Pan-ROCK and ROCK2 Inhibitors Affect Dexamethasone-Treated 2D- and 3D-Cultured Human Trabecular Meshwork (HTM) Cells in Opposite Manners
Abstract
:1. Introduction
2. Results
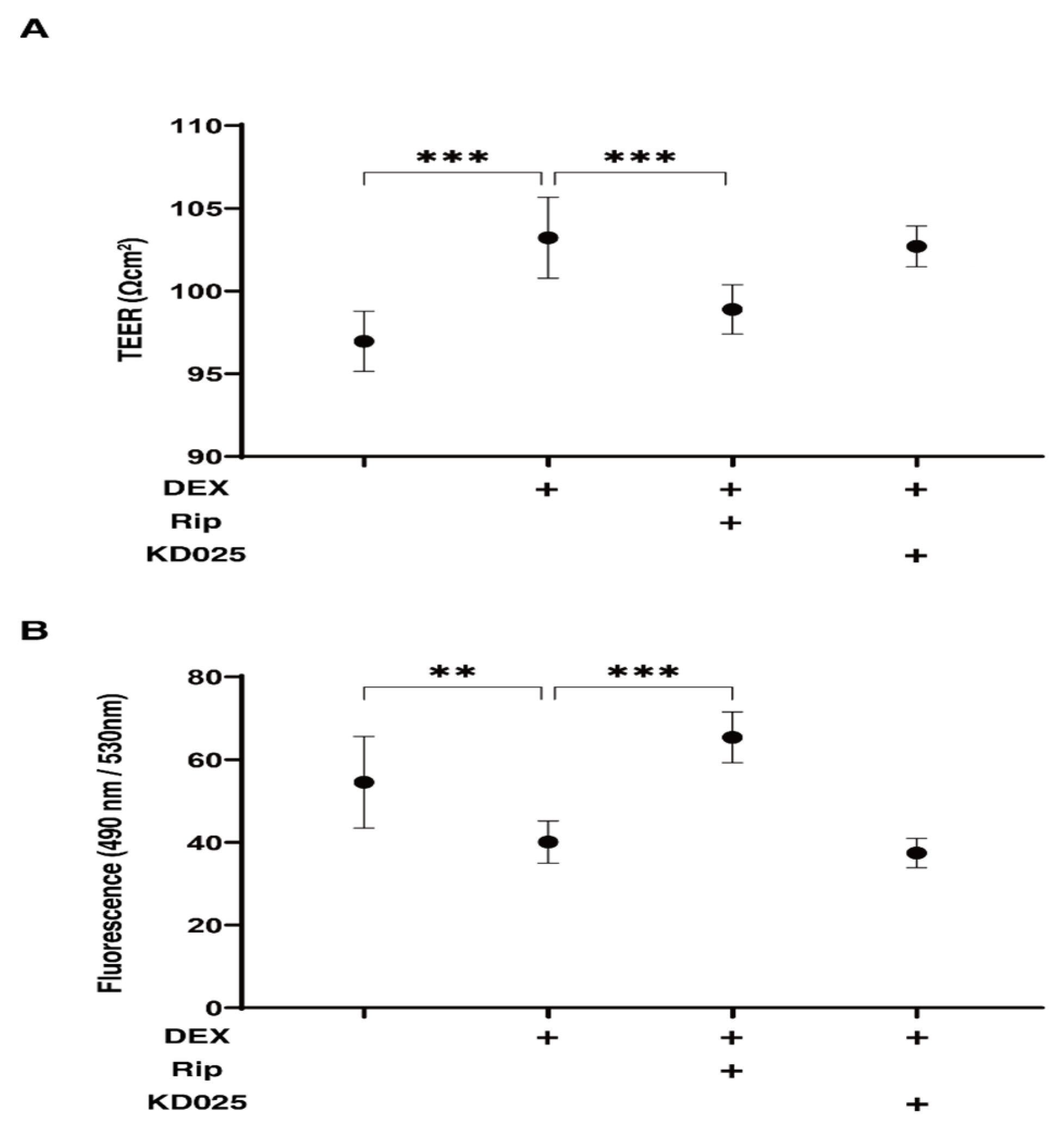
2.1. Effects of Pan-ROCK-i, Rip and ROCK2-i, KD025 on TEER and FITC–Dextran Permeability Values for the 2D DEX-Treated HTM Monolayers
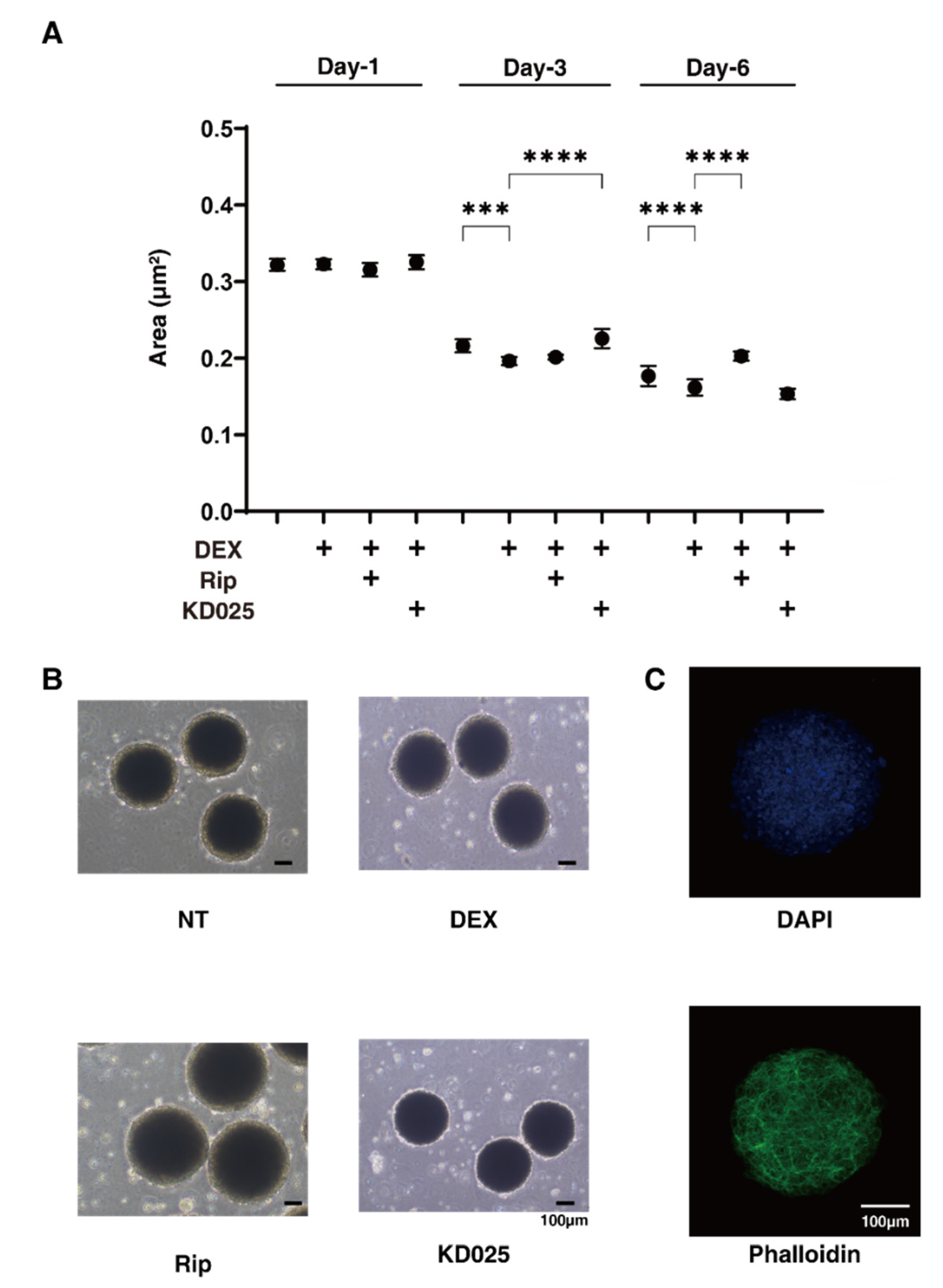
2.2. Effects of Pan-ROCK-i, Rip and ROCK2-i, KD025 on the Physical Properties, Size and Stiffness, of the 3D DEX-Treated HTM Spheroids
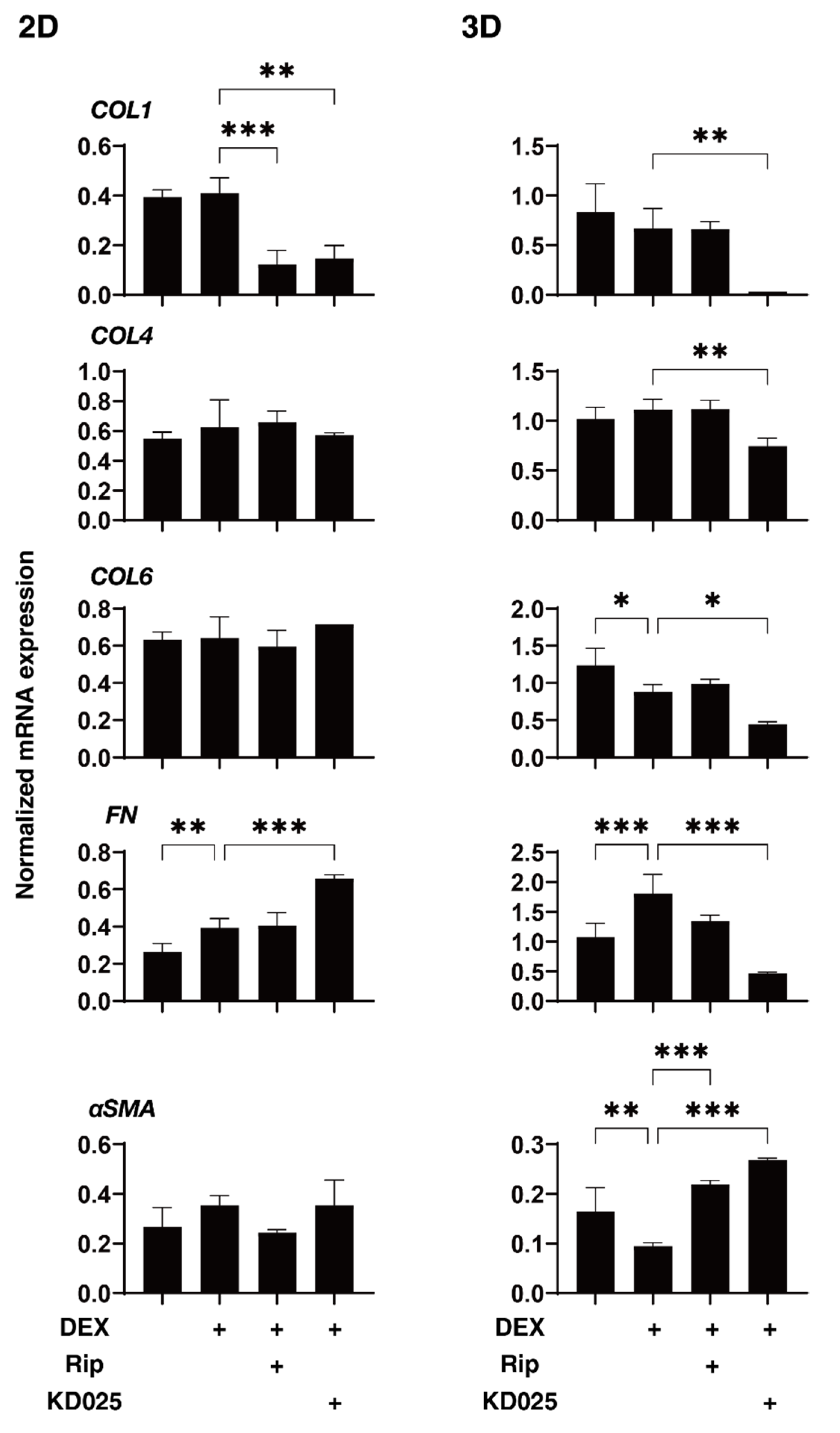
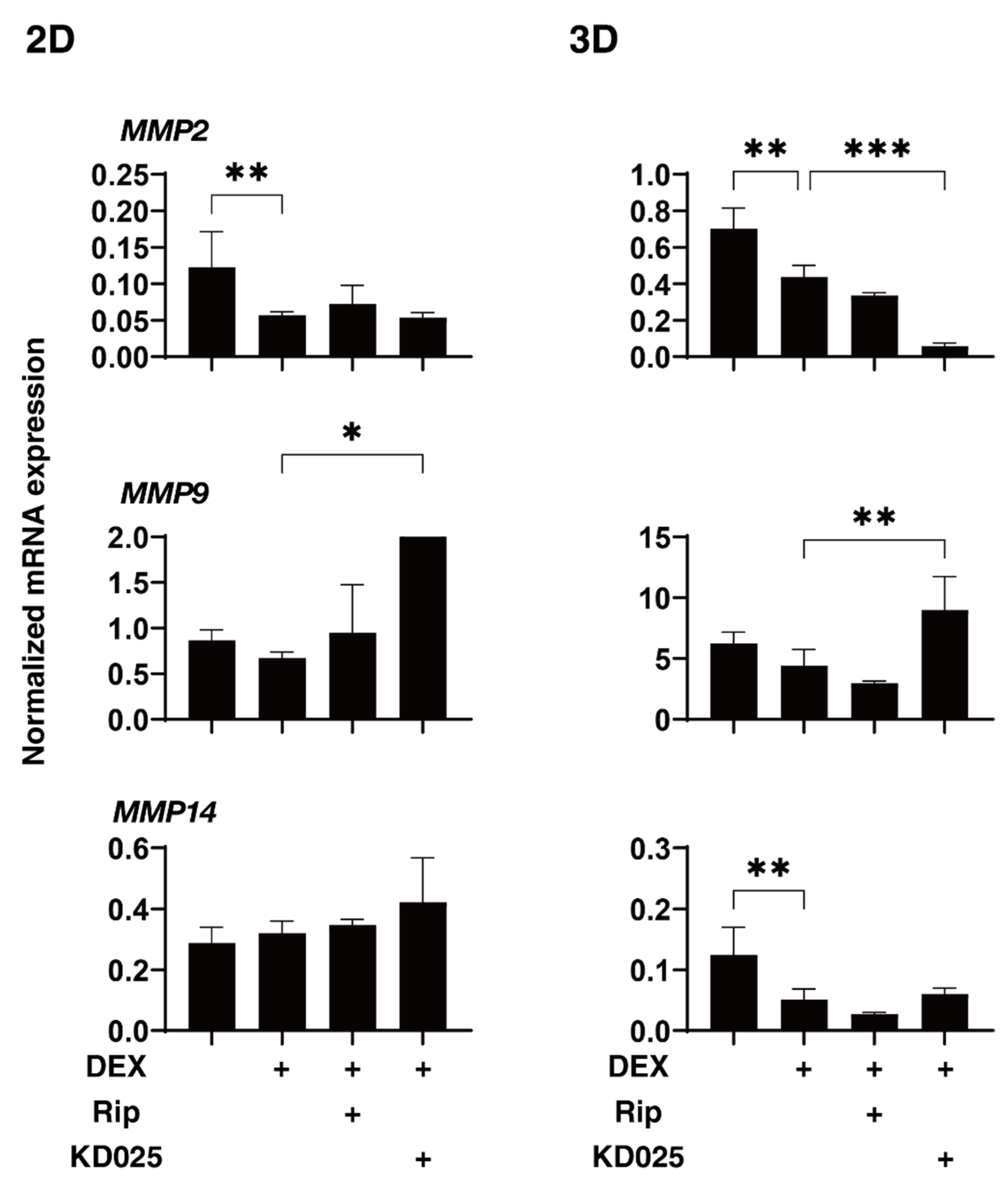
2.3. Effects of Pan-ROCK-i, Rip and ROCK2-i, KD025 on the Gene Expressions of ECM and ECM Regulatory Factors of the 2D and 3D DEX-Treated HTM Cells
3. Discussion
4. Materials and Methods
4.1. Human Trabecular Meshwork (HTM) Cells
4.2. 2D and 3D Cultures of Human Trabecular Meshwork (HTM) Cells
4.3. Transendothelial Electron Resistance (TEER) Measurements and the Fluorescein Isothiocyanate (FITC)–Dextran Permeability of 2D-Cultured HTM Monolayers
4.4. Physical Property, Size and Solidity Measurements of 3D Spheroids
4.5. Quantitative PCR
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Inoue, T.; Tanihara, H. Rho-associated kinase inhibitors: A novel glaucoma therapy. Prog. Retin. Eye Res. 2013, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Suganami, H.; Araie, M. Intra-ocular pressure-lowering effects of a Rho kinase inhibitor, ripasudil (K-115), over 24 hours in primary open-angle glaucoma and ocular hypertension: A randomized, open-label, crossover study. Acta Ophthalmol. 2015, 93, e254–e260. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.; Winton, M.J.; Rodriguez-Hernandez, N.; Campenot, R.B.; McKerracher, L. Application of Rho antagonist to neuronal cell bodies promotes neurite growth in compartmented cultures and regeneration of retinal ganglion cell axons in the optic nerve of adult rats. J. Neurosci. 2005, 25, 1113–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.-B.; Zhong, Y.-S.; Cheng, Y.; Shen, X. Rho/ROCK pathway and neural regeneration: A potential therapeutic target for central nervous system and optic nerve damage. Int. J. Ophthalmol. 2011, 4, 652–657. [Google Scholar] [CrossRef]
- Sagawa, H.; Terasaki, H.; Nakamura, M.; Ichikawa, M.; Yata, T.; Tokita, Y.; Watanabe, M. A novel ROCK inhibitor, Y-39983, promotes regeneration of crushed axons of retinal ganglion cells into the optic nerve of adult cats. Exp. Neurol. 2007, 205, 230–240. [Google Scholar] [CrossRef]
- Watabe, H.; Abe, S.; Yoshitomi, T. Effects of Rho-associated protein kinase inhibitors Y-27632 and Y-39983 on isolated rabbit ciliary arteries. Jpn. J. Ophthalmol. 2011, 55, 411–417. [Google Scholar] [CrossRef]
- Stiles, J.M.; Kurisetty, V.; Mitchell, D.C.; Bryan, B.A. Rho kinase proteins regulate global miRNA expression in endothelial cells. Cancer Genom. Proteom. 2013, 10, 251–263. [Google Scholar]
- Nakagawa, O.; Fujisawa, K.; Ishizaki, T.; Saito, Y.; Nakao, K.; Narumiya, S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996, 392, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Fukiage, C.; Mizutani, K.; Kawamoto, Y.; Azuma, M.; Shearer, T.R. Involvement of phosphorylation of myosin phosphatase by ROCK in trabecular meshwork and ciliary muscle contraction. Biochem. Biophys. Res. Commun. 2001, 288, 296–300. [Google Scholar] [CrossRef]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef]
- Riento, K.; Ridley, A.J. Rocks: Multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 2003, 4, 446–456. [Google Scholar] [CrossRef]
- Chun, K.H.; Araki, K.; Jee, Y.; Lee, D.H.; Oh, B.C.; Huang, H.; Park, K.S.; Lee, S.W.; Zabolotny, J.M.; Kim, Y.B. Regulation of glucose transport by ROCK1 differs from that of ROCK2 and is controlled by actin polymerization. Endocrinology 2012, 153, 1649–1662. [Google Scholar] [CrossRef]
- Yokota, T.; Utsunomiya, K.; Taniguchi, K.; Gojo, A.; Kurata, H.; Tajima, N. Involvement of the Rho/Rho kinase signaling pathway in platelet-derived growth factor BB-induced vascular endothelial growth factor expression in diabetic rat retina. Jpn. J. Ophthalmol. 2007, 51, 424–430. [Google Scholar] [CrossRef]
- Arita, R.; Hata, Y.; Nakao, S.; Kita, T.; Miura, M.; Kawahara, S.; Zandi, S.; Almulki, L.; Tayyari, F.; Shimokawa, H.; et al. Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage. Diabetes 2009, 58, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Hollanders, K.; Van, B.T.; Kindt, N.; Castermans, K.; Leysen, D.; Vandewalle, E.; Moons, L.; Stalmans, I. The effect of AMA0428, a novel and potent ROCK inhibitor, in a model of neovascular age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1335–1348. [Google Scholar] [CrossRef] [Green Version]
- Okumura, N.; Koizumi, N.; Ueno, M.; Sakamoto, Y.; Takahashi, H.; Hamuro, J.; Kinoshita, S. The new therapeutic concept of using a rho kinase inhibitor for the treatment of corneal endothelial dysfunction. Cornea 2011, 30, S54–S59. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Inoue, T.; Inatani, M.; Fujimoto, T.; Honjo, M.; Kasaoka, N.; Inoue, M.M.; Yoshimura, N.; Tanihara, H. The effect of rho-associated protein kinase inhibitor on monkey schlemm’s canal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3092–3103. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, S.; Van Bergen, T.; Sijnave, D.; Hollanders, K.; Castermans, K.; Defert, O.; Leysen, D.; Vandewalle, E.; Moons, L.; Stalmans, I. AMA0076, a novel, locally acting rho kinase inhibitor, potently lowers intraocular pressure in New Zealand white rabbits with minimal hyperemia. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1006–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, C.; Ida, Y.; Ohguro, H.; Hikage, F. ROCK inhibitors beneficially alter the spatial configuration of TGFβ2-treated 3D organoids from a human trabecular meshwork (HTM). Sci. Rep. 2020, 10, 20292. [Google Scholar] [CrossRef]
- Flügel-Koch, C.; Ohlmann, A.; Fuchshofer, R.; Welge-Lüssen, U.; Tamm, E.R. Thrombospondin-1 in the trabecular meshwork: Localization in normal and glaucomatous eyes, and induction by TGF-beta1 and dexamethasone in vitro. Exp. Eye Res. 2004, 79, 649–663. [Google Scholar] [CrossRef]
- Han, H.; Wecker, T.; Grehn, F.; Schlunck, G. Elasticity-dependent modulation of TGF-β responses in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2889–2896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welge-Lüssen, U.; May, C.A.; Lütjen-Drecoll, E. Induction of tissue transglutaminase in the trabecular meshwork by TGF-beta1 and TGF-beta2. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2229–2238. [Google Scholar]
- Raghunathan, V.K.; Morgan, J.T.; Park, S.A.; Weber, D.; Phinney, B.S.; Murphy, C.J.; Russell, P. Dexamethasone stiffens trabecular meshwork, trabecular meshwork cells, and matrix. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4447–4459. [Google Scholar] [CrossRef]
- Torrejon, K.Y.; Papke, E.L.; Halman, J.R.; Bergkvist, M.; Danias, J.; Sharfstein, S.T.; Xie, Y. TGFβ2-induced outflow alterations in a bioengineered trabecular meshwork are offset by a rho-associated kinase inhibitor. Sci. Rep. 2016, 6, 38319. [Google Scholar] [CrossRef] [Green Version]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef] [Green Version]
- Vernazza, S.; Tirendi, S.; Scarfì, S.; Passalacqua, M.; Oddone, F.; Traverso, C.E.; Rizzato, I.; Bassi, A.M.; Saccà, S.C. 2D- and 3D-cultures of human trabecular meshwork cells: A preliminary assessment of an in vitro model for glaucoma study. PLoS ONE 2019, 14, e0221942. [Google Scholar] [CrossRef]
- Torrejon, K.Y.; Papke, E.L.; Halman, J.R.; Stolwijk, J.; Dautriche, C.N.; Bergkvist, M.; Danias, J.; Sharfstein, S.T.; Xie, Y. Bioengineered glaucomatous 3D human trabecular meshwork as an in vitro disease model. Biotechnol. Bioeng. 2016, 113, 1357–1368. [Google Scholar] [CrossRef] [Green Version]
- Hikage, F.; Atkins, S.; Kahana, A.; Smith, T.J.; Chun, T.H. HIF2A-LOX pathway promotes fibrotic tissue remodeling in thyroid-associated orbitopathy. Endocrinology 2019, 160, 20–35. [Google Scholar] [CrossRef] [Green Version]
- Ida, Y.; Hikage, F.; Itoh, K.; Ida, H.; Ohguro, H. Prostaglandin F2α agonist-induced suppression of 3T3-L1 cell adipogenesis affects spatial formation of extra-cellular matrix. Sci. Rep. 2020, 10, 7958. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Hikage, F.; Ida, Y.; Ohguro, H. Prostaglandin F2α agonists negatively modulate the size of 3D organoids from primary human orbital fibroblasts. Investig. Ophthalmol. Vis. Sci. 2020, 61, 13. [Google Scholar] [CrossRef]
- Leung, T.; Chen, X.Q.; Manser, E.; Lim, L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol. Cell. Biol. 1996, 16, 5313–5327. [Google Scholar] [CrossRef] [Green Version]
- Yoneda, A.; Multhaupt, H.A.; Couchman, J.R. The Rho kinases I and II regulate different aspects of myosin II activity. J. Cell Biol. 2005, 170, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, A.; Ushakov, D.; Multhaupt, H.A.; Couchman, J.R. Fibronectin matrix assembly requires distinct contributions from Rho kinases I and -II. Mol. Biol. Cell 2007, 18, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Surma, M.; Wei, L.; Shi, J. Rho kinase as a therapeutic target in cardiovascular disease. Future Cardiol. 2011, 7, 657–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohenberger, P.; Eing, C.; Straessner, R.; Durst, S.; Frey, W.; Nick, P. Plant actin controls membrane permeability. Biochim. Biophys. Acta 2011, 1808, 2304–2312. [Google Scholar] [CrossRef] [Green Version]
- Sebbagh, M.; Renvoizé, C.; Hamelin, J.; Riché, N.; Bertoglio, J.; Bréard, J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat. Cell Biol. 2001, 3, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.L.; Sahai, E.A.; Yeo, M.; Bosch, M.; Dewar, A.; Olson, M.F. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat. Cell Biol. 2001, 3, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Amano, M.; Maeda, A.; Goto, H.; Takahashi, K.; Ito, M.; Kaibuchi, K. Identification of calponin as a novel substrate of Rho-kinase. Biochem. Biophys. Res. Commun. 2000, 273, 110–116. [Google Scholar] [CrossRef]
- Liu, X.; Yu, X.; Zack, D.J.; Zhu, H.; Qian, J. TiGER: A database for tissue-specific gene expression and regulation. BMC Bioinform. 2008, 9, 271. [Google Scholar] [CrossRef] [Green Version]
- Di Cunto, F.; Imarisio, S.; Hirsch, E.; Broccoli, V.; Bulfone, A.; Migheli, A.; Atzori, C.; Turco, E.; Triolo, R.; Dotto, G.P.; et al. Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron 2000, 28, 115–127. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Roberts, W.; Wang, L.; Yamada, M.; Zhang, S.; Zhao, Z.; Rivkees, S.A.; Schwartz, R.J.; Imanaka-Yoshida, K. Rho kinases play an obligatory role in vertebrate embryonic organogenesis. Development 2001, 128, 2953–2962. [Google Scholar] [CrossRef] [PubMed]
- Diep, D.T.V.; Duong, K.H.M.; Choi, H.; Jun, H.S.; Chun, K.H. KD025 (SLx-2119) suppresses adipogenesis at intermediate stage in human adipose-derived stem cells. Adipocyte 2019, 8, 114–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, K.E.; Bhattacharya, S.K.; Borrás, T.; Brunner, T.M.; Chansangpetch, S.; Clark, A.F.; Dismuke, W.M.; Du, Y.; Elliott, M.H.; Ethier, C.R.; et al. Consensus recommendations for trabecular meshwork cell isolation, characterization and culture. Exp. Eye Res. 2018, 171, 164–173. [Google Scholar] [CrossRef]
- Kaneko, Y.; Ohta, M.; Inoue, T.; Mizuno, K.; Isobe, T.; Tanabe, S.; Tanihara, H. Effects of K-115 (Ripasudil), a novel ROCK inhibitor, on trabecular meshwork and Schlemm’s canal endothelial cells. Sci. Rep. 2016, 6, 19640. [Google Scholar] [CrossRef] [PubMed]


| Sequence | Exon Location | RefSeq Number | ||
|---|---|---|---|---|
| human RPLP0 | Probe Primer2 Primer1 | 5′-/56-FAM/CCCTGTCTT/ZEN/CCCTGGGCATCAC/3IABkFQ/-3′ 5′-TCGTCTTTAAACCCTGCGTG-3′ 5′-TGTCTGCTCCCACAATGAAAC-3′ | 2–3 | NM_001002 |
| human COL1A1 | Probe Primer2 Primer1 | 5′-/56-FAM/TCGAGGGCC/ZEN/AAGACGAAGACATC/3IABkFQ/-3′ 5′-GACATGTTCAGCTTTGTGGAC-3′ 5′-TTCTGTACGCAGGTGATTGG-3′ | 1–2 | NM_000088 |
| human COL4A1 | Probe Primer2 Primer1 | 5′-/56-FAM/TCATACAGA/ZEN/CTTGGCAGCGGCT/3IABkFQ/-3′ 5′-AGAGAGGAGCGAGATGTTCA-3′ 5′-TGAGTCAGGCTTCATTATGTTCT-3′ | 51–52 | NM_001845 |
| human COL6A1 | Primer2 Primer1 | 5′-CCTCGTGGACAAAGTCAAGT-3′ 5′-GTGAGGCCTTGGATGATCTC-3′ | 2–3 | NM_001848 |
| human FN1 | Primer2 Primer1 | 5′-CGTCCTAAAGACTCCATGATCTG-3′ 5′-ACCAATCTTGTAGGACTGACC-3′ | 3–4 | NM_212482 |
| human αSMA | Probe Primer2 Primer1 | 5′-/56-FAM/AGACCCTGT/ZEN/TCCAGCCATCCTTC/3IABkFQ/-3′ 5′-AGAGTTACGAGTTGCCTGATG-3′ 5′-CTGTTGTAGGTGGTTTCATGGA-3′ | 8–9 | NM_001613 |
| human TIMP1 | Probe Primer2 Primer1 | 5′-/56-FAM/TCAACCAGA/ZEN/CCACCTTATACCAGCG/3IABkFQ/-3′ 5′-CCTTCTGCAATTCCGACCT-3′ 5′-GCTTGGAACCCTTTATACATCTTG-3′ | 2–4 | NM_003254 |
| human TIMP2 | Probe Primer2 Primer1 | 5′-/56-FAM/TCTCATTGC/ZEN/AGGAAAGGCCGAGG/3IABkFQ/-3′ 5′-GACGTTGGAGGAAAGAAGGA-3′ 5′-TGTGGTTCAGGCTCTTCTTC-3′ | 3–4 | NM_003255 |
| human TIMP3 | Probe Primer2 Primer1 | 5′-/56-FAM/CCTCCTTTA/ZEN/CCAGCTTCTTCCCCAC/3IABkFQ/-3′ 5′-CCTTCTGCAACTCCGACATC-3′ 5′-CGGTACATCTTCATCTGCTTGA-3′ | 1–3 | NM_000362 |
| human TIMP4 | Probe Primer2 Primer1 | 5′-/56-FAM/ACTGAGGAC/ZEN/CTGACCAGTCAAGAGA/3IABkFQ/-3′ 5′-GGTTTGAGAAAGTCAAGGATGTTC-3′ 5′-GTTGCACAGATGGATGAAGAC-3′ | 3–4 | NM_003256 |
| human MMP2 | Primer2 Primer1 | 5′-TCCACCACCTACAACTTTGAG-3′ 5′-GTGCAGCTGTCATAGGATGT-3′ | 6–7 | NM_004530 |
| human MMP9 | Primer2 Primer1 | 5′-ACATCGTCATCCAGTTTGGTG-3′ 5′-CGTCGAAATGGGCGTCT-3′ | 3–4 | NM_004994 |
| human MMP14 | Primer2 Primer1 | 5′-TTCGCCGACTAAGCAGAAG-3′ 5′-CTTGAATTCCTAGACCGCTGT-3′ | 1–1 | NM_004995 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, M.; Ida, Y.; Furuhashi, M.; Tsugeno, Y.; Hikage, F.; Ohguro, H. Pan-ROCK and ROCK2 Inhibitors Affect Dexamethasone-Treated 2D- and 3D-Cultured Human Trabecular Meshwork (HTM) Cells in Opposite Manners. Molecules 2021, 26, 6382. https://doi.org/10.3390/molecules26216382
Watanabe M, Ida Y, Furuhashi M, Tsugeno Y, Hikage F, Ohguro H. Pan-ROCK and ROCK2 Inhibitors Affect Dexamethasone-Treated 2D- and 3D-Cultured Human Trabecular Meshwork (HTM) Cells in Opposite Manners. Molecules. 2021; 26(21):6382. https://doi.org/10.3390/molecules26216382
Chicago/Turabian StyleWatanabe, Megumi, Yosuke Ida, Masato Furuhashi, Yuri Tsugeno, Fumihito Hikage, and Hiroshi Ohguro. 2021. "Pan-ROCK and ROCK2 Inhibitors Affect Dexamethasone-Treated 2D- and 3D-Cultured Human Trabecular Meshwork (HTM) Cells in Opposite Manners" Molecules 26, no. 21: 6382. https://doi.org/10.3390/molecules26216382
APA StyleWatanabe, M., Ida, Y., Furuhashi, M., Tsugeno, Y., Hikage, F., & Ohguro, H. (2021). Pan-ROCK and ROCK2 Inhibitors Affect Dexamethasone-Treated 2D- and 3D-Cultured Human Trabecular Meshwork (HTM) Cells in Opposite Manners. Molecules, 26(21), 6382. https://doi.org/10.3390/molecules26216382







