Natural Products and Their Derivatives against Human Herpesvirus Infection
Abstract
:1. Introduction
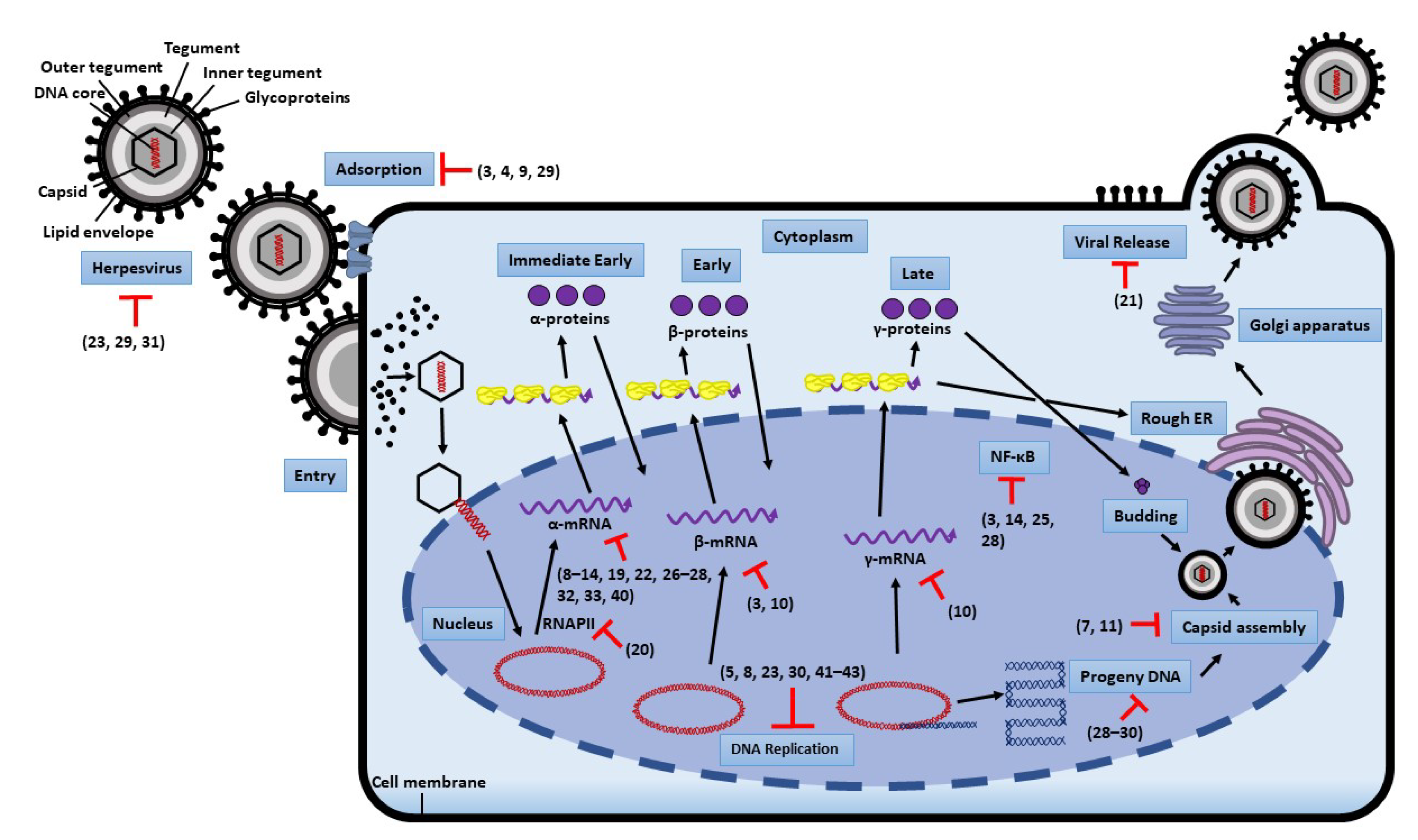
2. Replication Steps of Herpesvirus and Antiviral Targets
3. Natural Product-Derived Molecules with Anti-Herpetic Properties
4. Methodology
5. Compounds Showing Antiherpetic Activities
5.1. Alkaloids with Potential Anti-Herpetic Activities
5.2. Flavonoids with Potential Anti-Herpetic Activities
5.3. Terpenoids with Potential Anti-Herpetic Activities
5.4. Polyphenols with Potential Anti-Herpetic Activities
5.5. Anthraquinones and Anthracyclines with Potential Antiherpetic Activities
5.6. Miscellaneous Compounds with Potential Antiherpetic Activities
6. Mechanisms of Action
6.1. Blocking Viral Adsorption and Entry
6.2. Inhibition of Viral Replication
6.3. Inhibition of NF-κB Activity
6.4. Compounds Affecting Viral Replication by Other Mechanisms
6.5. Efficacy of Natural Compounds In Vivo
7. Summary and Additional Comment
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Connolly, S.A.; Jardetzky, T.S.; Longnecker, R. The structural basis of herpesvirus entry. Nat. Rev. Microbiol. 2021, 19, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Speck, S.H.; Ganem, D. Viral latency and its regulation: Lessons from the gamma-herpesviruses. Cell Host Microbe 2010, 8, 100–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parks, G. Genital Herpes. In Sexually Transmitted Diseases; Nelson, A.L., Woodward, J., Wysocki, S., Eds.; Humana Press: Totowa, NJ, USA, 2006. [Google Scholar]
- Weidner-Glunde, M.; Kruminis-Kaszkiel, E.; Savanagouder, M. Herpesviral latency—common themes. Pathogens 2020, 9, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitley, R.J. Herpesviruses. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Jiang, Y.C.; Feng, H.; Lin, Y.C.; Guo, X.R. New strategies against drug resistance to herpes simplex virus. Int. J. Oral Sci. 2016, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Su, S.H.; Martel-Laferrière, V.; Labbé, A.C.; Snydman, D.R.; Kent, D.; Laverdière, M.; Béliveau, C.; Logvinenko, T.; Cohen, S.; Lachance, S. High incidence of herpes zoster in nonmyeloablative hematopoietic stem cell transplantation. Biol. Blood and Marrow Transpl. 2011, 17, 1012–1017. [Google Scholar] [CrossRef] [Green Version]
- Arvin, A.M. Varicella-zoster virus. Clin. Rev. Microbiol. 1996, 9, 361–381. [Google Scholar] [CrossRef]
- Whitley, R.J. Herpes simplex virus infection. Semin. Pediatr. Infect. Dis. 2002, 13, 6–11. [Google Scholar] [CrossRef]
- Gershon, A.A.; Gershon, M.D.; Breuer, J.; Levin, M.J.; Oaklander, A.L.; Griffiths, P.D. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J. Clin. Virol. 2010, 48, S2–S7. [Google Scholar] [CrossRef] [Green Version]
- Howley, P.; Griffin, D. Herpesviruses: General features. In Desk encyclopedia of human and medical virology; Mahy, B.W., Van Regenmortel, M.H., Eds.; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Nikolich-Žugich, J.; van Lier, R.A. Cytomegalovirus (CMV) research in immune senescence comes of age: Overview of the 6th International workshop on CMV and immunosenescence. Geroscience 2017, 39, 245–249. [Google Scholar] [CrossRef]
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef]
- Meunier, Y. Infectious mononucleosis-like syndrome and gastrointestinal disorders in acute acquired cytomegalovirus infection. Singapore Med. J. 2005, 46, 421. [Google Scholar]
- Humar, A.; Lebranchu, Y.; Vincenti, F.; Blumberg, E.; Punch, J.; Limaye, A.; Abramowicz, D.; Jardine, A.; Voulgari, A.; Ives, J. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am. J. Transpl. 2010, 10, 1228–1237. [Google Scholar] [CrossRef]
- Allmon, A.; Deane, K.; Martin, K.L. Common skin rashes in children. Am. Fam Physician. 2015, 92, 211–216. [Google Scholar]
- Zerr, D.M.; Meier, A.S.; Selke, S.S.; Frenkel, L.M.; Huang, M.L.; Wald, A.; Rhoads, M.P.; Nguy, L.; Bornemann, R.; Morrow, R.A. A population-based study of primary human herpesvirus 6 infection. N. Engl. J. Med. 2005, 352, 768–776. [Google Scholar] [CrossRef]
- Lautenschlager, I.; Razonable, R.R. Human herpesvirus-6 infections in kidney, liver, lung, and heart transplantation. Transpl. Int. 2012, 25, 493–502. [Google Scholar] [CrossRef]
- Reid, G.E.; Lynch III, J.P.; Weigt, S.; Sayah, D.; Belperio, J.A.; Grim, S.A.; Clark, N.M. Herpesvirus respiratory infections in immunocompromised patients: Epidemiology, management, and outcomes. Semin. Respir. Crit. Care Med. 2016, 37, 603. [Google Scholar] [CrossRef]
- Ablashi, D.; Agut, H.; Alvarez-Lafuente, R.; Clark, D.A.; Dewhurst, S.; DiLuca, D.; Flamand, L.; Frenkel, N.; Gallo, R.; Gompels, U.A. Classification of HHV-6A and HHV-6B as distinct viruses. Arch. Virol. 2014, 159, 863–870. [Google Scholar] [CrossRef]
- Ward, K. The natural history and laboratory diagnosis of human herpesviruses-6 and-7 infections in the immunocompetent. J. Clin. Virol. 2005, 32, 183–193. [Google Scholar] [CrossRef]
- Ward, K.; Andrews, N.; Verity, C.; Miller, E.; Ross, E. Human herpesviruses-6 and -7 each cause significant neurological morbidity in Britain and Ireland. Arch. Dis. Childh. 2005, 90, 619–623. [Google Scholar] [CrossRef]
- Cohen, J.I. Chapter 142—Human herpesvirus types 6 and 7 (Exanthem Subitum). In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2015; pp. 1772–1776.e1. [Google Scholar]
- Prichard, M.N.; Whitley, R.J. The development of new therapies for human herpesvirus 6. Curr. Opin. Virol. 2014, 9, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.I. Epstein–Barr virus infection. N. Engl. J. Med. 2000, 343, 481–492. [Google Scholar] [CrossRef]
- Geng, L.; Wang, X. Epstein-Barr virus-associated lymphoproliferative disorders: Experimental and clinical developments. Int. J. Clin. Exp. Med. 2015, 8, 14656. [Google Scholar]
- Ganem, D. KSHV infection and the pathogenesis of Kaposi’s sarcoma. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 273–296. [Google Scholar] [CrossRef]
- Du, M.; Bacon, C.; Isaacson, P. Kaposi sarcoma-associated herpesvirus/human herpesvirus 8 and lymphoproliferative disorders. J. Clin Pathol. 2007, 60, 1350–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, H.C.; Banerjee, S.; Robertson, E.S. The role of gammaherpesviruses in cancer pathogenesis. Pathogens 2016, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Agelidis, A.M.; Shukla, D. Cell entry mechanisms of HSV: What we have learned in recent years. Future Virol. 2015, 10, 1145–1154. [Google Scholar] [CrossRef] [Green Version]
- Sathiyamoorthy, K.; Hu, Y.X.; Möhl, B.S.; Chen, J.; Longnecker, R.; Jardetzky, T.S. Structural basis for Epstein–Barr virus host cell tropism mediated by gp42 and gHgL entry glycoproteins. Nat. Commun. 2016, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Lanchy, J.M.; Ryckman, B.J. Human cytomegalovirus gH/gL/gO promotes the fusion step of entry into all cell types, whereas gH/gL/UL128-131 broadens virus tropism through a distinct mechanism. J. Virol. 2015, 89, 8999–9009. [Google Scholar] [CrossRef] [Green Version]
- Gruffat, H.; Marchione, R.; Manet, E. Herpesvirus late gene expression: A viral-specific pre-initiation complex is key. Front. Microbiol. 2016, 7, 869. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.H.; Luo, Z.; Liu, L.F.; Yan, C.; Yan, C.Y.; Chen, G.D.; Gao, H.; Duan, W.J.; Kurihara, H. Traditional Chinese medicine as a potential source for HSV-1 therapy by acting on virus or the susceptibility of host. Int. J. Mol. Sci. 2018, 19, 3266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teuton, J.R.; Brandt, C.R. Sialic acid on herpes simplex virus type 1 envelope glycoproteins is required for efficient infection of cells. J. Virol. 2007, 81, 3731–3739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, H.J.; Vere Hodge, R.A. Recent developments in anti-herpesvirus drugs. Br. Med. Bull. 2013, 106, 213–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrei, G.; Snoeck, R. Advances and perspectives in the management of Varicella-Zoster virus infections. Molecules 2021, 26, 1132. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.; Masarčíková, R.; Berchová, K. Bioactive natural products with anti-herpes simplex virus properties. J. Pharm. Pharm. 2015, 67, 1325–1336. [Google Scholar] [CrossRef]
- Chattopadhyay, D. Ethnomedicinal antivirals: Scope and opportunity. In Modern Phytomedicine: Turning Medicinal Plants into Drugs; Ahmad, I., Aqil, F., Owais, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 313–339. [Google Scholar]
- Li, T.; Peng, T. Traditional Chinese herbal medicine as a source of molecules with antiviral activity. Antivir. Res. 2013, 97, 1–9. [Google Scholar] [CrossRef]
- Álvarez, D.M.; Castillo, E.; Duarte, L.F.; Arriagada, J.; Corrales, N.; Farías, M.A.; Henríquez, A.; Agurto-Muñoz, C.; González, P.A. Current antivirals and novel botanical molecules interfering with herpes simplex virus infection. Front. Microbiol. 2020, 11, 139. [Google Scholar] [CrossRef]
- Treml, J.; Gazdová, M.; Šmejkal, K.; Šudomová, M.; Kubatka, P.; Hassan, S.T. Natural products-derived chemicals: Breaking barriers to novel anti-HSV drug development. Viruses 2020, 12, 154. [Google Scholar] [CrossRef] [Green Version]
- Garber, A.; Barnard, L.; Pickrell, C. Review of whole plant extracts with activity against herpes simplex viruses in vitro and in vivo. J. Evid.-Based Integr. Med. 2021, 26, 2515690X20978394. [Google Scholar] [CrossRef]
- Tremblay, C.; Hirsch, M.S.; McGovern, B.H. Human herpesvirus 7 infection. UpToDate. 2019. Available online: https://www.uptodate.com/contents/human-herpesvirus-7-infection?_escaped_fragment_= (accessed on 26 August 2021).
- Son, M.; Lee, M.; Sung, G.H.; Lee, T.; Shin, Y.S.; Cho, H.; Lieberman, P.M.; Kang, H. Bioactive activities of natural products against herpesvirus infection. J. Microbiol. 2013, 51, 545–551. [Google Scholar] [CrossRef]
- Van de Sand, L.; Bormann, M.; Schmitz, Y.; Heilingloh, C.S.; Witzke, O.; Krawczyk, A. Antiviral Active Compounds Derived from natural sources against herpes simplex viruses. Viruses 2021, 13, 1386. [Google Scholar] [CrossRef]
- Kim, J.E.; Song, Y.J. Anti-varicella-zoster virus activity of cephalotaxine esters in vitro. J. Microbiol. 2019, 57, 74–79. [Google Scholar] [CrossRef]
- Dong, H.J.; Wang, Z.H.; Meng, W.; Li, C.C.; Hu, Y.X.; Zhou, L.; Wang, X.J. The natural compound homoharringtonine presents broad antiviral activity in vitro and in vivo. Viruses 2018, 10, 601. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Qiu, M.; Chu, Y.; Chen, D.; Wang, X.; Su, A.; Wu, Z. Downregulation of cellular c-Jun N-terminal protein kinase and NF-κB activation by berberine may result in inhibition of herpes simplex virus replication. Antimicrob. Agents Chemother. 2014, 58, 5068–5078. [Google Scholar] [CrossRef] [Green Version]
- Chin, L.W.; Cheng, Y.W.; Lin, S.S.; Lai, Y.Y.; Lin, L.Y.; Chou, M.Y.; Chou, M.C.; Yang, C.C. Anti-herpes simplex virus effects of berberine from Coptidis rhizoma, a major component of a Chinese herbal medicine, Ching-Wei-San. Arch. Virol. 2010, 155, 1933–1941. [Google Scholar] [CrossRef]
- Duan, Q.; Liu, T.; Yuan, P.; Huang, C.; Shao, Q.; Xu, L.; Sun, J.; Huang, G.; Chen, Z. Antiviral effect of Chinese herbal prescription JieZe-1 on adhesion and penetration of VK2/E6E7 with herpes simplex viruses type 2. J. Ethnopharmacol. 2020, 249, 112405. [Google Scholar] [CrossRef]
- Kim, J.H.; Weeratunga, P.; Kim, M.S.; Nikapitiya, C.; Lee, B.H.; Uddin, M.B.; Kim, T.H.; Yoon, J.E.; Park, C.; Ma, J.Y. Inhibitory effects of an aqueous extract from Cortex Phellodendri on the growth and replication of broad-spectrum of viruses in vitro and in vivo. BMC Complement. Altern. Med. 2016, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wang, H.; Zhang, Y.; Guo, W.; Long, C.; Wang, J.; Liu, L.; Sun, X. Berberine inhibits the proliferation of human nasopharyngeal carcinoma cells via an Epstein-Barr virus nuclear antigen 1-dependent mechanism. Oncol. Rep. 2017, 37, 2109–2120. [Google Scholar] [CrossRef] [Green Version]
- Tsang, C.M.; Cheung, Y.C.; Lui, V.W.Y.; Yip, Y.L.; Zhang, G.; Lin, V.W.; Cheung, K.C.P.; Feng, Y.; Tsao, S.W. Berberine suppresses tumorigenicity and growth of nasopharyngeal carcinoma cells by inhibiting STAT3 activation induced by tumor associated fibroblasts. BMC Cancer 2013, 13, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Hu, J.; Dai, N.; Song, L.; Lin, T.; Liu, J.; Li, K.; Peng, Z.; He, Y.; Liao, D.F. Berberine and ginsenoside Rg3 act synergistically via the MAPK/ERK pathway in nasopharyngeal carcinoma cells. J. Funct. Foods 2020, 66, 103802. [Google Scholar] [CrossRef]
- Park, G.B.; Park, S.H.; Kim, D.; Kim, Y.S.; Yoon, S.H.; Hur, D.Y. Berberine induces mitochondrial apoptosis of EBV-transformed B cells through p53-mediated regulation of XAF1 and GADD45α. Int. J. Oncol. 2016, 49, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Luganini, A.; Mercorelli, B.; Messa, L.; Palù, G.; Gribaudo, G.; Loregian, A. The isoquinoline alkaloid berberine inhibits human cytomegalovirus replication by interfering with the viral Immediate Early-2 (IE2) protein transactivating activity. Antivir. Res. 2019, 164, 52–60. [Google Scholar] [CrossRef]
- Hayashi, K.; Minoda, K.; Nagaoka, Y.; Hayashi, T.; Uesato, S. Antiviral activity of berberine and related compounds against human cytomegalovirus. Bioorg. Med. Chem. Lett. 2007, 17, 1562–1564. [Google Scholar] [CrossRef]
- Nakagami, T.; Taji, S.; Takahashi, M.; Yamanishi, K. Antiviral activity of a bile pigment, biliverdin, against human herpesvirus 6 (HHV-6) in vitro. Microbiol. Immunol. 1992, 36, 381–390. [Google Scholar] [CrossRef]
- Gruffaz, M.; Zhou, S.; Vasan, K.; Rushing, T.; Michael, Q.L.; Lu, C.; Jung, J.U.; Gao, S.J. Repurposing Cytarabine for Treating Primary effusion lymphoma by targeting Kaposi’s Sarcoma-associated herpesvirus latent and lytic replications. MBio 2018, 9, e00756-18. [Google Scholar] [CrossRef]
- Ponnusamy, N.; Odumpatta, R.; Damodharan, P.; Arumugam, M. Computational investigation of marine bioactive compounds reveals frigocyclinone as a potent inhibitor of Kaposi’s Sarcoma associated herpesvirus (KSHV) targets. Biomed. Pharmacol. J. 2019, 12, 1289–1302. [Google Scholar] [CrossRef]
- Sookkongwaree, K.; Geitmann, M.; Roengsumran, S.; Petsom, A.; Danielson, U.H. Inhibition of viral proteases by Zingiberaceae extracts and flavones isolated from Kaempferia parviflora. Pharmazie. Int. J. Pharm. Sci. 2006, 61, 717–721. [Google Scholar]
- Akuzawa, K.; Yamada, R.; Li, Z.; Li, Y.; Sadanari, H.; Matsubara, K.; Watanabe, K.; Koketsu, M.; Tuchida, Y.; Murayama, T. Inhibitory effects of tricin derivative from Sasa albo-marginata on replication of human cytomegalovirus. Antivir. Res. 2011, 91, 296–303. [Google Scholar] [CrossRef]
- Murayama, T.; Li, Y.; Takahashi, T.; Yamada, R.; Matsubara, K.; Tuchida, Y.; Li, Z.; Sadanari, H. Anti-cytomegalovirus effects of tricin are dependent on CXCL11. Microbes Infect. 2012, 14, 1086–1092. [Google Scholar] [CrossRef]
- Akai, Y.; Sadanari, H.; Takemoto, M.; Uchide, N.; Daikoku, T.; Mukaida, N.; Murayama, T. Inhibition of human cytomegalovirus replication by tricin is associated with depressed CCL2 expression. Antivir. Res. 2017, 148, 15–19. [Google Scholar] [CrossRef]
- Itoh, A.; Sadanari, H.; Takemoto, M.; Matsubara, K.; Daikoku, T.; Murayama, T. Tricin inhibits the CCL5 induction required for efficient growth of human cytomegalovirus. Microbiol. Immunol. 2018, 62, 341–347. [Google Scholar] [CrossRef]
- Sadanari, H.; Fujimoto, K.J.; Sugihara, Y.; Ishida, T.; Takemoto, M.; Daikoku, T.; Murayama, T. The anti-human cytomegalovirus drug tricin inhibits cyclin-dependent kinase 9. FEBS Open Bio 2018, 8, 646–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, L.; Chiang, W.; Chang, M.; Ng, L.; Lin, C. Antiviral activity of Plantago major extracts and related compounds in vitro. Antivir. Res. 2002, 55, 53–62. [Google Scholar] [CrossRef]
- Lyu, S.Y.; Rhim, J.Y.; Park, W.B. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. Arch. Pharm. Res. 2005, 28, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, C. Inhibitory role of baicalin on human herpes virus type 6 in vitro. Procedia Eng. 2012, 37, 75–78. [Google Scholar] [CrossRef]
- Mercorelli, B.; Luganini, A.; Nannetti, G.; Tabarrini, O.; Palù, G.; Gribaudo, G.; Loregian, A. Drug repurposing approach identifies inhibitors of the prototypic viral transcription factor IE2 that block human cytomegalovirus replication. Cell Chem. Bio. 2016, 23, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Nukui, M.; O’Connor, C.M.; Murphy, E.A. The natural flavonoid compound deguelin inhibits HCMV lytic replication within fibroblasts. Viruses 2018, 10, 614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, L.C.; Ng, L.T.; Cheng, P.W.; Chiang, W.; Lin, C.C. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005, 32, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Rittà, M.; Marengo, A.; Civra, A.; Lembo, D.; Cagliero, C.; Kant, K.; Lal, U.R.; Rubiolo, P.; Ghosh, M.; Donalisio, M. Antiviral activity of a Arisaema tortuosum leaf extract and some of its constituents against herpes simplex virus type 2. Planta Med. 2020, 86, 267–275. [Google Scholar] [CrossRef]
- Angamuthu, D.; Swaminathan, R. Evaluation of antiviral efficacy of Punica granatum L. on human herpes virus-3 (Varicella Zoster virus). Asian J. Biol. Sci. 2019, 12, 917–926. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.C.; Fang, C.Y.; Cheng, Y.J.; Hsu, H.Y.; Chou, S.P.; Huang, S.Y.; Tsai, C.H.; Chen, J.Y. Inhibition of Epstein-Barr virus reactivation by the flavonoid apigenin. J. Biomed. Sci. 2017, 24, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Evers, D.L.; Chao, C.F.; Wang, X.; Zhang, Z.; Huong, S.M.; Huang, E.S. Human cytomegalovirus-inhibitory flavonoids: Studies on antiviral activity and mechanism of action. Antivir. Res 2005, 68, 124–134. [Google Scholar] [CrossRef]
- Granato, M.; Montani, M.S.G.; Santarelli, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Apigenin, by activating p53 and inhibiting STAT3, modulates the balance between pro-apoptotic and pro-survival pathways to induce PEL cell death. J. Exp. Clin. Cancer Res. 2017, 36, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.C.; Hohmann, J.; El-Shazly, M.; Chang, L.K.; Dankó, B.; Kúsz, N.; Hsieh, C.T.; Hunyadi, A.; Chang, F.R. Bioactive constituents of Lindernia crustacea and its anti-EBV effect via Rta expression inhibition in the viral lytic cycle. J. Ethnopharm. 2020, 250, 112493. [Google Scholar] [CrossRef]
- Chu, Y.; Lv, X.; Zhang, L.; Fu, X.; Song, S.; Su, A.; Chen, D.; Xu, L.; Wang, Y.; Wu, Z. Wogonin inhibits in vitro herpes simplex virus type 1 and 2 infection by modulating cellular NF-κB and MAPK pathways. BMC Microbiol. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Choi, E.J.; Lee, C.H.; Kim, Y.C.; Shin, O.S. Wogonin inhibits Varicella-Zoster (shingles) virus replication via modulation of type I interferon signaling and adenosine monophosphate-activated protein kinase activity. J. Funct. Foods 2015, 17, 399–409. [Google Scholar] [CrossRef]
- Wu, X.; Liu, P.; Zhang, H.; Li, Y.; Salmani, J.M.M.; Wang, F.; Yang, K.; Fu, R.; Chen, Z.; Chen, B. Wogonin as a targeted therapeutic agent for EBV (+) lymphoma cells involved in LMP1/NF-κB/miR-155/PU. 1 pathway. BMC Cancer 2017, 17, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Culenová, M.; Sychrová, A.; Hassan, S.T.S.; Berchová-Bímová, K.; Svobodová, P.; Helclová, A.; Michnová, H.; Hošek, J.; Vasilev, H.; Suchý, P. Multiple In vitro biological effects of phenolic compounds from Morus alba root bark. J. Ethnopharmacol. 2020, 248, 112296. [Google Scholar] [CrossRef]
- Murayama, T.; Eizuru, Y.; Yamada, R.; Sadanari, H.; Matsubara, K.; Rukung, G.; Tolo, F.M.; Mungai, G.M.; Kofi-Tsekpo, M. Anticytomegalovirus Activity of Pristimerin, a Triterpenoid Quinone Methide Isolated fromMaytenus Heterophylla(Eckl. & Zeyh.). Antivir. Chem. Chemother. 2007, 18, 133–139. [Google Scholar]
- Pompei, R.; Flore, O.; Marccialis, M.A.; Pani, A.; Loddo, B. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature 1979, 281, 689–690. [Google Scholar] [CrossRef]
- Dargan, D.; Subak-Sharpe, J. The antiviral activity against herpes simplex virus of the triterpenoid compounds carbenoxolone sodium and cicloxolone sodium. J. Antimicrob. Chemother. 1986, 18, 185–200. [Google Scholar] [CrossRef]
- Hirabayashi, K.; Iwata, S.; Matsumoto, H.; Mori, T.; Shibata, S.; Baba, M.; Ito, M.; Shigeta, S.; Nakashima, H.; Yamamoto, N. Antiviral activities of glycyrrhizin and its modified compounds against human immunodeficiency virus type 1 (HIV-1) and herpes simplex virus type 1 (HSV-1) in vitro. Chem. Pharm. Bull. 1991, 39, 112–115. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, T.; Yokomizo, K.; Okawa, M.; Tsuchihashi, R.; Kinjo, J.; Nohara, T.; Uyeda, M. Anti-herpes virus type 1 activity of oleanane-type triterpenoids. Biol. Pharm. Bull. 2005, 28, 1779–1781. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.C. Mechanism of action of glycyrrhizic acid in inhibition of Epstein-Barr virus replication in vitro. Antivir Res. 2003, 59, 41–47. [Google Scholar] [CrossRef]
- Lin, J.C.; Cherng, J.M.; Hung, M.S.; Baltina, L.A.; Baltina, L.; Kondratenko, R. Inhibitory effects of some derivatives of glycyrrhizic acid against Epstein-Barr virus infection: Structure–activity relationships. Antivir. Res. 2008, 79, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Bentz, G.L.; Lowrey, A.J.; Horne, D.C.; Nguyen, V.; Satterfield, A.R.; Ross, T.D.; Harrod, A.E.; Uchakina, O.N.; McKallip, R.J. Using glycyrrhizic acid to target sumoylation processes during Epstein-Barr virus latency. PLoS ONE 2019, 14, e0217578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.; Lieberman, P.M. Mechanism of glycyrrhizic acid inhibition of Kaposi’s Sarcoma-associated herpesvirus: Disruption of CTCF-cohesin-mediated RNA polymerase II pausing and sister chromatid cohesion. J. Virol. 2011, 85, 11159–11169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Chen, Y.; Guo, Y.; Huang, Y.; Zhu, B. Allicin and glycyrrhizic acid display antiviral activity against latent and lytic Kaposi Sarcoma-associated herpesvirus. Infect. Microbes Dis. 2020, 2, 30–34. [Google Scholar] [CrossRef]
- Curreli, F.; Friedman-Kien, A.E.; Flore, O. Glycyrrhizic acid alters Kaposi sarcoma–associated herpesvirus latency, triggering p53-mediated apoptosis in transformed B lymphocytes. J. Clin. Invest. 2005, 115, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Guo, W.; Long, C.; Wang, H.; Wang, J.; Sun, X. Triptolide inhibits proliferation of Epstein–Barr virus-positive B lymphocytes by down-regulating expression of a viral protein LMP1. Biochem. Biophys. Res. Commun. 2015, 456, 815–820. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y.; Wang, C.; Liu, L.; Wang, H.; Zhang, Y.; Long, C.; Sun, X. Triptolide inhibits Epstein-Barr nuclear antigen 1 expression by increasing sensitivity of mitochondria apoptosis of nasopharyngeal carcinoma cells. J. Exp. Clin. Cancer Res. 2018, 37, 1–17. [Google Scholar] [CrossRef]
- Long, C.; Xu, Q.B.; Ding, L.; Yang, L.; Ji, W.; Gao, F.; Ji, Y. Triptolide inhibits human telomerase reverse transcriptase by downregulating translation factors SP1 and c-Myc in Epstein-Barr virus-positive B lymphocytes. Oncol. Lett. 2021, 21, 1. [Google Scholar] [CrossRef]
- Long, C.; Guo, W.; Zhou, H.; Wang, J.; Wang, H.; Sun, X. Triptolide decreases expression of latency-associated nuclear antigen 1 and reduces viral titers in Kaposi’s Sarcoma-associated and herpesvirus-related primary effusion lymphoma cells. Int. J. Oncol. 2016, 48, 1519–1530. [Google Scholar] [CrossRef] [Green Version]
- Pilau, M.R.; Alves, S.H.; Weiblen, R.; Arenhart, S.; Cueto, A.P.; Lovato, L.T. Antiviral activity of the Lippia graveolens (Mexican oregano) essential oil and its main compound carvacrol against human and animal viruses. Braz. J. Microbiol. 2011, 42, 1616–1624. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, J.; Salehi, B.; Baghalpour, N.; Kobarfard, F.; Sharifi-Rad, M.; Mohammadizade, M. Antiviral activity of monoterpenes thymol, carvacrol and p-cymene against herpes simplex virus in vitro. Int. Pharm. Acta 2018, 1, 73. [Google Scholar]
- Kamalabadi, M.; Astani, A.; Nemati, F. Anti-viral effect and mechanism of carvacrol on herpes simplex virus type 1. Int. J. Med. Lab. 2018, 5, 113–122. [Google Scholar]
- Lai, W.L.; Chuang, H.S.; Lee, M.H.; Wei, C.L.; Lin, C.F.; Tsai, Y.C. Inhibition of herpes simplex virus type 1 by thymol-related monoterpenoids. Planta Med. 2012, 78, 1636–1638. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, D.; Wu, X.; Xu, R.; Li, Y. Antiviral mechanism of carvacrol on HSV-2 infectivity through inhibition of RIP3-mediated programmed cell necrosis pathway and ubiquitin-proteasome system in BSC-1 cells. BMC Infect. Dis. 2020, 20, 1–16. [Google Scholar] [CrossRef]
- Brezani, V.; Lelakova, V.; Hassan, S.T.S.; Berchova-Bimova, K.; Novy, P.; Kloucek, P.; Marsik, P.; Dall’Acqua, S.; Hosek, J.; Smejkal, K. Anti-Infectivity against Herpes Simplex Virus and Selected Microbes and Anti-Inflammatory Activities of Compounds Isolated from Eucalyptus globulus Labill. Viruses 2018, 10, 360. [Google Scholar] [CrossRef] [Green Version]
- Docherty, J.J.; Fu, M.M.H.; Stiffler, B.S.; Limperos, R.J.; Pokabla, C.M.; DeLucia, A.L. Resveratrol inhibition of herpes simplex virus replication. Antivir. Res. 1999, 43, 145–155. [Google Scholar] [CrossRef]
- Docherty, J.J.; Smith, J.S.; Fu, M.M.; Stoner, T.; Booth, T. Effect of topically applied resveratrol on cutaneous herpes simplex virus infections in hairless mice. Antivir. Res. 2004, 61, 19–26. [Google Scholar] [CrossRef]
- Docherty, J.J.; Fu, M.M.; Hah, J.M.; Sweet, T.J.; Faith, S.A.; Booth, T. Effect of resveratrol on herpes simplex virus vaginal infection in the mouse. Antivir. Res. 2005, 67, 155–162. [Google Scholar] [CrossRef]
- Faith, S.A.; Sweet, T.J.; Bailey, E.; Booth, T.; Docherty, J.J. Resveratrol suppresses nuclear factor-κB in herpes simplex virus infected cells. Antivir. Res. 2006, 72, 242–251. [Google Scholar] [CrossRef]
- Chuanasa, T.; Phromjai, J.; Lipipun, V.; Likhitwitayawuid, K.; Suzuki, M.; Pramyothin, P.; Hattori, M.; Shiraki, K. Anti-herpes simplex virus (HSV-1) activity of oxyresveratrol derived from Thai medicinal plant: Mechanism of action and therapeutic efficacy on cutaneous HSV-1 infection in mice. Antivir Res. 2008, 80, 62–70. [Google Scholar] [CrossRef]
- Chen, X.; Qiao, H.; Liu, T.; Yang, Z.; Xu, L.; Xu, Y.; Ge, H.M.; Tan, R.X.; Li, E. Inhibition of herpes simplex virus infection by oligomeric stilbenoids through ROS generation. Antivir. Res. 2012, 95, 30–36. [Google Scholar] [CrossRef]
- Docherty, J.J.; Sweet, T.J.; Bailey, E.; Faith, S.A.; Booth, T. Resveratrol inhibition of varicella-zoster virus replication in vitro. Antivir. Res. 2006, 72, 171–177. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Azuine, M.A.; Tokuda, H.; Takasaki, M.; Mukainaka, T.; Konoshima, T.; Nishino, H. Chemopreventive effect of resveratrol, sesamol, sesame oil and sunflower oil in the Epstein–Barr virus early antigen activation assay and the mouse skin two-stage carcinogenesis. Pharmacol. Res. 2002, 45, 499–505. [Google Scholar] [CrossRef]
- Yiu, C.Y.; Chen, S.Y.; Chang, L.K.; Chiu, Y.F.; Lin, T.P. Inhibitory effects of resveratrol on the Epstein-Barr virus lytic cycle. Molecules 2010, 15, 7115. [Google Scholar] [CrossRef] [Green Version]
- De Leo, A.; Arena, G.; Lacanna, E.; Oliviero, G.; Colavita, F.; Mattia, E. Resveratrol inhibits Epstein Barr Virus lytic cycle in Burkitt’s lymphoma cells by affecting multiple molecular targets. Antivir. Res. 2012, 96, 196–202. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Takami, A.; Trung, L.Q.; Kato, S.; Nakao, S. Resveratrol prevents EBV transformation and inhibits the outgrowth of EBV-immortalized human B cells. PLoS ONE 2012, 7, e51306. [Google Scholar] [CrossRef] [Green Version]
- Evers, D.L.; Wang, X.; Huong, S.M.; Huang, D.Y.; Huang, E.S. 3, 4’ 5-Trihydroxy-trans-stilbene (resveratrol) inhibits human cytomegalovirus replication and virus-induced cellular signaling. Antivir. Res. 2004, 63, 85–95. [Google Scholar] [CrossRef]
- Dyson, O.F.; Walker, L.R.; Whitehouse, A.; Cook, P.P.; Akula, S.M. Resveratrol inhibits KSHV reactivation by lowering the levels of cellular EGR-1. PLoS ONE 2012, 7, e33364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, F.Y.; Chen, C.Y.; Shyu, H.W.; Hong, S.; Chen, H.M.; Chiou, Y.H.; Lin, K.H.; Chou, M.C.; Wang, L.Y.; Wang, Y.F. Resveratrol induces cell death and inhibits human herpesvirus 8 replication in primary effusion lymphoma cells. Chem. Biol. Interact. 2015, 242, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, C.E.; Wen, G.Y.; Xu, W.; Jia, J.H.; Rohan, L.; Corbo, C.; Di Maggio, V.; Jenkins Jr, E.C.; Hillier, S. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob. Agents Chemother. 2008, 52, 962–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaacs, C.E.; Xu, W.; Merz, G.; Hillier, S.; Rohan, L.; Wen, G.Y. Digallate dimers of (−)-epigallocatechin gallate inactivate herpes simplex virus. Antimicrob. Agents Chemother. 2011, 55, 5646–5653. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, A.; Adams, S.D.; Lee, L.H.; Murray, S.R.; Hsu, S.D.; Hammond, J.R.; Dickinson, D.; Chen, P.; Chu, T.C. Inhibition of herpes simplex virus type 1 with the modified green tea polyphenol palmitoyl-epigallocatechin gallate. Food Chem. Toxicol. 2013, 52, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Colpitts, C.C.; Schang, L.M. A small molecule inhibits virion attachment to heparan sulfate-or sialic acid-containing glycans. J. Virol. 2014, 88, 7806–7817. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, P.; Nguyen, M.L. Herpes simplex virus virucidal activity of MST-312 and epigallocatechin gallate. Virus Res. 2018, 249, 93–98. [Google Scholar] [CrossRef]
- Wu, C.Y.; Yu, Z.Y.; Chen, Y.C.; Hung, S.L. Effects of epigallocatechin-3-gallate and acyclovir on herpes simplex virus type 1 infection in oral epithelial cells. J. Formos. Med. Assoc. 2020. (In press) [Google Scholar] [CrossRef]
- Chang, L.K.; Wei, T.T.; Chiu, Y.F.; Tung, C.P.; Chuang, J.Y.; Hung, S.K.; Li, C.; Liu, S.T. Inhibition of Epstein–Barr virus lytic cycle by (−)-epigallocatechin gallate. Biochem. Biophys. Res. Commun. 2003, 301, 1062–1068. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Chen, L.; Yang, L.; Li, L.; Tao, Y.; Li, W.; Li, Z.; Liu, H.; Tang, M. (-)-Epigallocatechin-3-gallate inhibition of Epstein–Barr virus spontaneous lytic infection involves ERK1/2 and PI3-K/Akt signaling in EBV-positive cells. Carcinogenesis 2013, 34, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Li, H.; Tang, M.; Cao, Y. (-)-Epigallocatechin-3-gallate inhibition of Epstein-Barr virus spontaneous lytic infection involves downregulation of latent membrane protein 1. Exp. Ther. Med. 2018, 15, 1105–1112. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.Y.; Chen, C.Y.; Chiou, Y.H.; Shyu, H.W.; Lin, K.H.; Chou, M.C.; Huang, M.H.; Wang, Y.F. Epigallocatechin-3-gallate suppresses human herpesvirus 8 replication and induces ROS leading to apoptosis and autophagy in primary effusion lymphoma cells. Int. J. Mol. Sci. 2018, 19, 16. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Wang, Q.; Gu, Q.; Zhang, H.; Jiang, C.; Hu, J.; Wang, Y.; Yan, Y.; Xu, J. Semisynthesis of (-)-Rutamarin derivatives and their inhibitory activity on Epstein–Barr virus lytic replication. J. Nat. Prod. 2017, 80, 53–60. [Google Scholar] [CrossRef]
- Xu, B.; Wang, L.; González-Molleda, L.; Wang, Y.; Xu, J.; Yuan, Y. Antiviral activity of (+)-rutamarin against Kaposi’s Sarcoma-associated herpesvirus by inhibition of the catalytic activity of human topoisomerase II. Antimicrob. Agents Chemother. 2014, 58, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Sochocka, M.; Sobczyński, M.; Ochnik, M.; Zwolińska, K.; Leszek, J. Hampering herpesviruses HHV-1 and HHV-2 infection by extract of Ginkgo biloba (EGb) and its phytochemical constituents. Front. Microbiol. 2019, 10, 2367. [Google Scholar] [CrossRef] [Green Version]
- Bhutta, M.S.; Shechter, O.; Gallo, E.S.; Martin, S.D.; Jones, E.; Doncel, G.F.; Borenstein, R. Ginkgolic acid inhibits herpes simplex virus type 1 skin infection and prevents zosteriform spread in mice. Viruses 2021, 13, 86. [Google Scholar] [CrossRef]
- Borenstein, R.; Hanson, B.A.; Markosyan, R.M.; Gallo, E.S.; Narasipura, S.D.; Bhutta, M.; Shechter, O.; Lurain, N.S.; Cohen, F.S.; Al-Harthi, L. Ginkgolic acid inhibits fusion of enveloped viruses. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Hsiang, C.Y.; Ho, T.Y. Emodin is a novel alkaline nuclease inhibitor that suppresses herpes simplex virus type 1 yields in cell cultures. Br. J. Pharmacol. 2008, 155, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Xiong, H.R.; Luo, J.; Hou, W.; Xiao, H.; Yang, Z.Q. The effect of emodin, an anthraquinone derivative extracted from the roots of Rheum tanguticum, against herpes simplex virus in vitro and in vivo. J. Ethnopharmacol. 2011, 133, 718–723. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Pan, C.; Cheng, W.; Wang, X.; Yang, Z.; Zheng, L. The intervention mechanism of emodin on TLR3 pathway in the process of central nervous system injury caused by herpes virus infection. Neurol. Res. 2021, 43, 307–313. [Google Scholar] [CrossRef]
- Yiu, C.Y.; Chen, S.Y.; Yang, T.H.; Chang, C.J.; Yeh, D.B.; Chen, Y.J.; Lin, T.P. Inhibition of Epstein-Barr virus lytic cycle by an ethyl acetate subfraction separated from Polygonum cuspidatum root and its major component, emodin. Molecules 2014, 19, 1258. [Google Scholar] [CrossRef]
- Wu, C.C.; Chen, M.S.; Cheng, Y.J.; Ko, Y.C.; Lin, S.F.; Chiu, I.M.; Chen, J.Y. Emodin inhibits EBV reactivation and represses NPC tumorigenesis. Cancers 2019, 11, 1795. [Google Scholar] [CrossRef] [Green Version]
- Barnard, D.L.; Huffman, J.H.; Morris, J.L.; Wood, S.G.; Hughes, B.G.; Sidwell, R.W. Evaluation of the antiviral activity of anthraquinones, anthrones and anthraquinone derivatives against human cytomegalovirus. Antivir. Res. 1992, 17, 63–77. [Google Scholar] [CrossRef]
- Kang, H.; Song, J.; Choi, K.; Kim, H.; Choi, M.; Lee, S.Y.; Kim, C.; Lee, S.J.; Song, M.J.; Kang, H. Efficient lytic induction of Kaposi’s Sarcoma-associated herpesvirus (KSHV) by the anthracyclines. Oncotarget 2014, 5, 8515. [Google Scholar] [CrossRef] [Green Version]
- Bora, P.P.; Baruah, N.; Bez, G.; Barua, N.C. New method for the synthesis of ether derivatives of artemisinin. Synth. Commun. 2012, 42, 1218–1225. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Zhang, Y.; Zhu, H.; Liu, J.; Li, H.; Li, X.N.; Sun, W.; Zeng, J.; Xue, Y.; Zhang, Y. Filicinic acid based meroterpenoids with anti-Epstein–Barr virus activities from Hypericum japonicum. Org. Lett. 2016, 18, 2272–2275. [Google Scholar] [CrossRef]
- Wu, R.; Le, Z.; Wang, Z.; Tian, S.; Xue, Y.; Chen, Y.; Hu, L.; Zhang, Y. Hyperjaponol H, a new bioactive filicinic acid-based meroterpenoid from Hypericum japonicum Thunb. ex Murray. Molecules 2018, 23, 683. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Xue, Y.; Zhang, J.; Zhu, H.; Chen, C.; Li, X.N.; Liu, J.; Wang, Z.; Zhang, Y.; Zhang, Y. (±)-Japonicols A–D, acylphloroglucinol-based meroterpenoid enantiomers with anti-KSHV activities from Hypericum japonicum. J. Nat. Prod. 2016, 79, 1322–1328. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Y.; Wang, Y.; Wang, Z.; Huang, J.; Xue, Y.; Liu, J.; Liu, Z.; Chen, Y.; Zhang, Y. Discovery of acylphloroglucinol-based meroterpenoid enantiomers as KSHV inhibitors from Hypericum japonicum. RSC Adv. 2018, 8, 24101–24109. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Zhu, H.; Li, L.; Huang, J.; Sun, W.; Liu, J.; Li, H.; Luo, Z.; Wang, J.; Xue, Y. (±)-Japonones A and B, two pairs of new enantiomers with anti-KSHV activities from Hypericum japonicum. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Wang, Z.; Zhang, J.; Lu, Y.; Wang, K.; Xue, Y.; Zhang, Y.; Zhang, Y. Two new bioactive α-pyrones from Hypericum japonicum. Molecules 2016, 21, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da-Cheng, H.; Xu-Dong, H.; Xiao-Jie, G.; Pei-Gen, X.; Guang-Bo, G. Ethnopharmacology, chemodiversity, and bioactivity of Cephalotaxus medicinal plants. Chin. J. Nat. Med. 2021, 19, 321–338. [Google Scholar]
- Šudomová, M.; Berchová-Bímová, K.; Marzocco, S.; Liskova, A.; Kubatka, P.; Hassan, S.T. Berberine in human oncogenic herpesvirus infections and their linked cancers. Viruses 2021, 13, 1014. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.F.; Su, J.; Ouyang, S. Marine-derived drugs: Recent advances in cancer therapy and immune signaling. Biomed. Pharmacother. 2021, 134, 111091. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Mohamed, T.A.; Essa, A.F.; Gawad, A.E.; Ahmed, M.; Alqahtani, A.S.; Shahat, A.A.; Yoneyama, T.; Farrag, A.R.H.; Noji, M. Recent advances in Kaempferia phytochemistry and biological activity: A comprehensive review. Nutrients 2019, 11, 2396. [Google Scholar] [CrossRef] [Green Version]
- Sabit, H.; Dahan, A.; Sun, J.; Provoda, C.J.; Lee, K.D.; Hilfinger, J.H.; Amidon, G.L. Cytomegalovirus protease targeted prodrug development. Mol. Pharm. 2013, 10, 1417–1424. [Google Scholar] [CrossRef]
- Sakai, A.; Watanabe, K.; Koketsu, M.; Akuzawa, K.; Yamada, R.; Li, Z.; Sadanari, H.; Matsubara, K.; Murayama, T. Anti-human cytomegalovirus activity of constituents from Sasa albo-marginata (Kumazasa in Japan). Antivir. Chem. Chemother. 2008, 19, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Ma, C.; Fan, S.; Deng, N.; Lian, Y.; Tan, L.; Du, W.; Zhang, S.; Liu, S.; Ren, B. Systematic understanding of the mechanism of baicalin against ischemic stroke through a network pharmacology approach. Evid. Based Complementary Altern. Med. 2018, 2018, 2582843. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, W.; Zheng, W. Deguelin, a novel anti-tumorigenic agent targeting apoptosis, cell cycle arrest and anti-angiogenesis for cancer chemoprevention. Mol. Clin. Oncol. 2013, 1, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Mercorelli, B.; Luganini, A.; Palù, G.; Gribaudo, G.; Loregian, A. Drug repurposing campaigns for human cytomegalovirus identify a natural compound targeting the immediate-early 2 (IE2) protein: A comment on “the natural flavonoid compound deguelin inhibits HCMV lytic replication within fibroblasts”. Viruses 2019, 11, 117. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.H.; Yang, S.J.; Liang, N.; Hu, D.Y.; Jin, L.H.; Xue, W.; Yang, S. Chemical constituents of Caesalpinia decapetala (Roth) alston. Molecules 2013, 18, 1325. [Google Scholar] [CrossRef]
- Wanjala, C.C.; Majinda, R.R. Flavonoid glycosides from Crotalaria podocarpa. Phytochemistry 1999, 51, 705–707. [Google Scholar] [CrossRef]
- Pukalskas, A.; Venskutonis, P.R.; Salido, S.; de Waard, P.; van Beek, T.A. Isolation, identification and activity of natural antioxidants from horehound (Marrubium vulgare L.) cultivated in Lithuania. Food Chem. 2012, 130, 695–701. [Google Scholar] [CrossRef]
- Huynh, D.L.; Ngau, T.H.; Nguyen, N.H.; Tran, G.B.; Nguyen, C.T. Potential therapeutic and pharmacological effects of wogonin: An updated review. Mol. Biol. Rep. 2020, 47, 9779–9789. [Google Scholar] [CrossRef]
- Huan, C.; Xu, Y.; Zhang, W.; Guo, T.; Pan, H.; Gao, S. Research progress on the antiviral activity of glycyrrhizin and its derivatives in liquorice. Front. Pharmacol. 2021, 12, 1706. [Google Scholar] [CrossRef]
- Yang, X.W. Antiviral effect of glycyrrhizic acid. 2020. World Health Organization. Available online: https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/ppcovidwho-5285 (accessed on 20 August 2021).
- Bishop, G.A.; Busch, L.K. Molecular mechanisms of B-lymphocyte transformation by Epstein–Barr virus. Microb. Infect. 2002, 4, 853–857. [Google Scholar] [CrossRef]
- Ahsan, N.; Kanda, T.; Nagashima, K.; Takada, K. Epstein-Barr virus transforming protein LMP1 plays a critical role in virus production. J. Virol 2005, 79, 4415–4424. [Google Scholar] [CrossRef] [Green Version]
- Xiong, L.; Peng, C.; Zhou, Q.M.; Wan, F.; Xie, X.F.; Guo, L.; Li, X.H.; He, C.J.; Dai, O. Chemical composition and antibacterial activity of essential oils from different parts of Leonurus japonicus Houtt. Molecules 2013, 18, 963. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, A.M.; Heimfarth, L.; Pereira, E.W.M.; Oliveira, F.S.; Menezes, I.R.; Coutinho, H.D.; Picot, L.; Antoniolli, A.R.; Quintans, J.S.; Quintans-Júnior, L.J. Phytol, a chlorophyll component, produces antihyperalgesic, anti-inflammatory, and antiarthritic effects: Possible NFκB pathway involvement and reduced levels of the proinflammatory cytokines TNF-α and IL-6. J. Nat. Prod. 2020, 83, 1107–1117. [Google Scholar] [CrossRef]
- Ma, L.; Yao, L. Antiviral effects of plant-derived essential oils and their components: An updated review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef]
- Ito, H.; Koreishi, M.; Tokuda, H.; Nishino, H.; Yoshida, T. Cypellocarpins A−C, Phenol Glycosides Esterified with Oleuropeic Acid, from Eucalyptus Cypellocarpa. J. Nat. Prod. 2000, 63, 1253–1257. [Google Scholar] [CrossRef]
- Hakki, Z.; Cao, B.; Heskes, A.M.; Goodger, J.Q.; Woodrow, I.E.; Williams, S.J. Synthesis of the monoterpenoid esters cypellocarpin C and cuniloside B and evidence for their widespread occurrence in Eucalyptus. Carbohydr. Res. 2010, 345, 2079–2084. [Google Scholar] [CrossRef]
- Wang, H.; Fujimoto, Y. Triterpene esters from Eucalyptus tereticornis. Phytochemistry 1993, 33, 151–153. [Google Scholar]
- Calis, I.; Lahloub, M.F.; Rogenmoser, E.; Sticher, O. Isomartynoside, a phenylpropanoid glycoside from Galeopsis pubescens. Phytochemistry 1984, 23, 2313–2315. [Google Scholar] [CrossRef]
- Abba, Y.; Hassim, H.; Hamzah, H.; Noordin, M.M. Antiviral activity of resveratrol against human and animal viruses. Adv. Virol. 2015, 2015, 184241. [Google Scholar] [CrossRef] [Green Version]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Resveratrol as a novel anti-herpes simplex virus nutraceutical agent: An overview. Viruses 2018, 10, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, S. Compounds derived from epigallocatechin-3-gallate (EGCG) as a novel approach to the prevention of viral infections. Inflamm. Allergy Drug Targets 2015, 14, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, Y.; Yuan, Y. Antiviral activity of topoisomerase II catalytic inhibitors against Epstein–Barr virus. Antivir. Res. 2014, 107, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gao, J.; Pang, X. Molecular mechanism of emodin action: As an anti-cardiovascular disease drug. Front. Pharmacol. 2020, 11, 1363. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zeng, Y.; Liu, Y.; You, L.; Yin, X.; Fu, J.; Ni, J. Aloe-emodin: A review of its pharmacology, toxicity, and pharmacokinetics. Phytother. Res. 2020, 34, 270–281. [Google Scholar] [CrossRef]
- Fujiwara, A.; Hoshino, T.; Westley, J.W. Anthracycline antibiotics. Crit. Rev. Biotechnol. 1985, 3, 133–157. [Google Scholar] [CrossRef]
- Aviello, G.; Abenavoli, L.; Borrelli, F.; Capasso, R.; Izzo, A.A.; Lembo, F.; Romano, B.; Capasso, F. Garlic: Empiricism or science? Nat. Prod. Commun. 2009, 4, 1934578X0900401231. [Google Scholar] [CrossRef] [Green Version]
- Rouf, R.; Uddin, S.J.; Sarker, D.K.; Islam, M.T.; Ali, E.S.; Shilpi, J.A.; Nahar, L.; Tiralongo, E.; Sarker, S.D. Anti-viral potential of garlic (Allium sativum) and it’s organosulfur compounds: A systematic update of pre-clinical and clinical data. Trends Food Sci. Technol. 2020, 104, 219–234. [Google Scholar] [CrossRef]
- Hirano, T.; Gotoh, M.; Oka, K. Natural flavonoids and lignans are potent cytostatic agents against human leukemic HL-60 cells. Life Sci. 1994, 55, 1061–1069. [Google Scholar] [CrossRef]
- Pauletti, P.M.; Araújo, A.R.; Young, M.C.M.; Giesbrecht, A.M.; da Silva Bolzani, V. Nor-lignans from the leaves of Styrax ferrugineus (Styracaceae) with antibacterial and antifungal activity. Phytochemistry 2000, 55, 597–601. [Google Scholar] [CrossRef]
- Timmers, M.A.; Guerrero-Medina, J.L.; Esposito, D.; Grace, M.H.; Paredes-López, O.; García-Saucedo, P.A.; Lila, M.A. Characterization of phenolic compounds and antioxidant and anti-inflammatory activities from mamuyo (Styrax ramirezii Greenm.) fruit. J. Agric. Food Chem. 2015, 63, 10459–10465. [Google Scholar] [CrossRef]
- De Oliveira, P.F.; Damasceno, J.L.; Bertanha, C.S.; Araújo, A.R.B.; Pauletti, P.M.; Tavares, D.C. Study of the cytotoxic activity of Styrax camporum extract and its chemical markers, egonol and homoegonol. Cytotechnology 2016, 68, 1597–1602. [Google Scholar] [CrossRef] [Green Version]
- Efferth, T.; Dunstan, H.; Sauerbrey, A.; Miyachi, H.; Chitambar, C.R. The anti-malarial artesunate is also active against cancer. Int. J. Oncol. 2001, 18, 767–773. [Google Scholar] [CrossRef]
- Slack, R.D.; Jacobine, A.M.; Posner, G.H. Antimalarial peroxides: Advances in drug discovery and design. MedChemComm 2012, 3, 281–297. [Google Scholar] [CrossRef]
- Kim, B.J.; Sasaki, T. Synthesis of O-aminodihydroartemisinin via TMS triflate catalyzed C− O coupling reaction. J. Org. Chem. 2004, 69, 3242–3244. [Google Scholar] [CrossRef]
- Karagöz, A.Ç.; Reiter, C.; Seo, E.J.; Gruber, L.; Hahn, F.; Leidenberger, M.; Klein, V.; Hampel, F.; Friedrich, O.; Marschall, M. Access to new highly potent antileukemia, antiviral and antimalarial agents via hybridization of natural products (homo) egonol, thymoquinone and artemisinin. Bioorg. Med. Chem. 2018, 26, 3610–3618. [Google Scholar] [CrossRef]
- Takeda, Y.; Zhang, H.; Masuda, T.; Honda, G.; Otsuka, H.; Sezik, E.; Yesilada, E.; Sun, H. Megastigmane glucosides from Stachys byzantina. Phytochemistry 1997, 44, 1335–1337. [Google Scholar] [CrossRef]
- Matsunami, K.; Otsuka, H.; Takeda, Y. Structural revisions of blumenol C glucoside and byzantionoside B. Chem. Pharm. Bull. 2010, 58, 438–441. [Google Scholar] [CrossRef] [Green Version]
- Vincent, O.M.; Nguta, J.M.; Mitema, E.S.; Musila, F.M.; Nyak, D.M.; Mohammed, A.H.; Gervason, M.A. Ethnopharmacology, pharmacological activities, and chemistry of the Hypericum genus. J. Phytopharmacol. 2021, 10, 105–113. [Google Scholar] [CrossRef]
- Agostinho, K.F.; Rechenchoski, D.Z.; Faccin-Galhardi, L.C.; de Sousa, A.L.N.; Cunha, A.P.; Ricardo, N.M.P.S.; Linhares, R.E.C.; Nozawa, C. Cucumis melo pectin as potential candidate to control herpes simplex virus infection. FEMS Microbiol. Lett. 2021, 368, fnab013. [Google Scholar] [CrossRef] [PubMed]
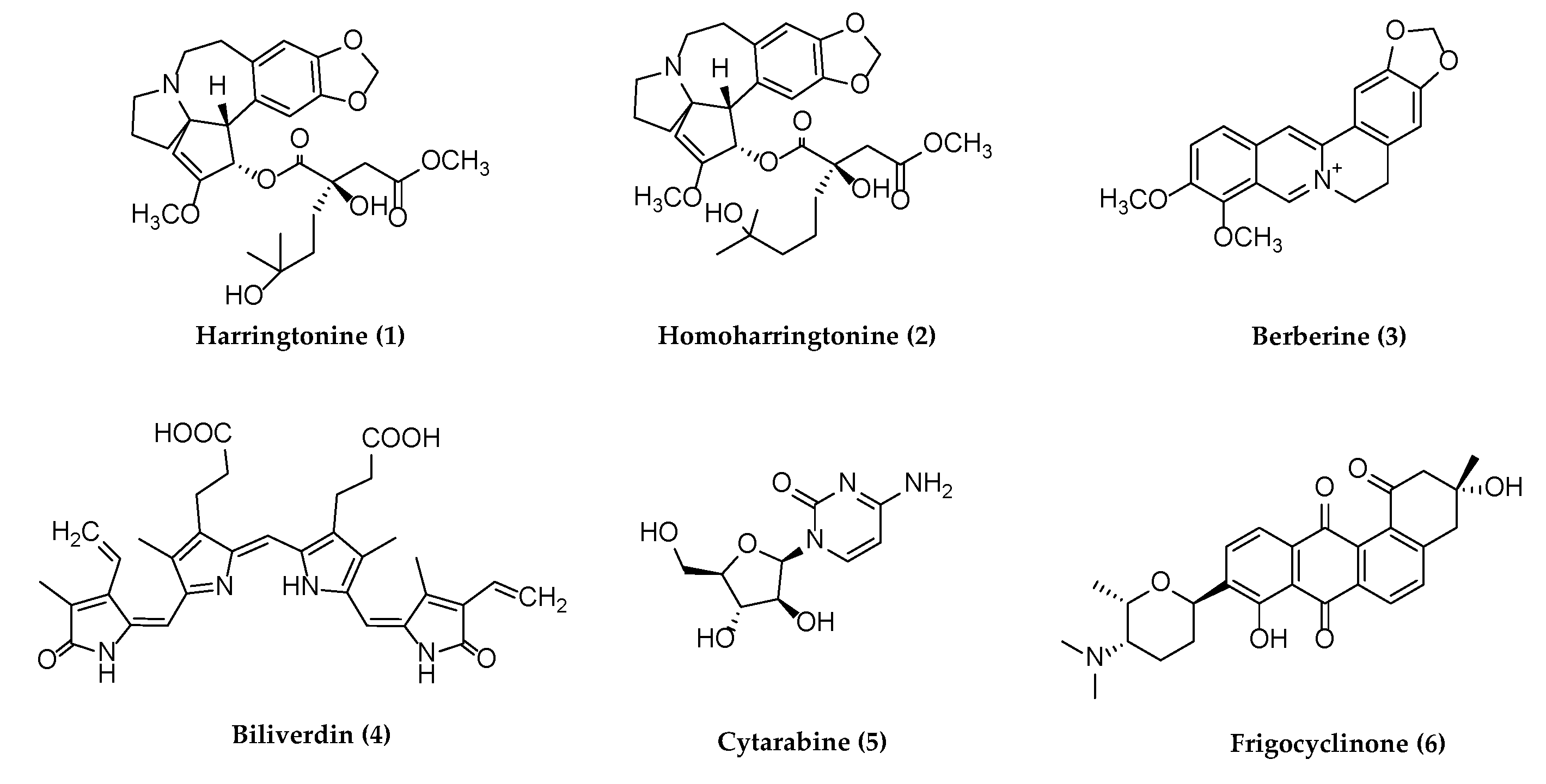
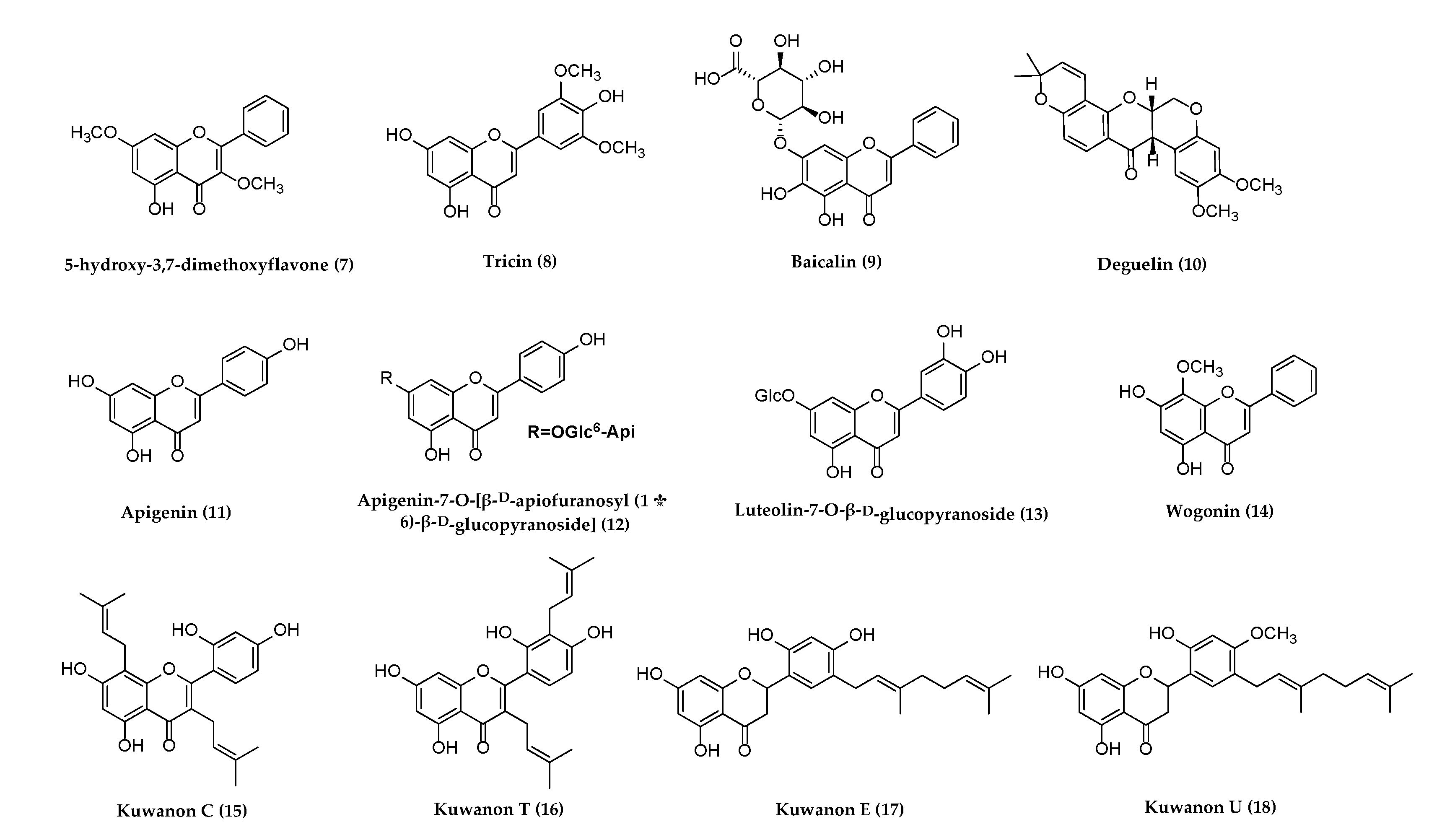




| No. | Compound | Origin | Virus (Strain) | Cell Line/Animal | EC50/IC50; SI, CC50 | Mode of Action | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Harringtonine (1) | Cephalotaxus harringtonii | VZV (pOka-luciferase, YC01) | HFF | EC50: 9.574 and 4.654 ng/mL of 1 and 2, CC50 = 45.82 and 74.71 ng/mL, respectively. | Induced down-regulation of VZV lytic genes by 1 and 2. | [47] |
| 2 | Homoharringtonine (2) | HSV-1 (F); PRV (Fa) | Vero, HEK293T, HeLa | IC50: 139 and 789 nM of 2 and acyclovir respectively in HSV-1. | Antagonizes the phosphorylation level of endogenous and exogenous eIF4E. | [48] | |
| 3 | Berberine (3) | Berberis vulgaris | HSV-1 (HF, Blue); HSV-2 (G) | HEK293T, Vero, HEC-1-A | EC50: 6.77, 5.04 μM of 3 in HSV-1, HSV-2, respectively. | Modulating cellular JNK and NF-κB pathways. | [49] |
| HSV-1 (F); HSV-2 (333) | Vero | IC50: 8.2 × 10−2 and 9.0 × 10−2 mg/mL of 3 in HSV-1 and HSV-2, respectively; SI = 147–161, CC50 = 13.2 mg/mL. | Inhibited HSV-1 and HSV-2 adsorption, gB, gE via western blot analysis. | [50] | |||
| HSV-2 (333) | VK2/E6E7 | - | Weak anti-HSV-2 activity | [51] | |||
| HSV (GFP) | RAW264.7 | - | Induction of antiviral state via type I IFN stimulation. | [52] | |||
| EBV | HONE1, HK1, NOD/SCID mice | IC50: 101.3 and 56.7 µM for HONE1 cells, IC50 = 124.5 and 43.1 µM for HK1-EBV cells when treated at 24, 48 h, respectively. | Decreases the expression of EBNA1. | [53] | |||
| HK1, HONE1, C666-1, NP460, Nude mice | IC50: ~ 100 μM for HONE1. IC50 ~ 400 μM for HK1, C666-1, NP460 NPC cell line. | Inhibition of STAT3 activation in NPC cells. | [54] | ||||
| CNE2, BALB/C-NU male mice | IC50: 21.71 μM | Regulated the expression of key protein in MAPK/ERK pathway by 3 combined with Rg3. | [55] | ||||
| IM-9 | - | Upregulation of XAF1 and GADD45α expression by MAPK and functional p53. | [56] | ||||
| HCMV (AD169, clinical isolates, drug-resistant) | HFF, NIH 3T3, HELF, U373-MG | EC50: 2.65 μM; SI: 147, CC50: 390 μM. | Interference with the transactivating activity of viral IE2 protein. | [57] | |||
| HCMV | MRC-5 | IC50: 0.68 and 0.91 μM of 3 and ganciclovir, respectively. | Berberine Chloride inhibited HCMV replication. | [58] | |||
| 4 | Biliverdin (4) | A chicken bile pigment | HHV-6 (HST) | MT-4 | - | Anti-HHV-6 activity in vitro during viral adsorption. | [59] |
| 5 | Cytarabine (5) | Cryptotheca crypta | KSHV | BCBL1, BC3, JSC1, BCP1, BJAB | - | Inducing cell cycle arrest, apoptosis, rapid degradation of KSHV LANA. | [60] |
| 6 | Frigocyclinone (6) | Streptomyces griseus strain NTK 97 | KSHV | - | - | Inhibited KSHV LANA1 via computational investigation. | [61] |
| 7 | 5-hydroxy-3,7- dimethoxyflavone (7) | Kaempferia parviflora | HCMV (AD169) | Serine protease | IC50: 250 μM | Inhibited HCMV protease. | [62] |
| 8 | Tricin (8) | Sasa albomarginata | HCMV (AD169) | MRC-5 | EC50: 0.17 μg/mL/IC50: 205 μg/mL; SI: 1205.8. | Anti-HCMV activity in vitro. | [63] |
| HCMV (Towne) | MRC-5 | - | Inhibited CXCL11 mRNA expression of IE and/or E stages. | [64] | |||
| HEL | - | Depressing CCL2 expression. | [65] | ||||
| - | Inhibits the CCL5 induction. | [66] | |||||
| EC50: 2.09 μM/IC50: 1.38 μM. | Inhibited kinase activity of CDK9. | [67] | |||||
| 9 | Baicalin (9) | Scutellariae baicalensis | HSV-1 (KOS); HSV-2 (196) | BCC-1/KMC | EC50 > 50 and 61.5 μg/mL in HSV-1 and HSV-2, respectively, CC50 = 41.1 μg/mL. | Anti-HSV-1 and HSV-2 activities. | [68] |
| HSV-1 (KOS) | Vero | EC50: 5 μM; SI: 200, CC50: 1000 μM. | Anti-HSV-1 activity involved the intracellular effect. | [69] | |||
| HHV-6 (GS) | HSB2 | - | Inhibited viral proliferation and adsorption. | [70] | |||
| 10 | Deguelin (10) | Derris trifoliata | HCMV (AD169) | HFF | EC50 and EC90: 1.3 and 6.5 μM respectively, CC50 > 500, SI > 357. | Inhibited the viral transcription factor Immediate-Early 2 (IE2). | [71] |
| HCMV (Ganciclovir-resistant) | NuFF-1 | - | Inhibits HCMV lytic replication. | [72] | |||
| 11 | Apigenin (11) | Punica granatum | HSV-1 (KOS); HSV-2 (196) | BCC-1/KMC | EC50: 6.7 and 9.7 mg/L, SI: 9.0 and 6.2 respectively. Both CC50: 59.9 mg/L. | Anti-HSV-1 and HSV-2 activities. | [73] |
| HSV-1 (KOS) | Vero | CC50: 250 μM, EC50: 5 μM, and SI: 50. | Anti-HSV-1 activity. | [69] | |||
| HSV-1 (Clinical isolates); HSV-2 (Clinical isolates, acyclovir-resistant) | EC50: 7.04 and 0.05 µg/mL respectively. EC50: 2.33 µg/mL in acyclovir-resistant strain. | Reduced viral progeny production; interfered with cell-to-cell virus spread. | [74] | ||||
| VZV (Clinical isolates) | Hep-2 | - | Interact with the protease of HHV-3 through discovery studio. | [75] | |||
| EBV | NA, HA, P3HR1 | - | Inhibits EBV reactivation by suppressing the promoter activities of two viral IE genes. | [76] | |||
| HCMV (Towne) | HEL 299 | IC50: 22 and 6.4 µM. | Inhibitory effect against HCMV. | [77] | |||
| KSHV | BC3, BCBL1, B cells | - | Modulate pro-apoptotic and pro-survival pathways. | [78] | |||
| 12 | Apigenin-7-O-[β-d-apiofuranosyl (1 → 6)-β-D-glucopyranoside] (12) | Lindernia crustacea | EBV | P3HR-1 | - | Significant inhibitory effect on the EBV Rta lytic cycle. | [79] |
| 13 | Luteolin-7-O-β-d-glucopyranoside (13) | - | |||||
| 14 | Wogonin (14) | Scutellaria baicalensis | HSV-1 (HF); HSV-2 (G) | Vero, HEC-1-A | - | Modulating cellular NF-κB and MAPK pathways. | [80] |
| VZV (YC01, YC03) | HFF | - | Modulation of type I interferon signaling and adenosine monophosphate-activated protein kinase activity. | [81] | |||
| EBV | Raji, four-week-old male BALB/c nude mice | - | Downregulating the expression of NF-κB through LMP1/miR-155/NF-κB/PU.1 pathway. | [82] | |||
| 15 | Kuwanon C (15) | Morus alba | HSV-1 (KOS) | Vero | IC50: 0.91 and 1.45 µg/mL; SI: 230.8 and 144.8 for 15 and acyclovir, respectively, CC50 > 210 µg/mL. | Molecular docking: targeting HSV-1 DNA polymerase and HSV-2 protease. | [83] |
| 16 | Kuwanon T (16) | HSV-1 (KOS) | IC50: 0.64 and 1.45 µg/mL; SI 238.1 and 144.8 for 16 and acyclovir, respectively, CC50 > 210 µg/mL. | Molecular docking: targeting HSV-1 DNA polymerase and HSV-2 protease. | |||
| 17 | Kuwanon E (17) | HSV-2 (Clinical isolates) | EC50: 1.61 and 1.65 µg/mL; SI 130.4 and 127.3 for 17 and acyclovir, respectively, CC50 > 210 µg/mL. | Molecular docking: targeting HSV-1 DNA polymerase and HSV-2 protease. | |||
| 18 | Kuwanon U (18) | HSV-1 (KOS) | IC50: 1.93 and 1.45 µg/mL; SI 108.8 and 144.8 for 18 and acyclovir, respectively, CC50 > 210 µg/mL. | Molecular docking: targeting HSV-1 DNA polymerase and HSV-2 protease. | |||
| 19 | Pristimerin (19) | Maytenus heterophylla | HCMV (93-1R, 91-7S) | MRC-5 | IC50: 0.53 μg/mL; SI: 27.9, CC50:14.8 μg/ml. | Inhibited viral replication. | [84] |
| 20 | Glycyrrhizin (20) | Glycyrrhiza glabra | HSV-1 | HEp-2 | - | Inhibitory effect on the growth of HSV-1. | [85] |
| HSV-1 (17 syn+); HSV-2 (HG52) | BHK, Vero | - | Reduced of HSV-1 and HSV-2 infectious virus. | [86] | |||
| HSV-1 (KOS) | HEF | IC50: 3.6 µM. | Inhibitory effect on HSV-1 replication. | [87] | |||
| Vero | IC50: 225 µM; SI > 2.7 CC50 > 608 µM. | Anti-HSV-1 activity. | [88] | ||||
| VZV, EBV | Raji, P3HR-1 | IC50: 0.71 and 0.04 mM for viral inhibition, IC50 = 21.3 and 4.8 mM for cell growth, TI: 30 and 120, respectively. | Inhibited VZV and EBV replication mainly at the early stage. | [89] | |||
| EBV | - | Inhibited EBV infection. | [90] | ||||
| HEK | - | Targeted the first step of the sumoylation process. | [91] | ||||
| KSHV | BC-3, BCBL-1, BCP-1, BC-1, BC-2, keratinocytes, CB33, Ramos, SLK, KS2616, HUVEC, BJAB | - | Downregulating LANA, upregulating vCyclin, and inducing cell death in KSHV-infected cells. | [92] | |||
| BCBL-1, 293T | - | Disruption of CTCF-cohesin-mediated RNA polymerase II pausing and sister chromatid cohesion. | [93] | ||||
| BC-3 | - | Showed antiviral activity against both latent and lytic KSHV. | [94] | ||||
| 21 | Triptolide (21) | Tripterygium wilfordii | EBV | B95-8 P3HR-1 HONE1/Akata C666-1 293T HeLa, BALB/c male mice | - | Reduced LMP1 expression in EBV-positive B lymphocytes. | [95] |
| HONE1/Akata, HK1/Akata, C666-1 CNE1/Akata, CNE1, BALB/c male mice | IC50: 55.43, 76.56, 1.12, 11.04, 10.66 for C666-1, HONE1/Akata, HK1/Akata, CNE1/Akata and CNE1 cells. | Increasing sensitivity of mitochondria apoptosis of nasopharyngeal carcinoma cells. | [96] | ||||
| B95-8, P3HR-1, 293T | - | Downregulating translation factors SP1 and c-Myc. | [97] | ||||
| KSHV | BCBL-1, JSC-1, BC-3, BJAB, P3HR-1, NOD/SCID mice | - | Downregulated LANA1 expression, reduced viral titers. | [98] | |||
| 22 | Phytol (22) | Lindernia crustacea | EBV | P3HR-1 | - | Inhibited Rta expression. | [79] |
| 23 | Carvacrol (23) | Lippia graveolens | HSV-1 (KOS, acyclovir-resistant) | HEp-2 | CC50: 250 µg/mL, EC50: 48.6 and 28.6 µg/ mL against KOS and acyclovir-resistant strain, respectively. | Anti-HSV-1 activity. | [99] |
| HSV-1 | Vero | IC50: 0.037%. | Interact with viral envelope before the adsorption. | [100] | |||
| HSV-1 (KOS) | - | 70% decrease in pretreatment of virus. | [101] | ||||
| HSV-1 (Clinical isolates) | IC50: 7 µM (1.05 µg/mL), CC50: 300 µM (45 µg/mL), SI: 43. | Anti-HSV-1 activity. | [102] | ||||
| HSV-2 (G) | BSC-1 | - | Inhibited HSV-2 induced RIP3-mediated programmed cell necrosis pathway and ubiquitin-proteasome system. | [103] | |||
| 24 | Cypellocarpin C (24) | Eucalyptus globulus | HSV-2 (Clinical Isolates) | Vero | EC50: 0.73 and 1.75 µg/mL; SI > 287.7 and >120 of 24 and acyclovir, respectively, CC50 > 210 µg/mL. | Stronger anti-HSV-2 compared to acyclovir. | [104] |
| 25 | Tereticornate A (25) | HSV-1 (KOS) | IC50: 0.96 and 1.92 µg/mL; SI > 218.8 and >109.4 of 25 and acyclovir, respectively, CC50 > 210 µg/mL. | Inhibited NF-κB activity. | |||
| 26 | Cis/trans-martynoside (26) | Lindernia crustacea | EBV | P3HR-1 | - | Inhibitory effect on the EBV lytic cycle. | [79] |
| 27 | Cis/trans- isomartynoside (27) | ||||||
| 28 | Resveratrol (28) | Polygonum cuspidatum | HSV-1/2 (Clinical isolates) | Vero, MRC-5 | Inhibited ICP4 expression. | [105] | |
| HSV-1 (Oral lesion, adult brain) | SKH1 mice | Inhibited HSV-induced skin lesion formation. | [106] | ||||
| HSV-1 (Oral lesion); HSV-2 (Genital lesion) | Inhibits or reduces HSV replication. | [107] | |||||
| HSV-1 (Acyclovir-resistant); HSV-2 | Vero | Inhibited NF–kB activation. | [108] | ||||
| HSV-1 (KOS, 7401H, TK-deficient, PAA-resistant, acyclovir-resistant, three clinical isolates); HSV-2 (Baylor186) | Vero, female BALB/c mice | IC50 = 19.8, 23.3, 23.5, 24.8, 25.5 and 21.7 µg/mL against three clinical isolates, TK-deficient and PAA-resistant HSV-1, respectively. | Exhibited the inhibitory activity at the early phase and late phase of replication of HSV-1 (KOS) and HSV-2. | [109] | |||
| HSV-1 (17); HSV-2 (G) | Vero | - | Promoted rapid and transient release of reactive oxygen species (ROS). | [110] | |||
| VZV (Ellen) | MRC-5 | EC50: 4 and 19 µM for acyclovir and 28 treatments, respectively. | Inhibited VZV IE62 synthesis. | [111] | |||
| EBV | Raji, female mice | IC50: 16.38 µg/mL, LD50: 143.75 µg/ml. | Inhibited TPA-induced Epstein–Barr early antigen activation. | [112] | |||
| P3HR-1 | EC50 ~ 24 µM. | Preventing the proliferation of the virus. | [113] | ||||
| Raji, Akata | - | Inhibited protein synthesis, decreased reactive oxygen species (ROS) levels, and suppressed the EBV-induced activation of the redox-sensitive transcription factors NF–kB and AP-1. | [114] | ||||
| B95-8, Akata, B cells | - | Downregulation of the anti-apoptotic proteins Mcl-1 and survivin. | [115] | ||||
| HCMV (Towne, AD169) | HEL 299 | IC50: 1.7 μM, CC50 > 400 μM, SI ≥ 50. | Blocked virus-induced activation of the EGFR and phosphatidylinositol-3-kinase signal transduction. | [116] | |||
| KSHV | HEK293, BCBL-1 | - | Lowered ERK1/2 activity, Egr1 expression. | [117] | |||
| BCBL-1, BC-1, P3HR1, BJAB | - | Inhibited HHV8 gene expression and replication | [118] | ||||
| 29 | Epigallocatechin gallate (29) | Camellia sinensis | HSV-1 (KOS) | Vero | CC50: 100 μM, EC50: 2.5 μM, and SI: 40. | Anti-HSV-1 activity. | [69] |
| HSV-1 (F1); HSV-2 (333) | Vero, CV-1 | - | Targeted gB, gD, or another enveloped glycoprotein. | [119] | |||
| - | Inactivated Class I, II, and III fusion proteins of enveloped viruses. | [120] | |||||
| HSV-1 (17) | Vero | - | p-EGCG inhibited HSV-1 production via viral adsorption in vitro. | [121] | |||
| HSV-1/2 (Clinical isolates) | Vero, MDCK | - | Inhibited virion surface proteins. | [122] | |||
| HSV-1 (KOS) | Vero | - | Direct virucidal properties on HSV-1. | [123] | |||
| Vero, OC3 | - | Reduced the levels of viral particles and viral DNA during viral entry phase. | [124] | ||||
| EBV | P3HR1 | - | Inhibited the expressions of EBV lytic proteins. | [125] | |||
| B95.8, CNE1-LMP1 | - | Involve the suppression of the activation of MEK/ERK1/2 and PI3-K/Akt signaling. | [126] | ||||
| B95.8, CNE1-LMP1 | IC50: 20 µM. | Involving in downregulation of LMP1. | [127] | ||||
| KSHV | BCBL-1, BC-1 | - | Induced cell death and ROS generation. | [128] | |||
| BC-3 | - | Displayed the activity against lytic KSHV infection. | [94] | ||||
| 30 | (+)-Rutamarin (30) | Ruta graveolens | EBV | P3HR-1 | - | Exhibited anti-EBV lytic DNA replication. | [129] |
| KSHV | BCBL-1, JSC-1, BJAB | IC50: 1.12 μM, EC50: 1.62 μM, SI: 84.14 and CC50: 94.24 μM. | Inhibition of the catalytic activity of human topoisomerase II. | [130] | |||
| 31 | Ginkgolic acid (31) | Ginkgo Biloba | HSV-1 (MacIntyre); HSV-2 (MS) | A549 | - | Anti-HSV-1 and 2 activities before viral adsorption to cell surface. | [131] |
| HSV-1 (Acyclovir-resistant-GFP-17+) | Vero, BALB/cJ female mice | - | Virucidal activity and fusion inhibition. | [132] | |||
| HSV-1 (F); HCMV (CH19, B16) | HEp2, 293T, HFF | - | Inhibited viral fusion. | [133] | |||
| 32 | Emodin (32) | Polygonum cuspidatum | HSV-1 (17) | Vero | EC50 = 21.54 μM in plaque reduction assay. | Inhibited nuclease activity of HSV-1 UL12. | [134] |
| HSV-1 (F); HSV-2 (333) | HEp-2, specific pathogen-free BALB/c mice | - | Inhibit the replication of HSV-1 and HSV-2. | [135] | |||
| HSV-1 (Laboratory) | HeLa, male BALB/c mice | - | Decreased TLR3 pathway and its downstream molecules. | [136] | |||
| EBV | P3HR-1 | - | Inhibitory effect on the EBV lytic cycle. | [79] | |||
| EC50: 1.2 μg/mL. | Inhibit the transcription of EBV immediate early genes, the expression of EBV lytic proteins and reduces EBV DNA replication. | [137] | |||||
| NA, HA, TW01, HONE-1 | CC50: 31, 58, 65, 79 μM for Tw01, HONE-1, HA, and NA, respectively. | Restricting EBV reactivation and NPC recurrence. | [138] | ||||
| HCMV (AD169, ganciclovir-resistant) | MRC-5 | EC50: 4.1 and 3.7 μM/IC50 = 9.6 and 12.6 for HCMV AD-169 and ganciclovir-resistant strain, respectively | Anti-HCMV activity. | [139] | |||
| 33 | Aloe-emodin (33) | Lindernia crustacea | EBV | P3HR-1 | Inhibitory effect on the EBV lytic cycle. | [79] | |
| HCMV (AD169) | MRC-5 | EC50 > 37.0 μM and IC50 > 37.0 μM. | Anti-HCMV activity | [139] | |||
| 34 | Daunorubicin (34) | Streptomyces peucetius | KSHV | BCBL-1, HEK293, Vero, PAN-LUC | - | Induced the luciferase expression under the control of the PAN or RTA promoters, induced the expressions of lytic genes. | [140] |
| 35 | Doxorubicin (35) | ||||||
| 36 | Epirubicin (36) | ||||||
| 37 | Allicin (37) | Allium sativum | KSHV | BC-3 | Antiviral activity against latent and lytic KSHV. | [94] | |
| 38 | Artemisinin-egonol (38) | Artemisia annua | HCMV (AD169-GFP) | Type A-positive human erythrocytes | EC50 = 0.17 and 0.13 μM, respectively | Strong anti-HCMV activity. | [141] |
| 39 | Artemisinin-homoegonol (39) | ||||||
| 40 | Byzantionoside B (40) | Lindernia crustacea | EBV | P3HR-1 | Inhibitory effect on the EBV lytic cycle. | [79] | |
| 41 | (+)-Hyperjaponicol B (41) | Hypericum japonicum | EBV | B95-8 | EC50: 0.57, 0.49, 2.86 μM, SI > 52.63, 106.78, 104.50 for 41, 42, and ganciclovir, respec-tively. | Inhibited EBV DNA replication. | [142] |
| 42 | Hyperjaponicol D (42) | ||||||
| 43 | Hyperjaponicol H (43) | EBV | B95-8 | EC50: 25.00 µM; SI > 2., CC50 > 50 µM | Moderately inhibited EBV lytic DNA replication. | [143] | |
| 44 | (+)-Japonicol B (44) | KSHV | Human-iSLK.219 | EC50: 8.75 μM; SI = 16.06, CC50 = 140.60 μM. | Moderately exhibited anti-KSHV activities. | [144] | |
| 45 | (+)-Japonicol E (45) | KSHV | Human-iSLK.219, Vero | IC50: 8.3 and 4.9 μM, SI = 23.49 and 25.7 for 45 and 46, respectively. | Inhibitory effects on KSHV lytic replication. | [145] | |
| 46 | (+)-Japonicol H (46) | ||||||
| 47 | (+)-Japonone A (47) | KSHV | Human-iSLK.219, Vero | IC50: 166.0 μM; SI > 3.01, CC50 > 500 μM. | Inhibitory effect on KSHV lytic replication | [146] | |
| 48 | Japopyrones B(48) | KSHV | Vero | IC50: 29.46 µM, CC50 > 200, SI > 6.79. | Inhibitory effect on TPA-induced KSHV lytic replication. | [147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruchawapol, C.; Yuan, M.; Wang, S.-M.; Fu, W.-W.; Xu, H.-X. Natural Products and Their Derivatives against Human Herpesvirus Infection. Molecules 2021, 26, 6290. https://doi.org/10.3390/molecules26206290
Ruchawapol C, Yuan M, Wang S-M, Fu W-W, Xu H-X. Natural Products and Their Derivatives against Human Herpesvirus Infection. Molecules. 2021; 26(20):6290. https://doi.org/10.3390/molecules26206290
Chicago/Turabian StyleRuchawapol, Chattarin, Man Yuan, Si-Min Wang, Wen-Wei Fu, and Hong-Xi Xu. 2021. "Natural Products and Their Derivatives against Human Herpesvirus Infection" Molecules 26, no. 20: 6290. https://doi.org/10.3390/molecules26206290
APA StyleRuchawapol, C., Yuan, M., Wang, S.-M., Fu, W.-W., & Xu, H.-X. (2021). Natural Products and Their Derivatives against Human Herpesvirus Infection. Molecules, 26(20), 6290. https://doi.org/10.3390/molecules26206290





