Anticancer Activity of Aqueous Extracts from Asparagus officinalis L. Byproduct on Breast Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Asparagus Extracts
2.2. LC-MS/MS Analysis of Asparagus Extracts
2.2.1. Free Amino Acid Profile
2.2.2. Organic Acids, Phenolic Acids, Polyphenols and Derivatives
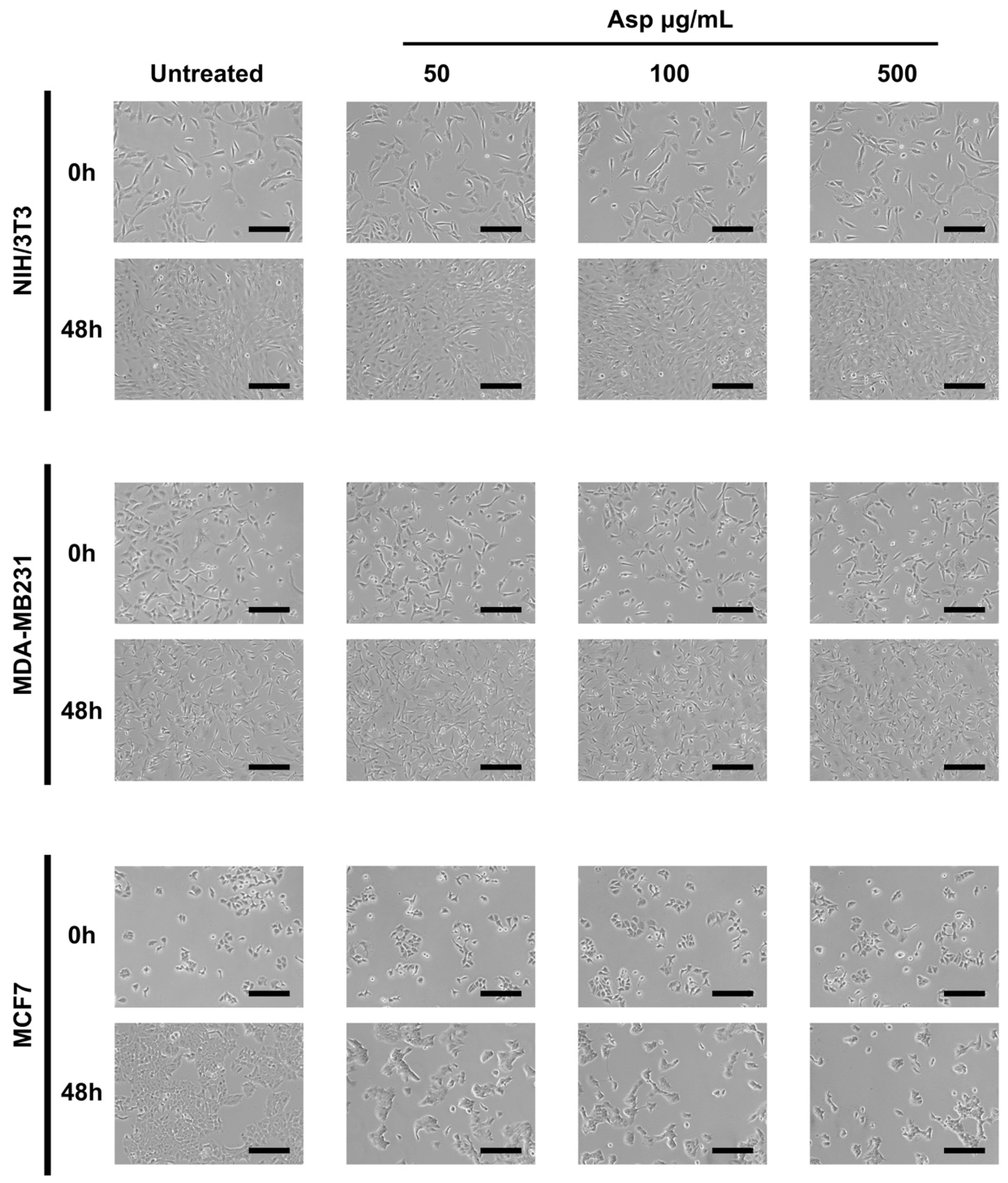
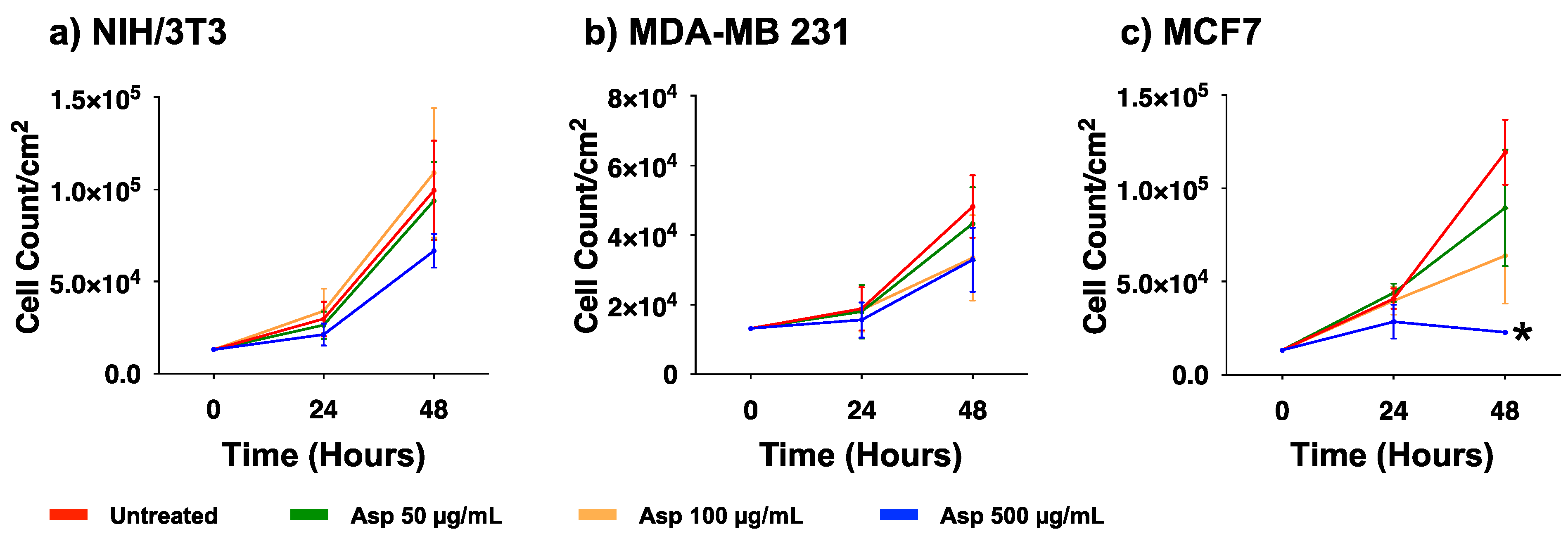
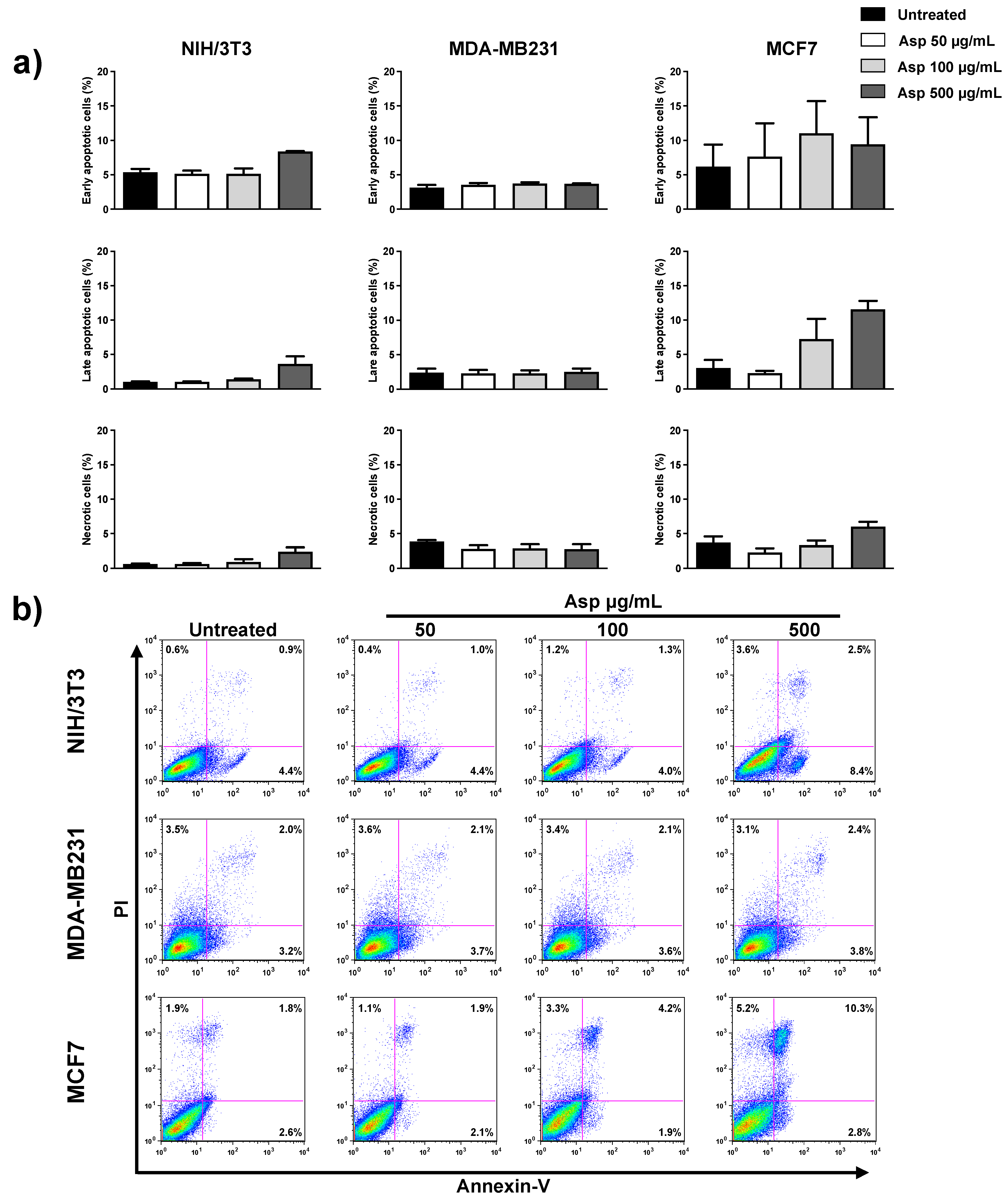
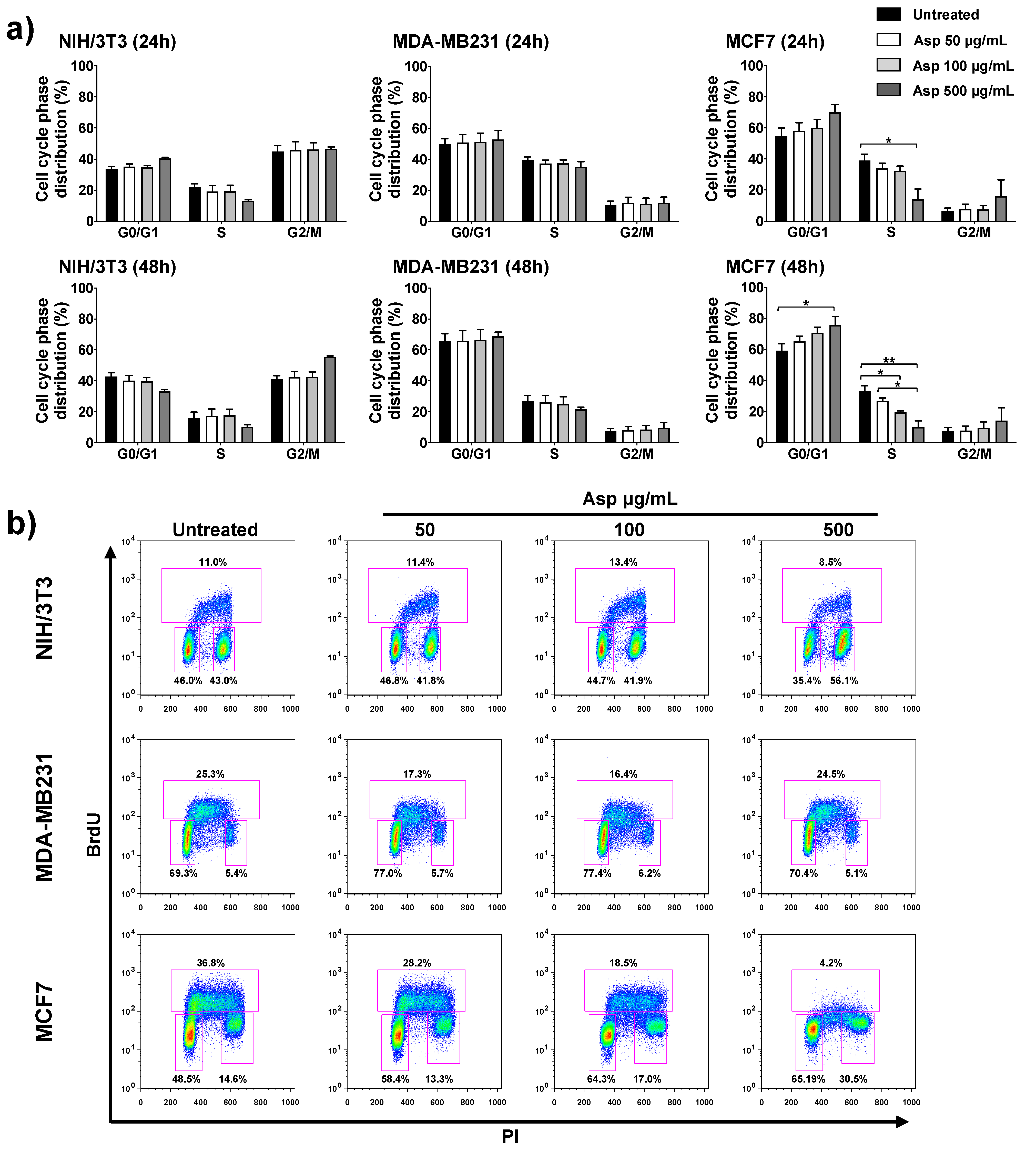
2.3. Asparagus Extracts Affect Viability of MCF-7 Breast Cancer Cells through Cell Cycle Block
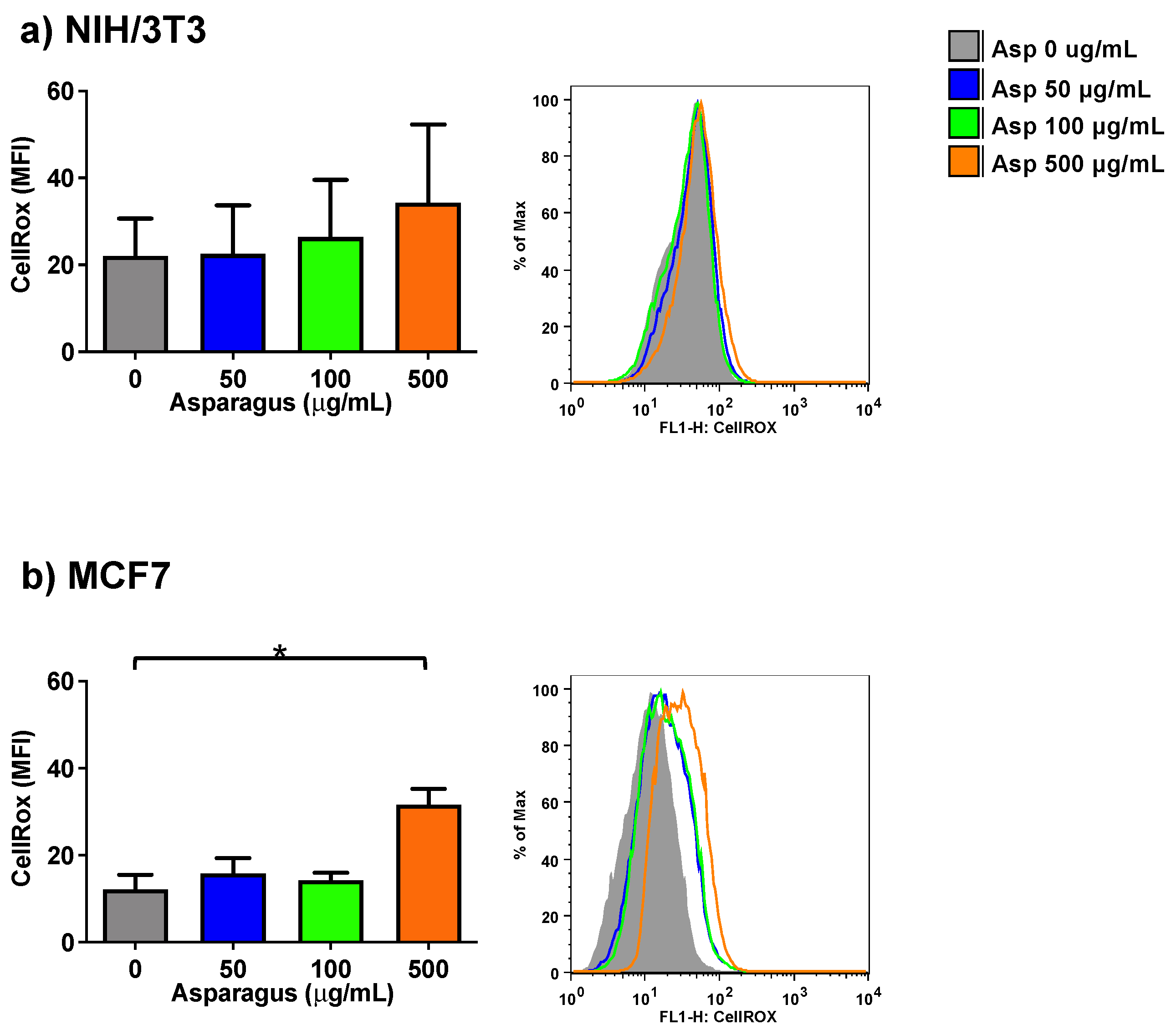
2.4. Asparagus Extracts Cause ROS Production in Breast Cancer Cells but Not in Normal Cells
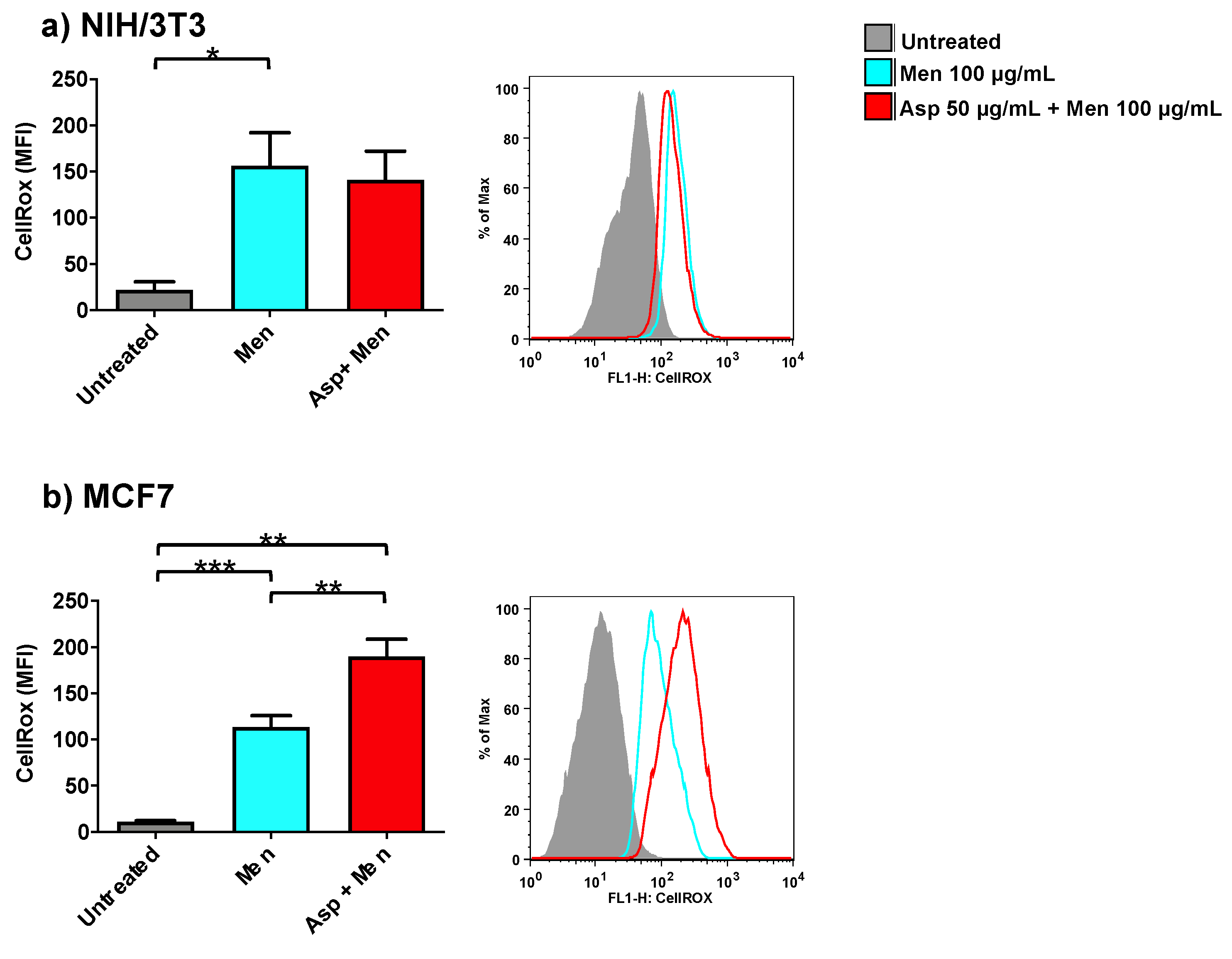
2.5. Asparagus Extracts Increase Oxidant Activity of Menadione in Breast Cancer Cells
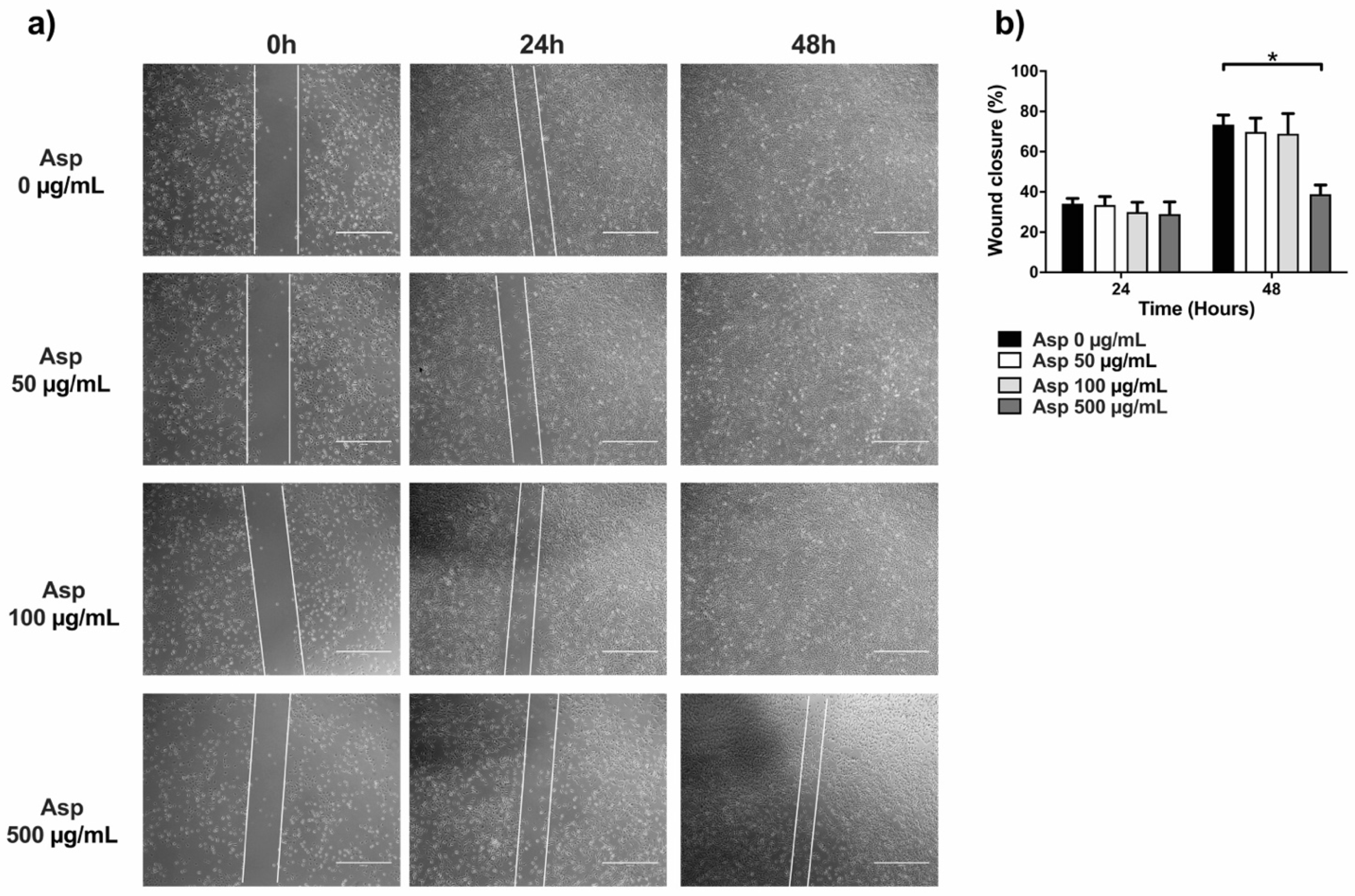
2.6. Asparagus Extracts Impair Cell Migration
3. Discussion
4. Materials and Methods
4.1. Preparation of Asparagus Extracts, Phenolic Content, Total Flavonoids and Antioxidant Activity
4.2. LC-MS/MS Analysis of Asparagus Extracts
4.2.1. Free Amino Acid Profile
4.2.2. Organic Acids, Phenolic Acids, Polyphenols and Derivatives
4.3. Cell Culture and Treatment
4.4. Assessment of Cell Viability, Cell Cycle Profile and Apoptosis
4.5. Analysis of ROS
4.6. Wound Healing Assay
4.7. Assessment of Cell Migration
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, B.; Quaquarini, E.; Palumbo, R.; Balletti, E.; Presti, D.; Malovini, A.; Agozzino, M.; Teragni, C.M.; Terzoni, A.; Bernardo, A.; et al. Role of Androgen Receptor Expression in Early Stage ER+/PgR−/HER2– Breast Cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835920958355. [Google Scholar] [CrossRef] [PubMed]
- Kwapisz, D. Cyclin-Dependent Kinase 4/6 Inhibitors in Breast Cancer: Palbociclib, Ribociclib, and Abemaciclib. Breast Cancer Res. Treat. 2017, 166, 41–54. [Google Scholar] [CrossRef]
- Presti, D.; Quaquarini, E. The PI3K/AKT/MTOR and CDK4/6 Pathways in Endocrine Resistant HR+/HER2− Metastatic Breast Cancer: Biological Mechanisms and New Treatments. Cancers 2019, 11, 1242. [Google Scholar] [CrossRef] [PubMed]
- Almehdar, H.; Abdallah, H.M.; Osman, A.-M.M.; Abdel-Sattar, E.A. In Vitro Cytotoxic Screening of Selected Saudi Medicinal Plants. J. Nat. Med. 2012, 66, 406–412. [Google Scholar] [CrossRef]
- Sethi, G.; Shanmugam, M.; Warrier, S.; Merarchi, M.; Arfuso, F.; Kumar, A.; Bishayee, A. Pro-Apoptotic and Anti-Cancer Properties of Diosgenin: A Comprehensive and Critical Review. Nutrients 2018, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Chitrakar, B.; Zhang, M.; Adhikari, B. Asparagus (Asparagus officinalis): Processing Effect on Nutritional and Phytochemical Composition of Spear and Hard-Stem Byproducts. Trends Food Sci. Technol. 2019, 93, 1–11. [Google Scholar] [CrossRef]
- Adouni, K.; Chahdoura, H.; Mosbah, H.; Santos-Buelga, C.; González-Paramás, A.M.; Ciudad-Mulero, M.; Fernandes, Â.; Calhelha, R.C.; Morales, P.; Flamini, G.; et al. Revalorization of Wild Asparagus Stipularis Forssk. as a Traditional Vegetable with Nutritional and Functional Properties. Food Funct. 2018, 9, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Singh, R. Asparagus racemosus: A Review on Its Phytochemical and Therapeutic Potential. Nat. Prod. Res. 2016, 30, 1896–1908. [Google Scholar] [CrossRef]
- Rodríguez, R.; Jaramillo, S.; Rodríguez, G.; Espejo, J.A.; Guillén, R.; Fernández-Bolaños, J.; Heredia, A.; Jiménez, A. Antioxidant Activity of Ethanolic Extracts from Several Asparagus Cultivars. J. Agric. Food Chem. 2005, 53, 5212–5217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Y.-Y.; Sun, Y.-S.; Ma, R.-H.; Thakur, K.; Zhang, J.-G.; Wei, Z.-J. Asparanin A from Asparagus officinalis L. Induces G0/G1 Cell Cycle Arrest and Apoptosis in Human Endometrial Carcinoma Ishikawa Cells via Mitochondrial and PI3K/AKT Signaling Pathways. J. Agric. Food Chem. 2020, 68, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Mfengwana, P.H.; Mashele, S.S.; Manduna, I.T. Cytotoxicity and Cell Cycle Analysis of Asparagus Laricinus Burch. and Senecio Asperulus DC. on Breast and Prostate Cancer Cell Lines. Heliyon 2019, 5, e01666. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Alventosa, J.M.; Jaramillo-Carmona, S.; Rodríguez-Gutiérrez, G.; Guillén-Bejarano, R.; Jiménez-Araujo, A.; Fernández-Bolaños, J.; Rodríguez-Arcos, R. Preparation of Bioactive Extracts from Asparagus By-Product. Food Bioprod. Process. 2013, 91, 74–82. [Google Scholar] [CrossRef]
- Montone, C.M.; Zenezini Chiozzi, R.; Marchetti, N.; Cerrato, A.; Antonelli, M.; Capriotti, A.L.; Cavaliere, C.; Piovesana, S.; Laganà, A. Peptidomic Approach for the Identification of Peptides with Potential Antioxidant and Anti-Hyperthensive Effects Derived From Asparagus By-Products. Molecules 2019, 24, 3627. [Google Scholar] [CrossRef] [PubMed]
- Bergantin, C.; Maietti, A.; Tedeschi, P.; Font, G.; Manyes, L.; Marchetti, N. HPLC-UV/Vis-APCI-MS/MS Determination of Major Carotenoids and Their Bioaccessibility from “Delica” (Cucurbita Maxima) and “Violina” (Cucurbita Moschata) Pumpkins as Food Traceability Markers. Molecules 2018, 23, 2791. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, W.; Zhu, X.; Zhao, D.; Wang, K.; Wang, R.; Qu, W. The Aqueous Extract of Asparagus officinalis L. by-Product Exerts Hypoglycaemic Activity in Streptozotocin-Induced Diabetic Rats. J. Sci. Food Agric. 2011, 91, 2095–2099. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Yuan, F.; Wang, N.; Gao, Y.; Huang, Y. Extraction and Analysis of Antioxidant Compounds from the Residues of Asparagus officinalis L. J. Food Sci. Technol. 2015, 52, 2690–2700. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.; Jaramillo, S.; Guillén, R.; Jiménez, A.; Fernández-Bolaños, J.; Heredia, A. Cell Wall Phenolics of White and Green Asparagus. J. Sci. Food Agric. 2005, 85, 971–978. [Google Scholar] [CrossRef]
- Fuentes-Alventosa, J.M.; Jaramillo-Carmona, S.; Rodríguez-Gutiérrez, G.; Rodríguez-Arcos, R.; Fernández-Bolaños, J.; Guillén-Bejarano, R.; Espejo-Calvo, J.A.; Jiménez-Araujo, A. Effect of the Extraction Method on Phytochemical Composition and Antioxidant Activity of High Dietary Fibre Powders Obtained from Asparagus By-Product. Food Chem. 2009, 116, 484–490. [Google Scholar] [CrossRef]
- Fuentes-Alventosa, J.M.; Rodríguez-Gutiérrez, G.; Jaramillo-Carmona, S.; Espejo-Calvo, J.A.; Rodríguez-Arcos, R.; Fernández-Bolaños, J.; Guillén-Bejarano, R.; Jiménez-Araujo, A. Effect of Extraction Method on Chemical Composition and Functional Characteristics of High Dietary Fibre Powders Obtained from Asparagus By-Products. Food Chem. 2009, 2, 665–671. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food Waste: A Potential Bioresource for Extraction of Nutraceuticals and Bioactive Compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Beck, R.; Verrax, J.; Dejeans, N.; Taper, H.; Calderon, P.B. Menadione Reduction by Pharmacological Doses of Ascorbate Induces an Oxidative Stress That Kills Breast Cancer Cells. Int. J. Toxicol. 2009, 28, 33–42. [Google Scholar] [CrossRef]
- Bakalova, R.; Semkova, S.; Ivanova, D.; Zhelev, Z.; Miller, T.; Takeshima, T.; Shibata, S.; Lazarova, D.; Aoki, I.; Higashi, T. Selective Targeting of Cancerous Mitochondria and Suppression of Tumor Growth Using Redox-Active Treatment Adjuvant. Oxid. Med. Cell. Longev. 2020, 2020, e6212935. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-D.; Sun, Y.; Zhou, W.-J.; Xie, X.-Z.; Zhou, Q.-M.; Lu, Y.-Y.; Su, S.-B. Resveratrol Enhances Inhibition Effects of Cisplatin on Cell Migration and Invasion and Tumor Growth in Breast Cancer MDA-MB-231 Cell Models In Vivo and In Vitro. Molecules 2021, 26, 2204. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mazumdar, M.; Chakraborty, S.; Manna, A.; Saha, S.; Khan, P.; Bhattacharjee, P.; Guha, D.; Adhikary, A.; Mukhjerjee, S.; et al. Curcumin Inhibits Breast Cancer Stem Cell Migration by Amplifying the E-Cadherin/β-Catenin Negative Feedback Loop. Stem Cell Res. Ther. 2014, 5, 116. [Google Scholar] [CrossRef] [PubMed]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Takeyama, H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers 2015, 7, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Guan, S.; Hou, Y.; Qin, Y.; Zeng, H.; Yang, L.; Qiao, Y.; Liu, S.; Li, Q.; Jin, T.; et al. FOSL2 Promotes VEGF-Independent Angiogenesis by Transcriptionnally Activating Wnt5a in Breast Cancer-Associated Fibroblasts. Theranostics 2021, 11, 4975–4991. [Google Scholar] [CrossRef]
- Marchetti, N.; Bonetti, G.; Brandolini, V.; Cavazzini, A.; Maietti, A.; Meca, G.; Mañes, J. Stinging Nettle (Urtica dioica L.) as a Functional Food Additive in Egg Pasta: Enrichment and Bioaccessibility of Lutein and β-Carotene. J. Funct. Foods 2018, 47, 547–553. [Google Scholar] [CrossRef]
- Alonso-Garrido, M.; Tedeschi, P.; Maietti, A.; Font, G.; Marchetti, N.; Manyes, L. Mitochondrial Transcriptional Study of the Effect of Aflatoxins, Enniatins and Carotenoids in Vitro in a Blood Brain Barrier Model. Food Chem. Toxicol. 2020, 137, 111077. [Google Scholar] [CrossRef]
- Bergantin, C.; Maietti, A.; Cavazzini, A.; Pasti, L.; Tedeschi, P.; Brandolini, V.; Marchetti, N. Bioaccessibility and HPLC-MS/MS Chemical Characterization of Phenolic Antioxidants in Red Chicory (Cichorium Intybus). J. Funct. Foods 2017, 33, 94–102. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, R.P.; Beek, T.A. van Screening of Radical Scavenging Activity of Some Medicinal and Aromatic Plant Extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Chang, C.; Yang, M.; Wen, H.; Chern, J. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colometric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Trapella, C.; Rizzo, R.; Gallo, S.; Alogna, A.; Bortolotti, D.; Casciano, F.; Zauli, G.; Secchiero, P.; Voltan, R. HelixComplex Snail Mucus Exhibits Pro-Survival, Proliferative and pro-Migration Effects on Mammalian Fibroblasts. Sci. Rep. 2018, 8, 17665. [Google Scholar] [CrossRef]
- Voltan, R.; Rimondi, E.; Melloni, E.; Rigolin, G.M.; Casciano, F.; Arcidiacono, M.V.; Celeghini, C.; Cuneo, A.; Zauli, G.; Secchiero, P. Ibrutinib Synergizes with MDM-2 Inhibitors in Promoting Cytotoxicity in B Chronic Lymphocytic Leukemia. Oncotarget 2016, 7, 70623–70638. [Google Scholar] [CrossRef][Green Version]

| TPC (μgGAE/mgde) | 10.09 ± 1.23 |
| TFC (μgGAE/mgde) | 9.31 ± 0.74 |
| DPPH (μmolTE/mgde) | 0.010 ± 0.006 |
| ABTS (μmolTE/mgde) | 0.017 ± 0.006 |
| Amino Acid | Concentration (μg/mgde) | MRM (m/z) |
|---|---|---|
| ALA | 0.101 ± 0.023 | 90.2 → 44.2 |
| ARG | 0.369 ± 0.019 | 175.1 → 70.1 |
| ASN | 13.65 ± 1.12 | 133.1 → 74.1 |
| ASP | 0.322 ± 0.022 | 134.1 → 74.1 |
| GLU | 0.451 ± 0.027 | 148.2 → 84.2 |
| HIS | 0.254 ± 0.015 | 156.1 → 110.2 |
| LEU+ILE | 0.368 ± 0.018 | 132.1 → 86.1 |
| LYS | 25.33 ± 1.75 | 147.1 → 84.1 |
| MET | 0.069 ± 0.004 | 150.2 → 104.1 |
| PHE | 0.117 ± 0.012 | 166.2 → 120.2 |
| PRO | 1.434 ± 0.068 | 116.1 → 70.1 |
| SER | 0.364 ± 0.016 | 106.1 → 60.1 |
| THR | 0.253 ± 0.011 | 120.1 → 74.1 |
| TRP | 0.144 ± 0.009 | 205.2 → 146.2 |
| TYR | 0.039 ± 0.007 | 182.2 → 136.2 |
| VAL | 0.814 ± 0.034 | 118.1 → 72.1 |
| Compound | [M − H]− (m/z) | MS/MS Fragments (m/z) | Relative Abundance |
|---|---|---|---|
| Dicaffeoyltartaric acid | 473 | 311 (100), 293 (80) 179 (5), 149 (3) | 0.5% |
| Caftaric acid | 311 | 149 (100), 179 (55), 135 (5) | 6.7% |
| Caffeoylquinic acid | 353 | 191 (100), 179 (5) | 0.27% |
| Malic acid | 133 | 115 (100) | 48.8% |
| Myricetin-O-rhamnoside | 463 | 316 (100), 317 (50), 271 (15) | 0.40% |
| Quinic acid | 191 | 111 (100), 173 (20) | 38.0% |
| Feruloylquinic acid | 367 | 191 (100), 173 (5) | 0.04% |
| Quercetin | 301 | 179 (100), 151 (75), 273 (15), 257 (10) | 0.03% |
| Tricin-O-glucoside | 491 | 476 (100), 329 (70), 328 (15), 314 (5) | 0.2% |
| Isorhamnetin-O-glucuronide | 491 | 315 (100), 255 (10), 151 (5) | 0.02% |
| [M + H]+ (m/z) | |||
| Laricitrin-O-glucoside | 495 | 333 (100) | 0.04% |
| Syringetin | 347 | 291 (100), 153 (50), 287 (20) | 0.08% |
| Kaempferol | 287 | 153 (100), 121 (50), 213 (20), 229 (5) | 0.1% |
| Apigenin | 271 | 153 (100), 229 (30), 225 (30), 119 (10) | 0.3% |
| Rutin | 611 | 303 (100), 465 (30) | 2.4% |
| Genistein | 271 | 153 (100), 215 (85), 243 (60), 149 (25) | 0.2% |
| Prunetin | 285 | 229 (100), 257 (60), 267 (30), 163 (25) | 1.2% |
| [M]+ (m/z) | |||
| Petunidin-O-pentoside | 449 | 317 (100) | 0.4% |
| Malvidin-O-pentoside | 463 | 331 (100) | 0.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romani, A.; Casciano, F.; Stevanin, C.; Maietti, A.; Tedeschi, P.; Secchiero, P.; Marchetti, N.; Voltan, R. Anticancer Activity of Aqueous Extracts from Asparagus officinalis L. Byproduct on Breast Cancer Cells. Molecules 2021, 26, 6369. https://doi.org/10.3390/molecules26216369
Romani A, Casciano F, Stevanin C, Maietti A, Tedeschi P, Secchiero P, Marchetti N, Voltan R. Anticancer Activity of Aqueous Extracts from Asparagus officinalis L. Byproduct on Breast Cancer Cells. Molecules. 2021; 26(21):6369. https://doi.org/10.3390/molecules26216369
Chicago/Turabian StyleRomani, Arianna, Fabio Casciano, Claudia Stevanin, Annalisa Maietti, Paola Tedeschi, Paola Secchiero, Nicola Marchetti, and Rebecca Voltan. 2021. "Anticancer Activity of Aqueous Extracts from Asparagus officinalis L. Byproduct on Breast Cancer Cells" Molecules 26, no. 21: 6369. https://doi.org/10.3390/molecules26216369
APA StyleRomani, A., Casciano, F., Stevanin, C., Maietti, A., Tedeschi, P., Secchiero, P., Marchetti, N., & Voltan, R. (2021). Anticancer Activity of Aqueous Extracts from Asparagus officinalis L. Byproduct on Breast Cancer Cells. Molecules, 26(21), 6369. https://doi.org/10.3390/molecules26216369









