Extract of Triticum aestivum Sprouts Suppresses Acetaminophen-Induced Hepatotoxicity in Mice by Inhibiting Oxidative Stress
Abstract
:1. Introduction
2. Results
2.1. Chemical Properties of TAE
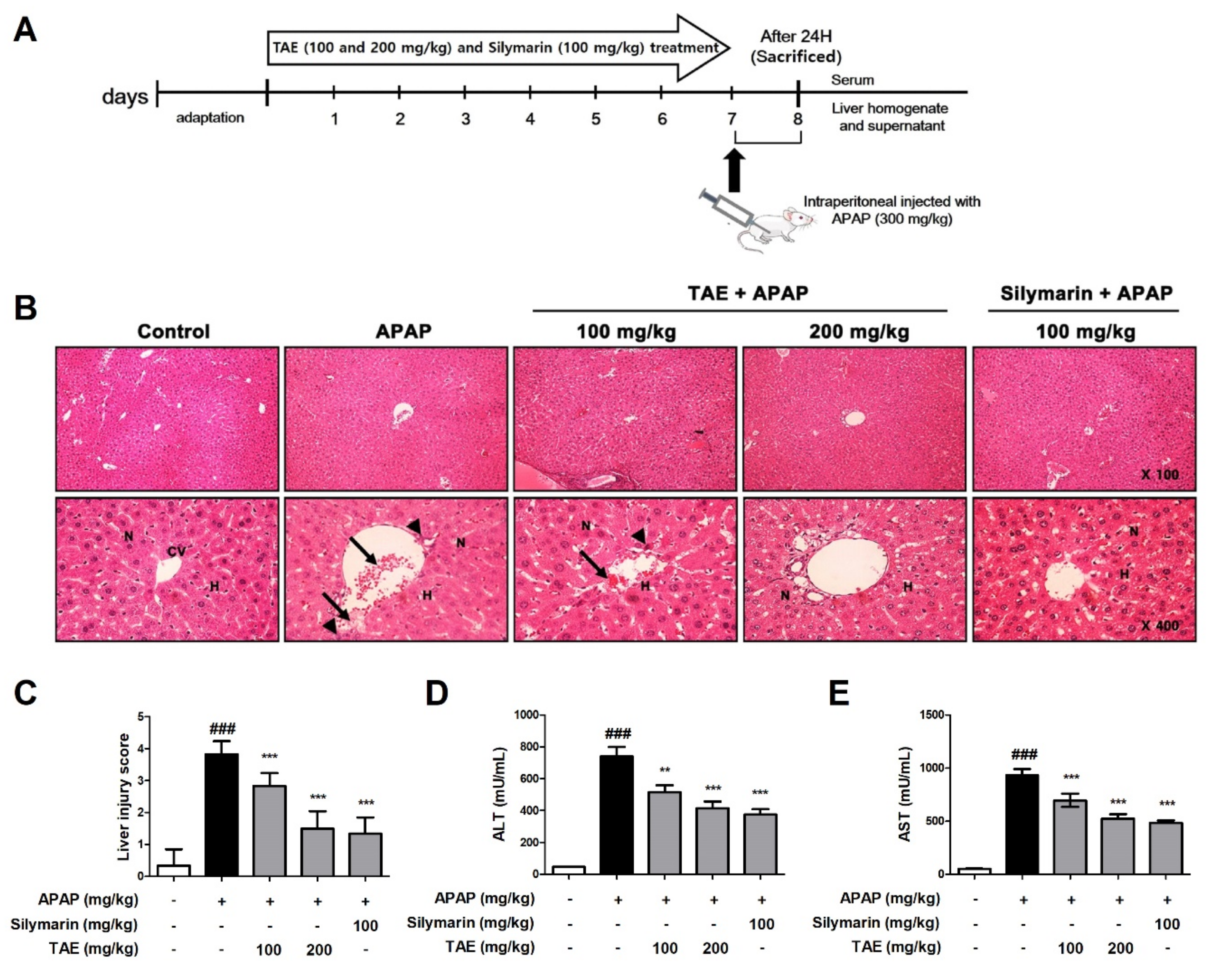
2.2. Effect of TAE on APAP-Induced Hepatotoxicity
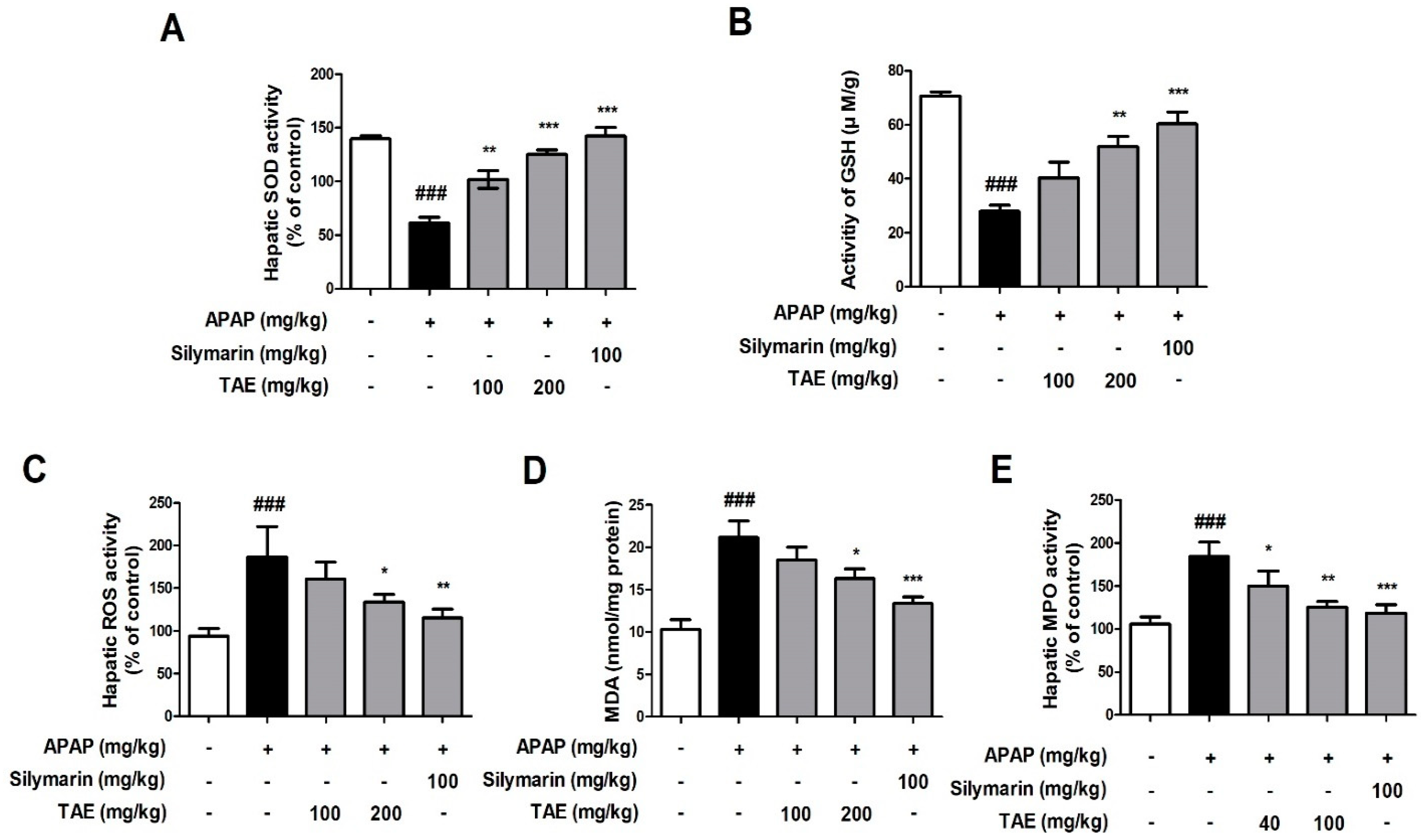
2.3. Effect of TAE on APAP-Induced Liver Oxidative Stress
2.4. Effect of TAE on APAP-Induced Inflammatory Cytokine
2.5. Effect of TAE on CYP2E1 and Nrf2 Pathway during APAP-Induced Hepatotoxicity
2.6. Effect of TAE on ASK1 and JNK Phosphorylation
2.7. Effect of TAE on Hepatocyte Apoptosis in APAP-Induced Hepatotoxicity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Ethanolic Extraction
4.3. Chemical Analysis
4.4. Experimental Design and Animals
4.5. Histological Observation
4.6. ALT and AST Assays
4.7. Cytokine Analysis
4.8. Gene Expression Analysis
4.9. Biochemical Analysis
4.10. Protein Expression Analysis
4.11. DNA Fragmentation Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| ALT | Alanine aminotransferase |
| APAP | Acetaminophen (N-acetyl-para-aminophenol) |
| AST | Aspartate aminotransferase |
| Bax | Bcl-2-associated X |
| Bcl2 | B-cell lymphoma 2 |
| Caspase | Cysteinyl aspartate specific proteinase |
| CYP2E1 | Cytochrome P4502E1 |
| GSH | Glutathione |
| HO-1 | Heme oxygenase-1 |
| HPLC | High-performance liquid chromatography |
| MDA | Malondialdehyde |
| MPO | Myeloperoxidase |
| NAPQI | N-acetyl-p-benzoquinone imine |
| NF-κB | Transcription factors nuclear factor-kappa B |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| qPCR | quantitative polymerase chain reaction |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TAE | Triticum aestivum sprouts extract |
| UPLC | Ultra-performance liquid chromatography |
References
- Miner, D.J.; Kissinger, P.T. Evidence for the involvement of N-acetyl-p-benzoquinoneimine in acetaminophen metabolism. Biochem. Pharmacol. 1979, 28, 3285–3290. [Google Scholar] [CrossRef]
- Nassini, R.; Materazzi, S.; Andrè, E.; Sartiani, L.; Aldini, G.; Trevisani, M.; Carnini, C.; Massi, D.; Pedretti, P.; Carini, M.; et al. Acetaminophen, via its reactive metabolite N-acetyl-p-benzo-quinoneimine and transient receptor potential ankyrin-1 stimulation, causes neurogenic inflammation in the airways and other tissues in rodents. FASEB J. 2010, 24, 4904–4916. [Google Scholar] [PubMed]
- Gentry, C.; Andersson, D.A.; Bevan, S. TRPA1 mediates the hypothermic action of acetaminophen. Sci. Rep. 2015, 5, 12771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeschke, H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: The protective effect of allopurinol. J. Pharmacol. Exp. Ther. 1990, 255, 935–941. [Google Scholar]
- Antoine, D.J.; Williams, D.P.; Park, B.K. Understanding the role of reactive metabolites in drug-induced hepatotoxicity: State of the science. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Akakpo, J.Y.; Ramachandran, A.; Kandel, S.E.; Ni, H.M.; Kumer, S.C.; Rumack, B.H.; Jaeschke, H. 4-Methylpyrazole protects against acetaminophen hepatotoxicity in mice and in primary human hepatocytes. Hum. Exp. Toxicol. 2018, 37, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Mcgill, M.R.; Sharpe, M.R.; Williams, C.D.; Taha, M.; Curry, S.C.; Jaeschke, H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Investig. 2012, 122, 1574–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Ki, H.H.; Kim, D.K.; Lee, Y.M. Triticum aestivum sprout extract attenuates 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in mice and the expression of chemokines in human keratinocytes. Mol. Med. Rep. 2018, 18, 3461–3468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gore, R.D.; Palaskar, S.J.; Bartake, A.R. Wheatgrass: Green blood can help to fight cancer. J. Clin. Diagn. Res. 2017, 11, ZC40. [Google Scholar] [CrossRef]
- Luyen, B.T.T.; Tai, B.H.; Thao, N.P.; Cha, J.Y.; Lee, Y.M.; Kim, Y.H. A new phenolic component from Triticum aestivum sprouts and its effects on LPS-stimulated production of nitric oxide and TNF-α in RAW 264.7 cells. Phytother. Res. 2014, 28, 1064–1070. [Google Scholar] [CrossRef]
- Das, A.; Raychaudhuri, U.; Chakraborty, R. Effect of freeze drying and oven drying on antioxidant properties of fresh wheatgrass. Int. J. Food Sci. Nutr. 2012, 63, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Nepali, S.; Ki, H.H.; Lee, J.H.; CHA, J.Y.; Lee, Y.M.; Kim, D.K. Triticum aestivum sprout-derived polysaccharide exerts hepatoprotective effects against ethanol-induced liver damage by enhancing the antioxidant system in mice. Int. J. Mol. Med. 2017, 40, 1243–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nepali, S.; Ki, H.H.; Lee, J.H.; LEE, H.Y.; Kim, D.K.; Lee, Y.M. Wheatgrass-derived polysaccharide has antiinflammatory, anti-oxidative and anti-apoptotic effects on LPS-induced hepatic injury in mice. Phytother. Res. 2017, 31, 1107–1116. [Google Scholar] [CrossRef]
- Ki, H.H.; Hwang, S.W.; Lee, J.H.; Kim, Y.H.; Kim, D.K.; Lee, Y.M. A dichloromethane fraction of Triticum aestivum sprouts reduces allergic immune response through inhibiting Th2 differentiation in ovalbumin-immunized mice. Mol. Med. Rep. 2017, 16, 3535–3541. [Google Scholar] [CrossRef]
- Nagaoka, H. Treatment of germinated wheat to increase levels of GABA and IP6 analyzed by endogenous enzymes. Biotechnol. Prog. 2005, 21, 405–410. [Google Scholar] [CrossRef]
- Im, J.Y.; Ki, H.H.; Xin, M.; Kwon, S.U.; Kim, Y.H.; Kim, D.K.; Hong, S.P.; Jin, J.S.; Lee, Y.M. Anti-obesity effect of Triticum aestivum sprout extract in high-fat-diet-induced obese mice. Biosci. Biotechnol. Biochem. 2015, 79, 1133–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bataille, A.M.; Manautou, J.E. Nrf2: A potential target for new therapeutics in liver disease. Clin. Pharmacol. Ther. 2012, 92, 340–348. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Wei, J.G.; Tu, M.J.; Gu, J.G.; Zhang, W. Fucoidan alleviates acetaminophen-induced hepatotoxicity via oxidative stress inhibition and Nrf2 translocation. Int. J. Mol. Sci. 2018, 19, 4050. [Google Scholar] [CrossRef] [Green Version]
- Nagai, H.; Noguchi, T.; Takeda, K.; Ichijo, H. Pathophysiological roles of ASK1-MAP kinase signaling pathways. J. Biochem. Mol. Biol. 2007, 40, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mirochnitchenko, O.; Weisbrot-Lefkowitz, M.; Reuhl, K.; Chen, L.; Yang, C.; Inouye, M. Acetaminophen toxicity. Opposite effects of two forms of glutathione peroxidase. J. Cell. Biol. Metab. 1999, 274, 10349–10355. [Google Scholar]
- Minsart, C.; Liefferinckx, C.; Lemmers, A.; Dressen, C.; Quertinmont, E.; Leclercq, I.; Devière, J.; Moreau, R.; Gustot, T. New insights in acetaminophen toxicity: HMGB1 contributes by itself to amplify hepatocyte necrosis in vitro through the TLR4-TRIF-RIPK3 axis. Sci. Rep. 2020, 10, 5557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Sumaya-Martínez, M.T.; Gutiérrez-Salinas, J.; Bautista, M.; Morales-González, A.; García-Luna y González-Rubio, Y.; Aguilar-Faisal, J.L.; Morales-González, J.A. Review of natural products with hepatoprotective effects. World J. Gastroenterol. 2014, 20, 14787. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, L.; Wang, Y.; Zhao, T.; Khan, A.; Wang, Y.; Cao, J.; Cheng, G. Protective effect of Que Zui tea hot-water and aqueous ethanol extract against acetaminophen-induced liver injury in mice via inhibition of oxidative stress, inflammation, and apoptosis. Food Funct. 2021, 12, 2468–2480. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; He, D.; Li, W.; Wang, M.; Zhao, S.; Liu, L.; Cao, Z.; Liu, R.; Huang, Y.; Li, H.; et al. Hepatoprotective effect of Citrus aurantium L. against APAP-induced liver injury by regulating liver lipid metabolism and apoptosis. Int. J. Biol. Sci. 2020, 16, 752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oz, H.S.; Chen, T.S. Green-tea polyphenols downregulate cyclooxygenase and Bcl-2 activity in acetaminophen-induced hepatotoxicity. Dig. Dis. Sci. 2008, 53, 2980–2988. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Chakraborti, K.K.; Handa, S.S. Anti hepatotoxic acitivity of some Indian herbal formulations as compared to silymarin. Fitoterapia 1991, 62, 229–235. [Google Scholar]
- Lim, J.Y.; Lee, J.H.; Yun, D.H.; Lee, Y.M.; Kim, D.K. Inhibitory effects of nodakenin on inflammation and cell death in lipopolysaccharide-induced liver injury mice. Phytomedicine 2021, 81, 153411. [Google Scholar] [CrossRef] [PubMed]





| Gene | Base sequence (5′-3′) | Size (bp) |
|---|---|---|
| mTNF-α-F | TAGCCAGGAGGGAGAACAGA | 127 |
| mTNF-α-R | TTTTCTGGAGGGAGATATGG | |
| mIL-6-F | GACAACCACGGCCTTCCCTA | 302 |
| mIL-6-R | GGTACTCCAGAAGACCAGAGGA | |
| mIL-1β-F | GCAACTGTTCCTGAACTCAACT | 89 |
| mIL-1β-R | ATCTTTTGGGGTCCGTCAACT | |
| mGAPDH-F | GAAGGTGAAGGTCGGAGT | 226 |
| mGAPDH-R | GAAGATGGTGATGGGATTTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.-Y.; Yun, D.-H.; Lee, J.-H.; Kwon, Y.-B.; Lee, Y.-M.; Lee, D.-H.; Kim, D.-K. Extract of Triticum aestivum Sprouts Suppresses Acetaminophen-Induced Hepatotoxicity in Mice by Inhibiting Oxidative Stress. Molecules 2021, 26, 6336. https://doi.org/10.3390/molecules26216336
Lim J-Y, Yun D-H, Lee J-H, Kwon Y-B, Lee Y-M, Lee D-H, Kim D-K. Extract of Triticum aestivum Sprouts Suppresses Acetaminophen-Induced Hepatotoxicity in Mice by Inhibiting Oxidative Stress. Molecules. 2021; 26(21):6336. https://doi.org/10.3390/molecules26216336
Chicago/Turabian StyleLim, Ji-Ye, Dae-Ho Yun, Ji-Hyun Lee, Young-Bae Kwon, Young-Mi Lee, Dong-Hyun Lee, and Dae-Ki Kim. 2021. "Extract of Triticum aestivum Sprouts Suppresses Acetaminophen-Induced Hepatotoxicity in Mice by Inhibiting Oxidative Stress" Molecules 26, no. 21: 6336. https://doi.org/10.3390/molecules26216336
APA StyleLim, J.-Y., Yun, D.-H., Lee, J.-H., Kwon, Y.-B., Lee, Y.-M., Lee, D.-H., & Kim, D.-K. (2021). Extract of Triticum aestivum Sprouts Suppresses Acetaminophen-Induced Hepatotoxicity in Mice by Inhibiting Oxidative Stress. Molecules, 26(21), 6336. https://doi.org/10.3390/molecules26216336





