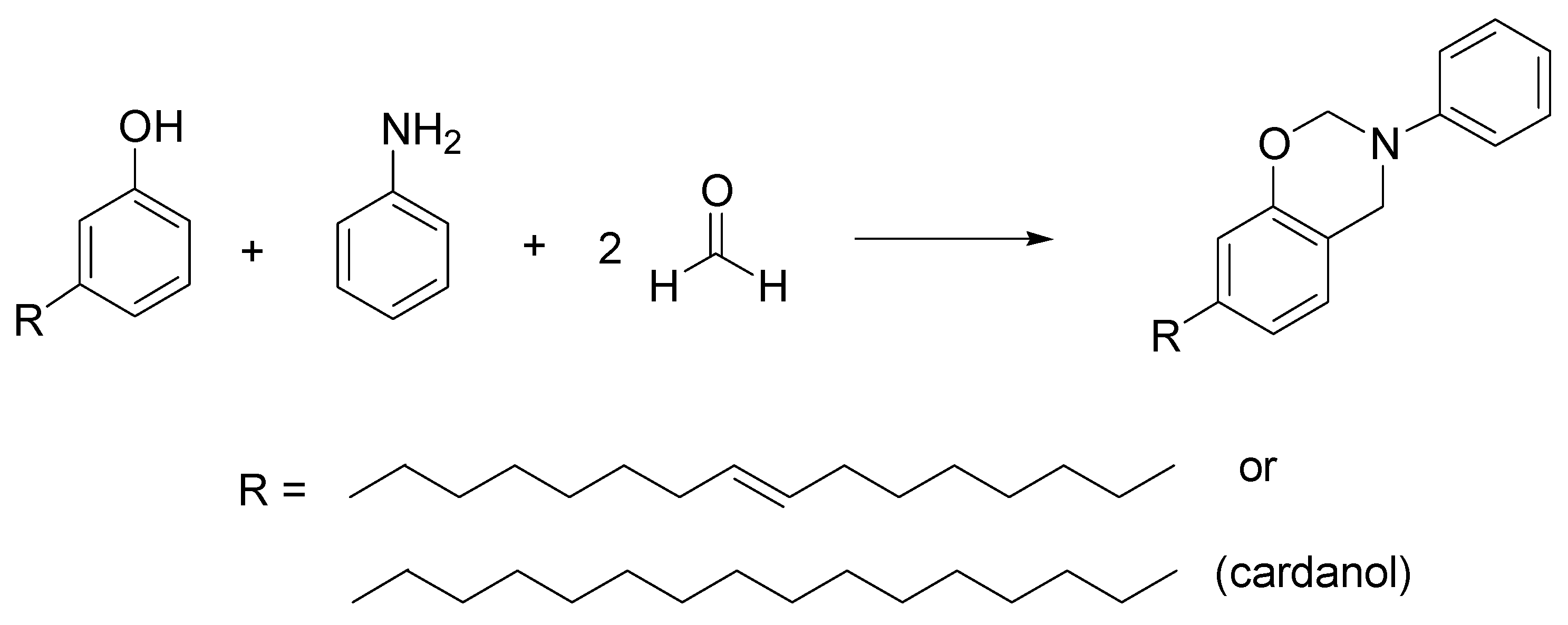
- Andrea Minigher, A.; Elena Benedetti, E.; De Giacomo, O.; Campaner, P.; Aroulmoji, V. Synthesis and characterization of novel cardanol based benzoxazines. Nat. Prod. Commun. 2009, 4, 521–528. [Google Scholar]
- Behalo, M.S.; Bloise, E.; Mele, G.; Salomone, A.; Messa, F.; Carbone, L.; Mazzetto, S.E.; Lomonaco, D. Bio-based benzoxazines synthesized in a deep eutectic solvent: A greener approach toward vesicular nanosystems. J. Heterocycl. Chem. 2020, 57, 768–773. [Google Scholar] [CrossRef]
- Günther, A.; Pełech, R. Bio-active pyridinium salts: A mini-review on properties and selected reactions. Mini-Rev. Org. Chem. 2019, 16, 610–616. [Google Scholar] [CrossRef]
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
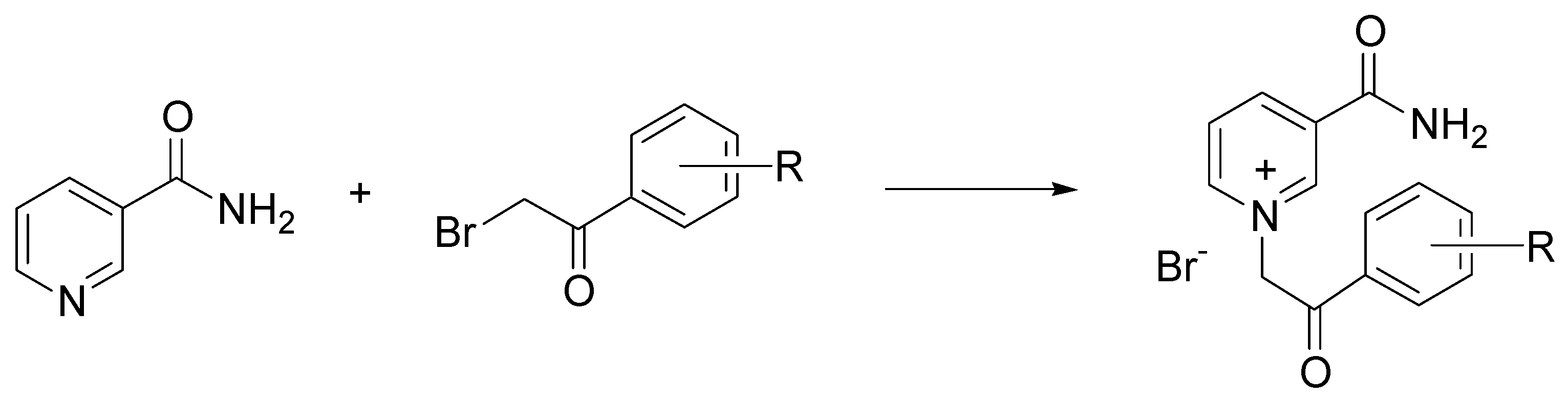
- Bušić, V.; Roca, S.; Vikić-Topić, D.; Vrandečić, K.; Ćosić, J.; Molnar, M.; Gašo-Sokač, D. Eco-friendly quaternization of nicotinamide and 2-bromoacetophenones in deep eutectic solvents. Antifungal activity of the products. Environ. Chem. Lett. 2020, 18, 889–894. [Google Scholar] [CrossRef]
- Popova, A.; Bondarenko, S.P.; Frasinyuk, M.S. Aurones: Synthesis and properties. Chem. Heterocycl. Comp. 2019, 55, 285–299. [Google Scholar] [CrossRef]
- Hawkins, I.; Handy, S.T. Synthesis of aurones under neutral conditions using a deep eutectic solvent. Tetrahedron 2013, 69, 9200–9204. [Google Scholar] [CrossRef]
- Dlugosz, A.; Janecka, A. Novobiocin analogs as potential anticancer agents. Mini Rev. Med. Chem. 2017, 17, 728–733. [Google Scholar] [CrossRef]
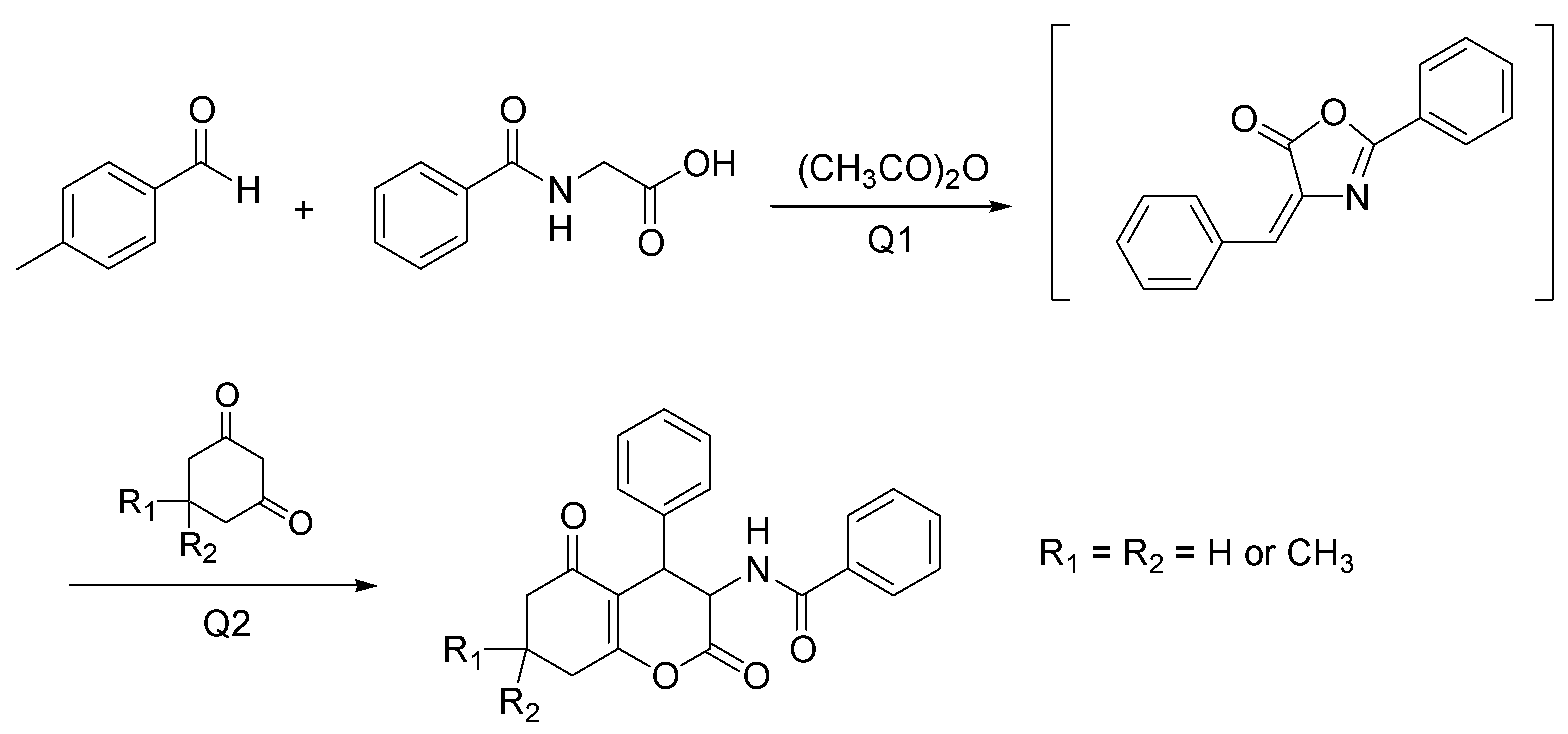
- Zamani, P.; Khosropour, A.R. A combination of natural deep eutectic solvents and microflow technology: A sustainable innovation for the tandem synthesis of 3-aminohexahydrocoumarins. Green Chem. 2016, 18, 6450–6455. [Google Scholar] [CrossRef]
- Conti, M. Cyclopentenone: A special moiety for anticancer drug design. Anticancer Drugs 2006, 17, 1017–1022. [Google Scholar] [CrossRef]
- Procopio, A.; Costanzo, P.; Curini, M.; Nardi, M.; Oliverio, M.; Sindona, G. Erbium(III) chloride in ethyl lactate as a smart ecofriendly system for efficient and rapid stereoselective synthesis of trans-4,5-diaminocyclopent-2-enones. ACS Sustain. Chem. Eng. 2013, 1, 541–544. [Google Scholar] [CrossRef]
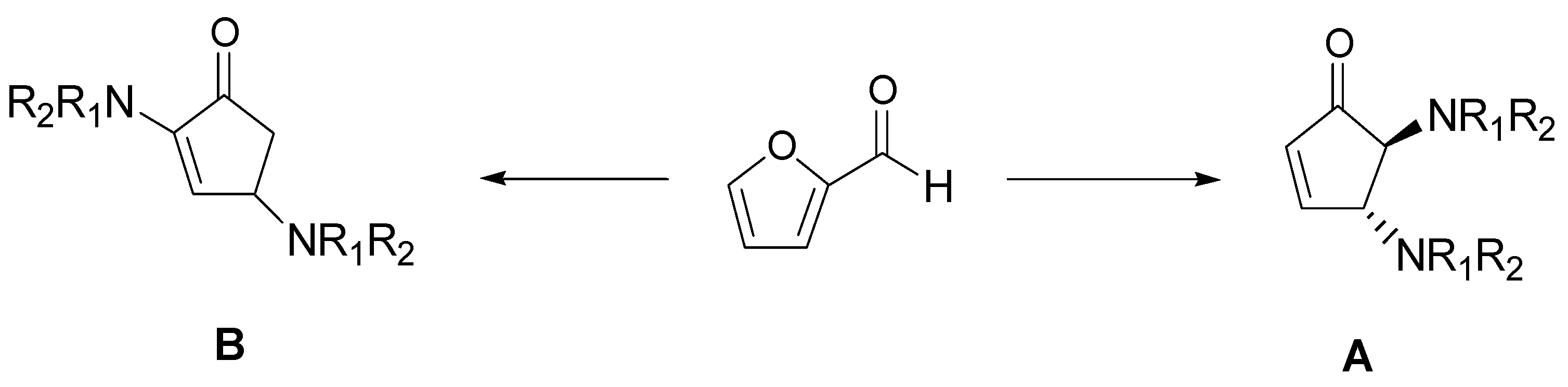
- Di Gioia, M.L.; Nardi, M.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Procopio, A. Biorenewable Deep Eutectic Solvent for selective and scalable conversion of furfural into cyclopentenone derivatives. Molecules 2018, 23, 1891. [Google Scholar] [CrossRef] [Green Version]
- Bora, D.; Kaushal, A.; Shankaraiah, N. Anticancer potential of spirocompounds in medicinal chemistry: A pentennial expedition. Eur. J. Med. Chem. 2021, 215, 113263. [Google Scholar] [CrossRef]
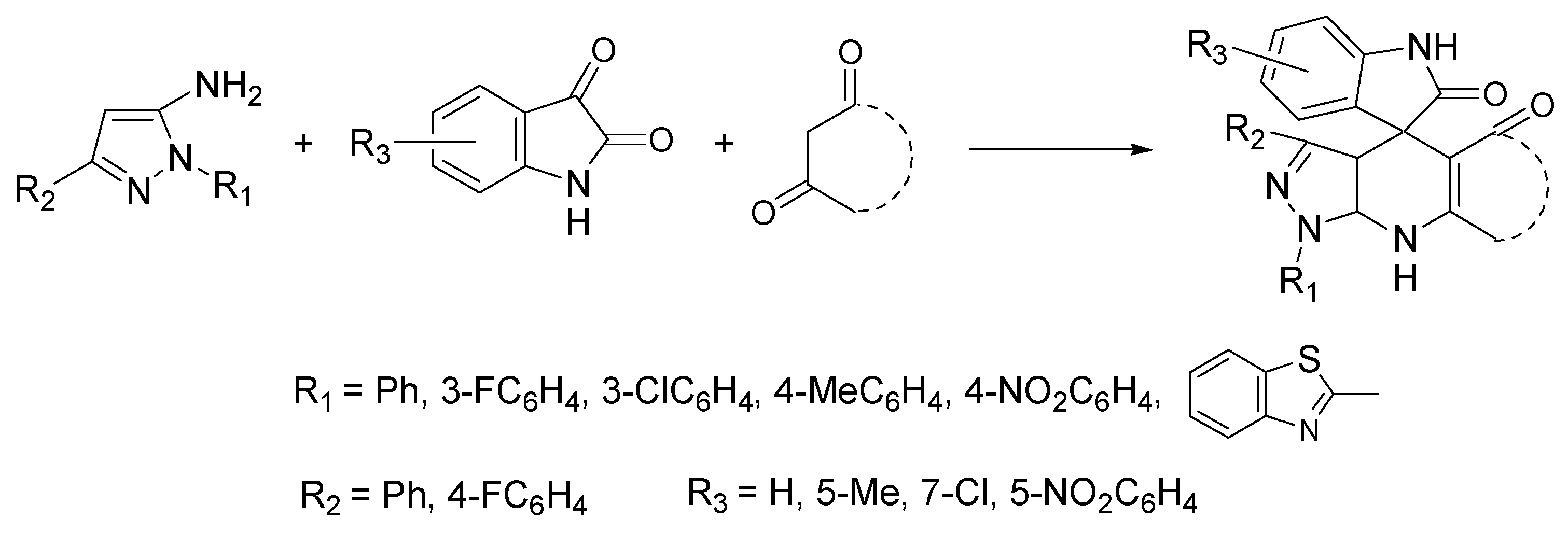
- Eldehna, W.M.; EL-Naggar, D.H.; Hamed, A.R.; Ibrahim, H.S.; Ghabbour, H.A.; Abdel-Aziz, H.A. One-pot three-component synthesis of novel spirooxindoles with potential cytotoxic activity against triple-negative breast cancer MDA-MB-231 cells. J. Enzyme Inhib. Med. Chem. 2018, 33, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.-H.; Chen, M.-N.; Hao, Y.; Jiang, X.; Zhou, X.-L.; Zhang, Z.-H. Choline chloride and lactic acid: A natural deep eutectic solvent for onepot rapid construction of spiro[indoline-3,4′-pyrazolo[3,4-b]pyridines]. J. Mol. Liq. 2019, 278, 124–129. [Google Scholar] [CrossRef]
- Lin, R.; Chiu, G.; Yu, Y.; Connolly, P.J.; Li, S.; Lu, Y.; Adams, M.; Fuentes-Pesquera, A.R.; Emanuel, S.L.; Greenberger, L.M. Design, synthesis, and evaluation of 3,4-disubstituted pyrazole analogues as anti-tumor CDK inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 4557–4561. [Google Scholar] [CrossRef]
- Kalla, R.M.N.; Kim, I. Highly efficient synthesis of pyrazolylphosphonate derivatives in biocompatible deep eutectic solvent. Mol. Catal. 2019, 473, 110396. [Google Scholar] [CrossRef]
- Singh, R.; Saini, M.R.; Bhardwaj, D.; Singh, A. An expedient synthesis of new iminothiazolidinone grafted dispiro-pyrrolidineoxindole/indeno hybrids via a multicomponent [3+2] cycloaddition reaction in a deep eutectic solvent. New J. Chem. 2020, 44, 7923–7931. [Google Scholar] [CrossRef]
- del Monte, F.; Carriazo, D.; Serrano, M.C.; Gutiérrez, M.C.; Ferrer, M.L. Deep eutectic solvents in polymerizations: A greener alternative to conventional syntheses. ChemSusChem 2014, 7, 999–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradeepkumar, P.; Elgorban, A.M.; Bahkali, A.H.; Rajan, M. Natural solvent-assisted synthesis of amphiphilic co-polymeric nanomicelles for prolonged release of camptothecin delivery. New J. Chem. 2018, 42, 10366–10375. [Google Scholar] [CrossRef]
- Gholap, S.S. Pyrrole: An emerging scaffold for construction of valuable therapeutic agents. Eur. J. Med. Chem. 2016, 110, 13–31. [Google Scholar] [CrossRef]
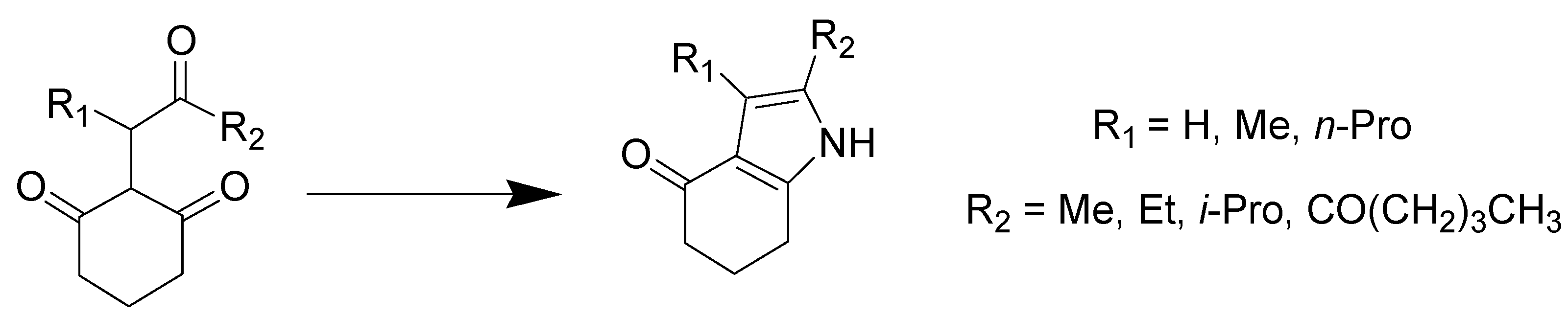
- Hu, L.; Luo, J.; Lu, D.; Tang, Q. Urea decomposition: Efficient synthesis of pyrroles using the deep eutectic solvent choline chloride/urea. Tetrahedron Lett. 2018, 59, 1698–1701. [Google Scholar] [CrossRef]
- Jiang, R.-W.; Lau, K.-M.; Hon, P.-M.; Mak, T.; Woo, K.-S.; Fung, K.-P. Chemistry and biological activities of caffeic acid derivatives from salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, A.-D.M.; Durand, E.; Laguerre, M.; Bayrasy, C.; Lecomte, J.; Villeneuve, P.; Jacobsen, C. Antioxidant properties and efficacies of synthesized alkyl caffeates, ferulates, and coumarates. J. Agric. Food Chem. 2014, 62, 12553–12562. [Google Scholar] [CrossRef]
- Wang, X.; Sun, S.; Hou, X. Synthesis of lipophilic caffeoyl alkyl ester using a novel natural deep eutectic solvent. ACS Omega 2020, 5, 11131–11137. [Google Scholar] [CrossRef] [PubMed]

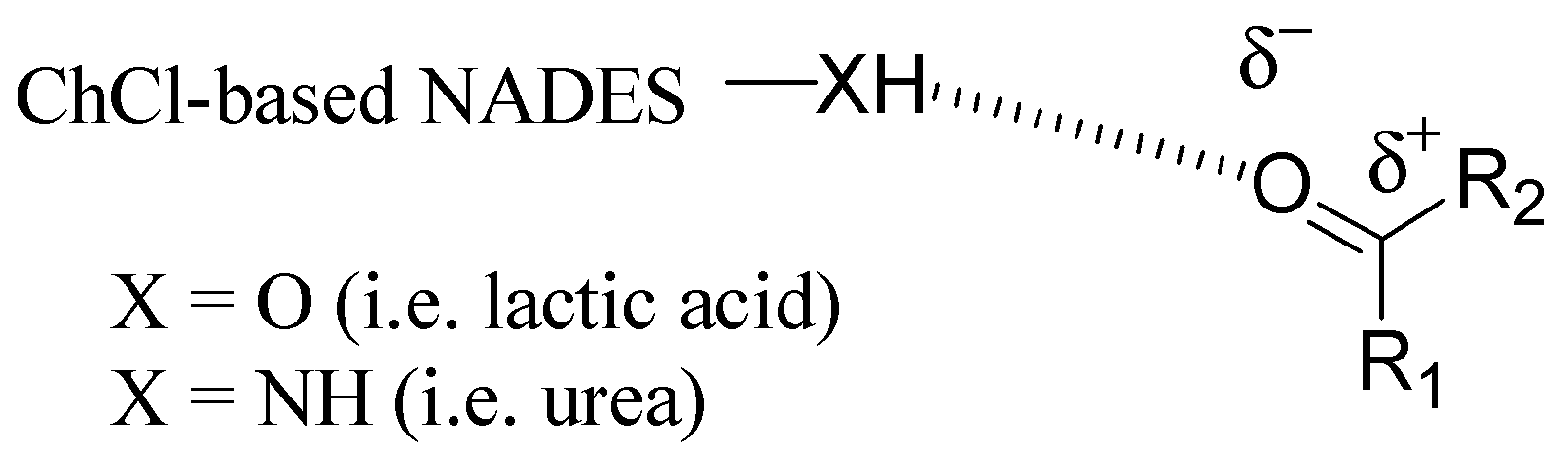
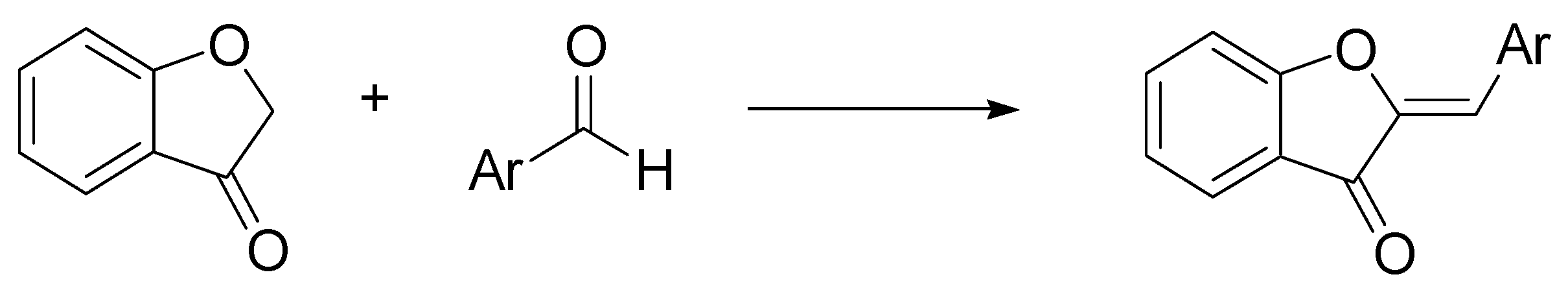
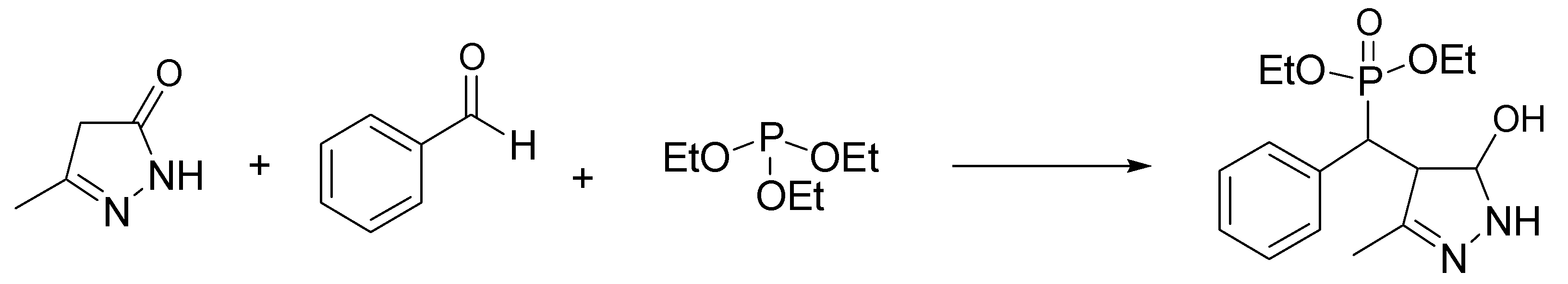



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amoroso, R.; Hollmann, F.; Maccallini, C. Choline Chloride-Based DES as Solvents/Catalysts/Chemical Donors in Pharmaceutical Synthesis. Molecules 2021, 26, 6286. https://doi.org/10.3390/molecules26206286
Amoroso R, Hollmann F, Maccallini C. Choline Chloride-Based DES as Solvents/Catalysts/Chemical Donors in Pharmaceutical Synthesis. Molecules. 2021; 26(20):6286. https://doi.org/10.3390/molecules26206286
Chicago/Turabian StyleAmoroso, Rosa, Frank Hollmann, and Cristina Maccallini. 2021. "Choline Chloride-Based DES as Solvents/Catalysts/Chemical Donors in Pharmaceutical Synthesis" Molecules 26, no. 20: 6286. https://doi.org/10.3390/molecules26206286
APA StyleAmoroso, R., Hollmann, F., & Maccallini, C. (2021). Choline Chloride-Based DES as Solvents/Catalysts/Chemical Donors in Pharmaceutical Synthesis. Molecules, 26(20), 6286. https://doi.org/10.3390/molecules26206286







