FTIR Spectroscopy as a Tool to Study Age-Related Changes in Cardiac and Skeletal Muscle of Female C57BL/6J Mice
Abstract
:1. Introduction
2. Results
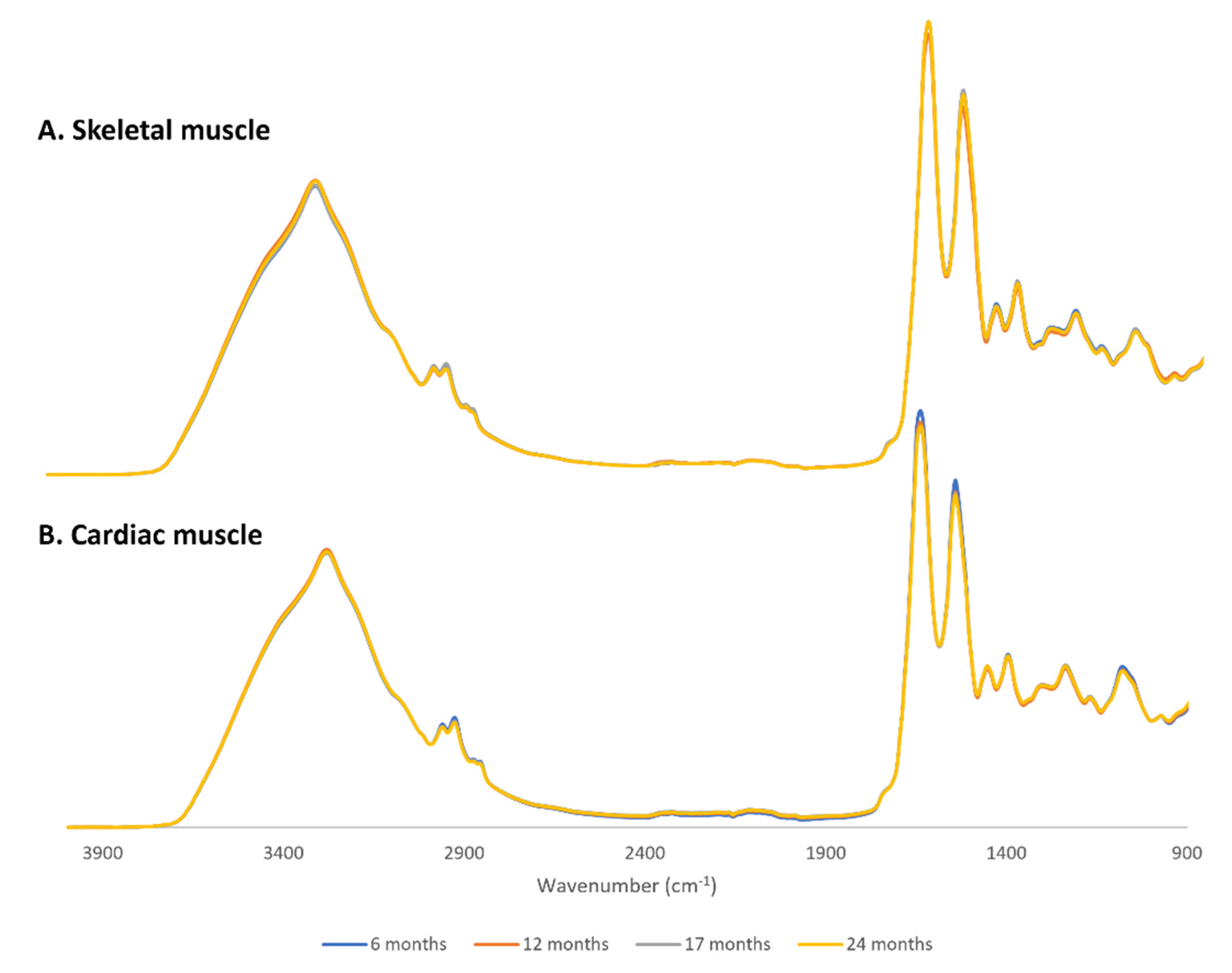
2.1. FTIR Spectra Overview and Pre-Treatments
2.2. Skeletal Muscle
2.3. Cardiac Muscle
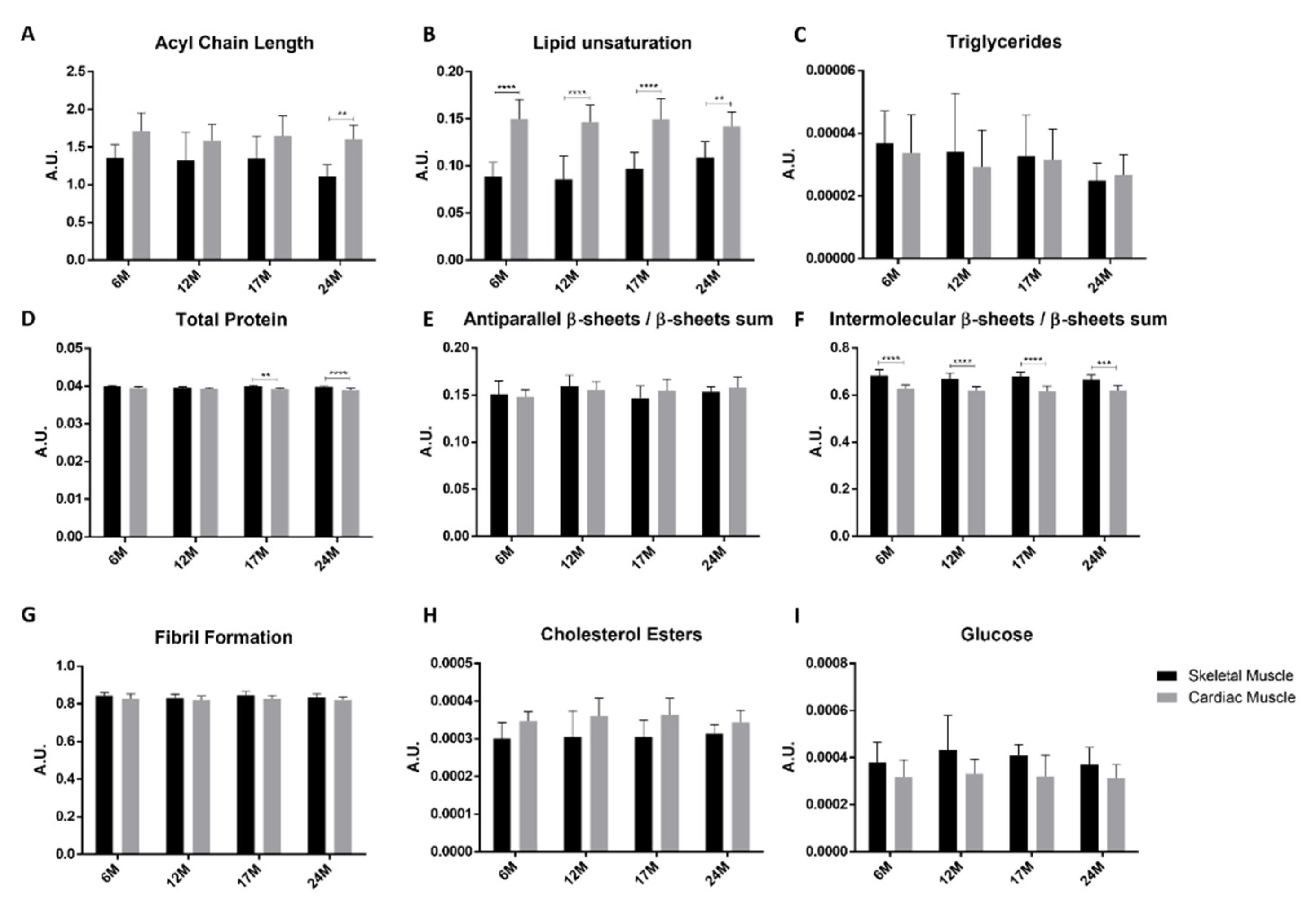
2.4. Skeletal vs. Cardiac Muscle
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Tissue Preparation
4.3. FTIR Measurements
4.4. FTIR Data Processing and Analysis
Preprocessing
4.5. Multivariate Analysis: PCA and PLS
4.6. Intensity of Spectral Bands
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, S.J.; Scheibye-Knudsen, M.; Longo, D.L.; de Cabo, R. Animal Models of Aging Research: Implications for Human Aging and Age-Related Diseases. Annu. Rev. Anim. Biosci. 2015, 3, 283–303. [Google Scholar] [CrossRef] [Green Version]
- Rocha, A.; Magalhães, S.; Nunes, A. Cell culture studies: A promising approach to metabolomic study of human aging. Curr. Metabolomics Syst. Biol. 2021, 8. [Google Scholar] [CrossRef]
- Flurkey, K.; Currer, J.M.; Harrison, D.E. Chapter 20—Mouse Models in Aging Research. In The Mouse in Biomedical Research, 2nd ed.; Fox, J.G., Davisson, M.T., Quimby, F.W., Barthold, S.W., Newcomer, C.E., Smith, A.L., Eds.; American College of Laboratory Animal Medicine; Academic Press: Burlington, MA, USA, 2007; pp. 637–672. [Google Scholar] [CrossRef]
- Lidzbarsky, G.; Gutman, D.; Shekhidem, H.A.; Sharvit, L.; Atzmon, G. Genomic Instabilities, Cellular Senescence, and Aging: In Vitro, In Vivo and Aging-Like Human Syndromes. Front. Med. 2018, 5, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brayton, C.F.; Treuting, P.M.; Ward, J.M. Pathobiology of Aging Mice and GEM. Vet. Pathol. 2012, 49, 85–105. [Google Scholar] [CrossRef] [PubMed]
- The Jackson Laboratory Guide: Aged C57BL/6J mice for research studies: Considerations, applications and best practices 2017.
- Graça, A.; Magalhães, S.; Nunes, A. Biological Predictors of Aging and Potential of FTIR to Study Age-related Diseases and Aging Metabolic Fingerprint. Curr. Metabolomics 2017, 5, 119–137. [Google Scholar] [CrossRef]
- Galkin, F.; Mamoshina, P.; Aliper, A.; de Magalhães, J.P.; Gladyshev, V.N.; Zhavoronkov, A. Biohorology and biomarkers of aging: Current state-of-the-art, challenges and opportunities. Ageing Res. Rev. 2020, 60, 101050. [Google Scholar] [CrossRef]
- Rivero-Segura, N.A.; Bello-Chavolla, O.Y.; Barrera-Vázquez, O.S.; Gutierrez-Robledo, L.M.; Gomez-Verjan, J.C. Promising biomarkers of human aging: In search of a multi-omics panel to understand the aging process from a multidimensional perspective. Ageing Res. Rev. 2020, 64, 101164. [Google Scholar] [CrossRef]
- Sitnikova, V.E.; Kotkova, M.A.; Nosenko, T.N.; Kotkova, T.N.; Martynova, D.M.; Uspenskaya, M.V. Breast cancer detection by ATR-FTIR spectroscopy of blood serum and multivariate data-analysis. Talanta 2020, 214, 120857. [Google Scholar] [CrossRef] [PubMed]
- Mateus, T.; Almeida, I.; Costa, A.; Viegas, D.; Magalhães, S.; Martins, F.; Herdeiro, M.T.; da Cruz e Silva, O.A.B.; Fraga, C.; Alves, I.; et al. Fourier-Transform Infrared Spectroscopy as a Discriminatory Tool for Myotonic Dystrophy Type 1 Metabolism: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 3800. [Google Scholar] [CrossRef]
- Magalhães, S.; Nunes, A. Fourier Transform Infrared Spectroscopy Applied to the Study of Unicellular Models. Curr. Metabolomics 2018, 6, 86–95. [Google Scholar] [CrossRef]
- Malonek, D.; Dekel, B.-Z.; Haran, G.; Reens-Carmel, R.; Groisman, G.M.; Hallak, M.; Bruchim, I. Rapid intraoperative diagnosis of gynecological cancer by ATR-FTIR spectroscopy of fresh tissue biopsy. J. Biophotonics 2020, 13, e202000114. [Google Scholar] [CrossRef] [PubMed]
- Perez-Guaita, D.; Richardson, Z.; Heraud, P.; Wood, B. Quantification and Identification of Microproteinuria Using Ultrafiltration and ATR-FTIR Spectroscopy. Anal. Chem. 2020, 92, 2409–2416. [Google Scholar] [CrossRef]
- Etienne, J.; Liu, C.; Skinner, C.M.; Conboy, M.J.; Conboy, I.M. Skeletal muscle as an experimental model of choice to study tissue aging and rejuvenation. Skelet. Muscle 2020, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Strait, J.B.; Lakatta, E.G. Aging-Associated Cardiovascular Changes and Their Relationship to Heart Failure. Heart Fail. Clin. 2012, 8, 143–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolon, B.; Brayton, C.; Cantor, G.H.; Kusewitt, D.F.; Loy, J.K.; Sartin, E.A.; Schoeb, T.R.; Sellers, R.S.; Schuh, J.C.L.; Ward, J.M. Editorial: Best Pathology Practices in Research Using Genetically Engineered Mice. Vet. Pathol. 2008, 45, 939–940. [Google Scholar] [CrossRef] [PubMed]
- Siparsky, P.N.; Kirkendall, D.T.; Garrett, W.E. Muscle Changes in Aging. Sport. Health A Multidiscip. Approach 2014, 6, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Chiao, Y.A.; Rabinovitch, P.S. The Aging Heart. Cold Spring Harb. Perspect. Med. 2015, 5, a025148. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.i.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Keng, B.M.H.; Gao, F.; Teo, L.L.Y.; Lim, W.S.; Tan, R.S.; Ruan, W.; Ewe, S.H.; Koh, W.; Koh, A.S. Associations between Skeletal Muscle and Myocardium in Aging: A Syndrome of “Cardio-Sarcopenia”? J. Am. Geriatr. Soc. 2019, 67, 2568–2573. [Google Scholar] [CrossRef]
- Yeo, D.; Kang, C.; Ji, L.L. Aging alters acetylation status in skeletal and cardiac muscles. GeroScience 2020, 42, 963–976. [Google Scholar] [CrossRef]
- Almeida, I.; Magalhães, S.; Nunes, A. Lipids: Biomarkers of healthy aging. Biogerontology 2021, 22, 273–295. [Google Scholar] [CrossRef]
- Zhou, J.; Chong, S.Y.; Lim, A.; Singh, B.K.; Sinha, R.A.; Salmon, A.B.; Yen, P.M. Changes in macroautophagy, chaperone-mediated autophagy, and mitochondrial metabolism in murine skeletal and cardiac muscle during aging. Aging 2017, 9, 583–599. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.W.K.; Braidy, N.; Pickford, R.; Vafaee, F.; Crawford, J.; Muenchhoff, J.; Schofield, P.; Attia, J.; Brodaty, H.; Sachdev, P.; et al. Plasma lipidome variation during the second half of the human lifespan is associated with age and sex but minimally with BMI. PLoS ONE 2019, 14, e0214141. [Google Scholar] [CrossRef] [Green Version]
- Magalhaes, S.; Goodfellow, B.J.; Nunes, A. Aging and Proteins: What Does Proteostasis Have to Do with Age? Curr. Mol. Med. 2018, 18, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.M.; Kasturi, P.; Zheng, M.; Pinkert, S.; Vecchi, G.; Ciryam, P.; Morimoto, R.I.; Dobson, C.M.; Vendruscolo, M.; Mann, M.; et al. Widespread proteome remodeling and aggregation in aging C. elegans. Cell 2015, 161, 919–932. [Google Scholar] [CrossRef] [Green Version]
- Tanase, M.; Urbanska, A.M.; Zolla, V.; Clement, C.C.; Huang, L.; Morozova, K.; Follo, C.; Goldberg, M.; Roda, B.; Reschiglian, P.; et al. Role of Carbonyl Modifications on Aging-Associated Protein Aggregation. Sci. Rep. 2016, 6, 19311. [Google Scholar] [CrossRef] [Green Version]
- Leeman, D.S.; Hebestreit, K.; Ruetz, T.; Webb, A.E.; McKay, A.; Pollina, E.A.; Dulken, B.W.; Zhao, X.; Yeo, R.W.; Ho, T.T.; et al. Lysosome activation clears aggregates and enhances quiescent neural stem cell activation during aging. Science 2018, 359, 1277–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, R.; Ward, J.E. Amyloid Cardiomyopathy. Circ. Res. 2017, 120, 1865–1867. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.B.; Liu, Y.-T.; Yeh, S.-Y.; Tsai, J.-W. Contributions of Animal Models to the Mechanisms and Therapies of Transthyretin Amyloidosis. Front. Physiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Tan, G.-J.; Peng, Z.-K.; Lu, J.-P.; Tang, F.-Q. Cathepsins mediate tumor metastasis. World J. Biol. Chem. 2013, 4, 91–101. [Google Scholar] [CrossRef]
- Uchitomi, R.; Hatazawa, Y.; Senoo, N.; Yoshioka, K.; Fujita, M.; Shimizu, T.; Miura, S.; Ono, Y.; Kamei, Y. Metabolomic Analysis of Skeletal Muscle in Aged Mice. Sci. Rep. 2019, 9, 10425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houtkooper, R.H.; Argmann, C.; Houten, S.M.; Cantó, C.; Jeninga, E.H.; Andreux, P.A.; Thomas, C.; Doenlen, R.; Schoonjans, K.; Auwerx, J. The metabolic footprint of aging in mice. Sci. Rep. 2011, 1, 134. [Google Scholar] [CrossRef] [PubMed]
- Denham, M.C. Choosing the number of factors in partial least squares regression: Estimating and minimizing the mean squared error of prediction. J. Chemom. 2000, 14, 351–361. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat. Protoc. 2015, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, S.; Trindade, D.; Martins, T.; Martins Rosa, I.; Delgadillo, I.; Goodfellow, B.J.; da Cruz E Silva, O.A.B.; Henriques, A.G.; Nunes, A. Monitoring plasma protein aggregation during aging using conformation-specific antibodies and FTIR spectroscopy. Clin. Chim. Acta 2020, 502, 25–33. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magalhães, S.; Almeida, I.; Martins, F.; Camões, F.; Soares, A.R.; Goodfellow, B.J.; Rebelo, S.; Nunes, A. FTIR Spectroscopy as a Tool to Study Age-Related Changes in Cardiac and Skeletal Muscle of Female C57BL/6J Mice. Molecules 2021, 26, 6410. https://doi.org/10.3390/molecules26216410
Magalhães S, Almeida I, Martins F, Camões F, Soares AR, Goodfellow BJ, Rebelo S, Nunes A. FTIR Spectroscopy as a Tool to Study Age-Related Changes in Cardiac and Skeletal Muscle of Female C57BL/6J Mice. Molecules. 2021; 26(21):6410. https://doi.org/10.3390/molecules26216410
Chicago/Turabian StyleMagalhães, Sandra, Idália Almeida, Filipa Martins, Fátima Camões, Ana R. Soares, Brian J. Goodfellow, Sandra Rebelo, and Alexandra Nunes. 2021. "FTIR Spectroscopy as a Tool to Study Age-Related Changes in Cardiac and Skeletal Muscle of Female C57BL/6J Mice" Molecules 26, no. 21: 6410. https://doi.org/10.3390/molecules26216410
APA StyleMagalhães, S., Almeida, I., Martins, F., Camões, F., Soares, A. R., Goodfellow, B. J., Rebelo, S., & Nunes, A. (2021). FTIR Spectroscopy as a Tool to Study Age-Related Changes in Cardiac and Skeletal Muscle of Female C57BL/6J Mice. Molecules, 26(21), 6410. https://doi.org/10.3390/molecules26216410








