Impact of Metallic Nanoparticles on In Vitro Culture, Phenolic Profile and Biological Activity of Two Mediterranean Lamiaceae Species: Lavandula viridis L’Hér and Thymus lotocephalus G. López and R. Morales
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biometric and Physiological Features
2.2. Phenolic Profile Analyzed by HPLC-HR-MS
2.3. Antioxidant and Enzymes Inhibitory Activities: Correlation with Phenolic Composition
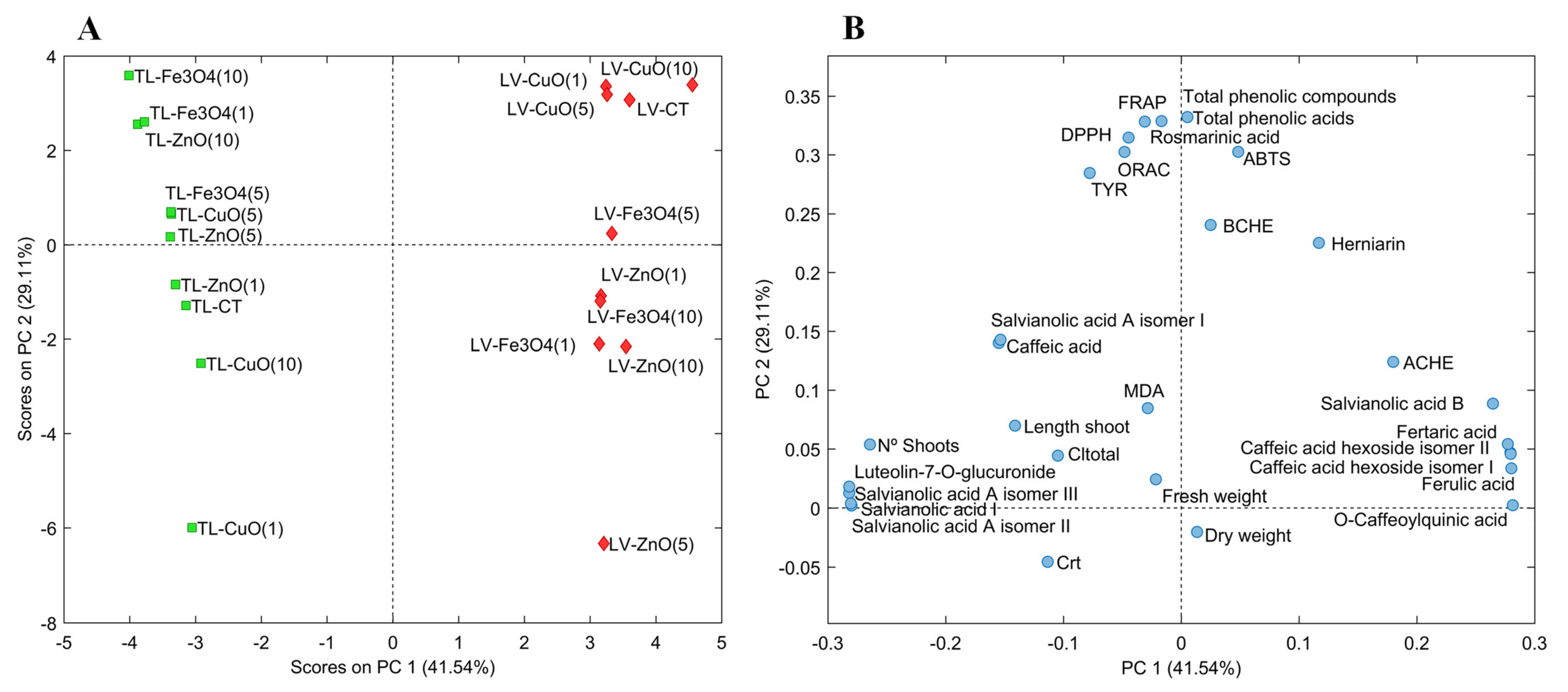
2.4. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material, Treatments and Culture Conditions
3.3. Biometric Features
3.4. Photosynthetic Pigments Analysis
3.5. Determination of Lipid Peroxidation
3.6. Extraction of Phenolics
3.7. HPLC-HR-MS Analysis of Phenolic Compounds
3.8. Antioxidant Activity
3.8.1. ABTS Free Radical Scavenging Assay
3.8.2. DPPH Free Radical Scavenging Assay
3.8.3. Ferric Reducing Antioxidant Power (FRAP)
3.8.4. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.9. Enzyme Inhibitory Capacity
3.9.1. Tyrosinase
3.9.2. Cholinesterases
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Torre, B.G.; Albericio, F. The pharmaceutical industry in 2018. an analysis of FDA drug approvals from the perspective of molecules. Molecules 2018, 24, 809. [Google Scholar] [CrossRef] [Green Version]
- Marchev, A.S.; Yordanova, Z.P.; Georgiev, M.I. Green (cell) factories for advanced production of plant secondary metabolites. Crit. Rev. Biotechnol. 2020, 40, 443–458. [Google Scholar] [CrossRef]
- Trivellini, A.; Lucchesini, M.; Maggini, R.; Mosadegh, H.; Villamarin, T.S.S.; Vernieri, P.; Mensuali-Sodi, A.; Pardossi, A. Lamiaceae phenols as multifaceted compounds: Bioactivity, industrialprospects and role of “positive-stress”. Ind. Crops Prod. 2016, 83, 241–254. [Google Scholar] [CrossRef]
- Tzima, K.; Brunton, N.P.; Rai, D.K. Qualitative and quantitative analysis of polyphenols in Lamiaceae plants—A review. Plants 2018, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Mnayer, D.; Özçelik, B.; Altin, G.; Kasapoğlu, K.N.; Daskaya-Dikmen, C.; Sharifi-Rad, M.; Selamoglu, Z.; Acharya, K.; Sen, S.; et al. Plants of the genus Lavandula: From farm to pharmacy. Nat. Prod. Commun. 2018, 13, 1385–1402. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; He, T.; Wang, X.; Shen, M.; Yan, X.; Fan, S.; Wang, L.; Wang, X.; Xu, X.; Sui, H.; et al. Traditional uses, chemical constituents and biological activities of plants from the genus Thymus. Chem. Biodiv. 2019, 16, e1900254. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Abu-Darwish, M.S.; Tarawneh, A.H.; Cabral, C.; Gadetskaya, A.V.; Salgueiro, L.; Hosseinabadi, T.; Rajabi, S.; Chanda, W.; Sharifi-Rad, M.; et al. Thymus spp. Plants—Food applications and phytopharmacy properties. Trends Food Sci. Technol. 2019, 85, 287–306. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Andrade, P.B.; Valentão, P.; Romano, A. Inhibitory effect of Lavandula viridis on Fe2+-induced lipid peroxidation, and antioxidant and anti cholinesterase properties. Food Chem. 2011, 126, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Gonçalves, S.; Grosso, C.; Andrade, P.B.; Valentão, P.; Bernardo-Gil, M.G.; Romano, A. Chemical profiling and biological screening of Thymus lotocephalus extracts obtained by supercritical fluid extraction and hydrodistillation. Ind. Crops Prod. 2012, 36, 246–256. [Google Scholar] [CrossRef]
- Costa, P.; Grosso, C.; Gonçalves, S.; Andrade, P.B.; Valentão, P.; Bernardo-Gil, M.G.; Romano, A. Supercritical fluid extraction and hydrodistillation for the recovery of bioactive compounds from Lavandula viridis L’Hér. Food Chem. 2012, 135, 112–121. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Coelho, N.; Romano, A. Thymus lotocephalus wild plants and in vitro cultures produce different profiles of phenolic compounds with antioxidant activity. Food Chem. 2012, 135, 1253–1260. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Romano, A. Accumulation of phenolic compounds in in vitro cultures and wild plants of Lavandula viridis L’Hér and their antioxidant and anticholinesterase potential. Food Chem. Toxicol. 2013, 57, 69–74. [Google Scholar] [CrossRef]
- Costa, P.; Sarmento, B.; Gonçalves, S.; Romano, A. Protective effects of Lavandula viridis L’Hér extracts and rosmarinic acid against H2O2-induced oxidative damage in A172 human astrocyte cell line. Ind. Crops Prod. 2013, 50, 361–365. [Google Scholar] [CrossRef]
- Gonçalves, S.; Mansinhos, I.; Rodríguez-Solana, R.; Pérez-Santín, E.; Coelho, N.; Romano, A. Elicitation improves rosmarinic acid content and antioxidant activity in Thymus lotocephalus shoot cultures. Ind. Crops Prod. 2019, 137, 214–220. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Romano, A. In vitro culture of lavenders (Lavandula spp.) and the production of secondary metabolites. Biotechnol. Adv. 2013, 31, 166–174. [Google Scholar] [CrossRef]
- Gonçalves, S.; Romano, A. Production of plant secondary metabolites by using biotechnological tools. In Secondary Metabolites: Sources and Applications; Vijayakumar, R., Raja, S.S.S., Eds.; InTech: Rijeka, Croatia, 2018. [Google Scholar]
- Yadav, V. Nanotechnology, big things from a tiny world: A review. Int. J. Serv. Sci. Technol. 2013, 3, 771–778. [Google Scholar]
- Kim, D.H.; Gopal, J.; Sivanesan, I. Nanomaterials in plant tissue culture: The disclosed and undisclosed. RSC Adv. 2017, 7, 36492–36505. [Google Scholar] [CrossRef] [Green Version]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isah, T.; Umar, S.; Mujib, A.; Sharma, M.P.; Rajasekharan, P.E.; Zafar, N.; Frukh, A. Secondary metabolism of pharmaceuticals in the plant in vitro cultures: Strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue Organ. Cult. 2018, 132, 239–265. [Google Scholar] [CrossRef]
- Dias, M.C.; Almeida, R.; Romano, A. Rapid clonal multiplication of Lavandula viridis L’Hér through in vitro axillary shoot proliferation. Plant Cell Tissue Organ. Cult. 2002, 68, 99–102. [Google Scholar] [CrossRef]
- Coelho, N.; Gonçalves, S.; González-Benito, M.E.; Romano, A. Establishment of an in vitro propagation protocol for Thymus lotocephalus, a rare aromatic species of the Algarve (Portugal). Plant Growth Regul. 2012, 66, 69–74. [Google Scholar] [CrossRef]
- Baskar, V.; Safia, N.; Preethy, K.S.; Dhivya, S.; Thiruvengadam, M.; Sathishkumar, R. A comparative study of phytotoxic effects of metal oxide (CuO, ZnO and NiO) nanoparticles on in-vitro grown Abelmoschus esculentus. Plant Biosyst. 2020, 155, 374–383. [Google Scholar] [CrossRef]
- El-Mahdy, M.T.; Elazab, D.S. Impact of zinc oxide nanoparticles on pomegranate growth under in vitro conditions. Russ. J. Plant Physiol. 2020, 67, 162–167. [Google Scholar] [CrossRef]
- Al-Mayahi, A.M.W. The effect of humic acid (HA) and zinc oxide nanoparticles (ZnO-NPS) on in vitro regeneration of date palm (Phoenix dactylifera L.) cv. Quntar. Plant Cell Tissue Organ. Cult. 2021, 145, 445–456. [Google Scholar] [CrossRef]
- Thunugunta, T.; Reddy, A.C.; Seetharamaiah, S.K.; Hunashikatti, L.R.; Chandrappa, S.G.; Kalathil, N.C.; Reddy, L.R.D.C. Impact of Zinc oxide nanoparticles on eggplant (S. melongena): Studies on growth and the accumulation of nanoparticles. IET Nanobiotechnol. 2018, 12, 706–713. [Google Scholar] [CrossRef]
- Da Costa, M.; Sharma, P. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 2016, 54, 110–119. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Yücesan, B.; Gürel, E. Abiotic stress of ZnO-PEG, ZnO-PVP, CuO-PEG and CuO-PVP nanoparticles enhance growth, sweetener compounds and antioxidant activities in shoots of Stevia rebaudiana Bertoni. IET Nanobiotechnol. 2017, 11, 898–902. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.-J. The components of plant tissue culture media I: Macro-and micro-nutrients. In Plant Propagation by Tissue Culture, 3rd ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 65–113. [Google Scholar]
- Mozafari, A.A.; Havas, F.; Ghaderi, N. Application of iron nanoparticles and salicylic acid in in vitro culture of strawberries (Fragaria × ananassa Duch.) to cope with drought stress. Plant Cell Tissue Organ. Cult. 2018, 132, 511–523. [Google Scholar] [CrossRef]
- Ngan, H.T.M.; Tung, H.T.; Lea, B.V.; Nhut, D.T. Evaluation of root growth, antioxidant enzyme activity and mineral absorbability of carnation (Dianthus caryophyllus “Express golem”) plantlets cultured in two culture systems supplemented with iron nanoparticles. Sci. Hortic. 2020, 272, 109612. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhang, F.D.; Feng, Z.B.; Zang, S.Q.; He, X.S.; Wang, R.F.; Wang, Y.J. Effects of nano-ferric oxide on the growth and nutrients absorption of peanut. Plant Nutr. Fertil. Sci. 2005, 11, 551–555. [Google Scholar]
- Joseph, S.; Anawar, H.M.; Storer, P.; Blackwell, P.; Chia, C.; Lin, Y.; Munroe, P.; Donne, S.; Horvat, J.; Wang, J.; et al. Effects of enriched biochars containing magnetic iron nanoparticles on mycorrhizal colonization, plant growth, nutrient uptake and soil quality improvement. Pedosphere 2015, 25, 749–760. [Google Scholar] [CrossRef]
- Jadczak, P.; Kulpa, D.; Bihun, M.; Przewodowski, W. Positive effect of AgNPs and AuNPs in in vitro cultures of Lavandula angustifolia Mill. Plant Cell Tissue Organ. Cult. 2019, 139, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.M.; Yang, J.C.; Zhang, J.F. Effects of external phosphorus on the cell ultrastructure and the chlorophyll content of maize under cadmium and zinc stress. Environ. Pollut. 2007, 147, 750–756. [Google Scholar] [CrossRef]
- Ebbs, S.; Uchil, S. Cadmium and zinc induced chlorosis in Indian mustard [Brassica juncea (L.) Czern] involves preferential loss of chlorophyll b. Photosynthetica 2008, 46, 49–55. [Google Scholar] [CrossRef]
- Abdel-Wahab, D.A.; Othman, N.A.R.M.; Hamada, A.M. Effects of copper oxide nanoparticles to Solanum nigrum and its potential for phytoremediation. Plant Cell Tissue Organ. Cult. 2019, 137, 525–539. [Google Scholar] [CrossRef]
- Chang, Y.N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef] [Green Version]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci. Total Environ. 2017, 589, 11–24. [Google Scholar] [CrossRef]
- Hawrył, A.; Hawrył, M.; Waksmundzka-Hajnos, M. Liquid chromatography fingerprint analysis and antioxidant activity of selected lavender species with chemometric calculations. PLoS ONE 2020, 14, e0218974. [Google Scholar] [CrossRef]
- Lopes, C.L.; Pereira, E.; Soković, M.; Carvalho, A.M.; Barata, A.M.; Lopes, V.; Rocha, F.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Phenolic composition and bioactivity of Lavandula pedunculata (Mill.) Cav. samples from different geographical origin. Molecules 2018, 23, 1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Ultrasonic-Assisted extraction and natural deep eutectic solvents combination: A Green strategy to improve the recovery of phenolic compounds from Lavandula pedunculata subsp. lusitanica (Chaytor) Franco. Antioxidants 2021, 10, 582. [Google Scholar] [CrossRef]
- Taghouti, M.; Martins-Gomes, C.; Félix, L.M.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Polyphenol composition and biological activity of Thymus citriodorus and Thymus vulgaris: Comparison with endemic Iberian Thymus species. Food Chem. 2020, 331, 127362. [Google Scholar] [CrossRef]
- Silva, A.M.; Martins-Gomes, C.; Souto, E.B.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M. Thymus zygis subsp. zygis an endemic portuguese plant: Phytochemical profiling, antioxidant, anti-proliferative and anti-inflammatory activities. Antioxidants 2020, 9, 482. [Google Scholar] [CrossRef] [PubMed]
- Sarfaraz, D.; Rahimmalek, M.; Saeidi, G. Polyphenolic and molecular variation in Thymus species using HPLC and SRAP analyses. Sci. Rep. 2021, 11, 5019. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.; Yucesan, B.; Zia, M.; Gurel, E. Elicitation of secondary metabolites in callus cultures of Stevia rebaudiana Bertoni grown under ZnO and CuO nanoparticles stress. Sugar Tech 2018, 20, 194–201. [Google Scholar] [CrossRef]
- Zafar, H.; Attarad, A.; Joham, S.A.; Haq, I.U.; Zia, M. Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: Growth dynamics and antioxidative response. Front. Plant Sic. 2016, 7, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosavat, N.; Golkar, P.; Yousefifard, M.; Javed, R. Modulation of callus growth and secondary metabolites in different Thymus species and Zataria multiflora micropropagated under ZnO nanoparticles stress. Biotechnol. Appl. Biochem. 2019, 66, 316–322. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Nasibi, F.; Kalantari, K.M.; Ghanati, F. Evaluation of secondary metabolites and antioxidant activity in Dracocephalum polychaetum Bornm. cell suspension culture under magnetite nanoparticles and static magnetic field elicitation. Plant Cell Tissue Organ. Cult. 2019, 136, 489–498. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Javed, R.; Adeel, M.; Rizwan, M.; Ao, Q.; Yang, Y. Engineered ZnO and CuO nanoparticles ameliorate morphological and biochemical response in tissue culture regenerants of candyleaf (Stevia rebaudiana). Molecules 2020, 25, 1356. [Google Scholar] [CrossRef] [Green Version]
- Javed, R.; Usman, M.; Yücesan, B.; Zia, M.; Gürel, E. Effect of zinc oxide (ZnO) nanoparticles on physiology and steviol glycosides production in micropropagated shoots of Stevia rebaudiana Bertoni. Plant Physiol. Biochem. 2017, 110, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Moharrami, F.; Hosseini, B.; Sharafi, A.; Farjaminezhad, M. Enhanced production of hyoscyamine and scopolamine from genetically trans-formed root culture of Hyoscyamus reticulatus L. elicited by iron oxide nanoparticles. Vitr. Cell Dev. Biol. Plant 2017, 53, 104–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, M.; Raja, N.I.; Mashwani, Z.; Iqbal, M.; Sabir, S.; Yasmeen, F. In vitro seed germination and biochemical profiling of Artemisia absinthium exposed to various metallic nanoparticles. 3 Biotech 2018, 130, 408–417. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-K.; Chang, C.-T.; Lu, H.-Y.; Chung, Y.-C. Inhibitory effects of the water extracts of Lavendula sp. on mushroom tyrosinase activity. Food Chem. 2007, 105, 1099–1105. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Biswas, R.; Sharma, A.; Banerjee, S.; Biswas, S.; Katiyar, C.K. Validation of medicinal herbs for anti-tyrosinase potential. J. Herb. Med. 2018, 14, 1–16. [Google Scholar] [CrossRef]
- Kang, H.-S.; Kim, H.-R.; Byun, D.-S.; Park, H.-J.; Choi, J.-S. Rosmarinic acid as a tyrosinase inhibitors from Salvia Miltiorrhiza. Nat. Prod. Sci. 2004, 10, 80–84. [Google Scholar]
- Karioti, A.; Protopappa, A.; Megoulas, N.; Skaltsa, H. Identification of tyrosinase inhibitors from Marrubium Velutinum and Marrubium Cylleneum. Bioorg. Med. Chem. 2007, 15, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Algieri, F.; Rodriguez-Nogales, A.; Gálvez, J.; Segura-Carretero, A. Phytochemical Profiling of Anti-Inflammatory Lavandula Extracts via RP-HPLC-DAD-QTOF-MS and -MS/MS: Assessment of Their Qualitative and Quantitative Differences. Electrophoresis 2018, 39, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Soler-Rivas, C.; Espín, J.C.; Wichers, H.J. An easy and fast test to compare total free radical scavenger capacity of foodstuffs. Phytochem. Anal. 2000, 11, 330–338. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [Green Version]
- Gillespie, K.M.; Chae, J.M.; Ainsworth, E.A. Rapid measurement of total antioxidant capacity in plants. Nat. Protoc. 2007, 2, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef] [Green Version]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]

| Treatment | NP Concentration (mg/L) | No. Shoots | Length of the Longest Shoot (mm) | Fresh Weight (mg) | Dry Weight (mg) | Cltotal (mg/gfresh weight) | Crt (mg/gfresh weight) | MDA (nmol/gfresh weight) |
|---|---|---|---|---|---|---|---|---|
| L. viridis | ||||||||
| Control | 0 | 4.26 ± 0.23 a | 33.5 ± 1.10 a | 1277 ± 196 b | 167 ± 19 b | 1.40 ± 0.08 a | 0.30± 0.02 a | 20.8 ± 1.5 d |
| CuO | 1 | 3.46 ± 0.22 b,c | 28.3 ± 0.9 b | 1836 ± 257 a,b | 207 ± 19 a,b | 1.40 ± 0.08 a | 0.30 ± 0.01 a | 24.0 ± 1.2 d |
| 5 | 2.90 ± 0.18 e,f | 24.4 ± 0.9 c | 2103 ± 451 a,b | 220 ± 29 a,b | 0.97 ± 0.02 b | 0.21 ± 0.01 b | 29.2 ± 1.6 c | |
| 10 | 2.84 ± 0.19 f | 21.4 ± 0.8 d | 1275 ± 152 b | 167 ± 16 b | 0.71 ± 0.05 c | 0.06 ± 0.01 e | 31.8 ± 2.4 b,c | |
| ZnO | 1 | 3.07 ± 0.21 c,d | 24.6 ± 1.01 c | 2711 ± 364 a | 282 ± 27 a | 0.87 ± 0.07 b,c | 0.19 ± 0.01 b,c | 19.9 ± 0.8 d |
| 5 | 2.68 ± 0.21 c,d,e | 28.7 ± 1.4 b | 2198 ± 366 a,b | 234 ± 32 a,b | 0.74 ± 0.05 b,c | 0.16 ± 0.01 c,d | 19.4 ± 1.6 d | |
| 10 | 2.33 ± 0.17 c,d,e | 20.6 ± 0.6 d | 2782 ± 330 a | 277 ± 25 a | 0.40 ± 0.05 d | 0.13 ± 0.01 d | 20.7 ± 1.0 d | |
| Fe3O4 | 1 | 4.41 ± 0.28 a | 27.5 ± 1.1 b,c | 2249 ± 356 a,b | 260 ± 29 a | 0.69 ± 0.04 c | 0.16 ±.0 01 c,d | 39.9 ± 2.2 a |
| 5 | 4.24 ± 0.29 a | 26.1 ± 1.3 b,c | 2787 ± 425 a | 251 ± 31 a,b | 0.75 ± 0.08 b,c | 0.15 ± 0.01 c,d | 36.6 ± 2.4 a,b | |
| 10 | 3.92 ± 0.24 a,b | 29.3 ± 1.3 b | 2761 ± 394 a | 268 ± 31 a | 0.77 ± 0.06 b,c | 0.19 ± 0.01 b,c | 41.2 ± 2.0 a | |
| T. lotocephalus | ||||||||
| Control | 0 | 16.28 ± 2.83 b,c | 38.30 ± 2.34 a | 1902.07 ± 443.18 b,c,d | 174.43 ± 29.21 b,c,d | 1.35 ± 0.05 b | 0.29 ± 0.02 b | 23.92 ± 1.86 e |
| CuO | 1 | 8.19 ± 1.02 d | 29.44 ± 1.55 b | 1397.72 ± 374.06 c,d | 214.41 ± 80.99 b,c,d | 1.57 ± 0.11 a | 0.34 ± 0.03 a | 21.76 ± 0.63 e |
| 5 | 8.66 ± 1.67 d | 17.54 ± 0.81 c | 1211.88 ± 292.58 d | 138.55 ± 21.28 c,d | 1.24 ± 0.09 b,c | 0.27 ± 0.02 b,c | 29.20 ± 1.73 d | |
| 10 | 11.19 ± 1.78 c,d | 20.79 ± 1.11 c | 1086.10 ± 193.89 d | 132.59 ± 20.65 d | 1.03 ± 0.07 c,d,e | 0.23 ± 0.01 c,d,e | 20.55 ± 2.00 e | |
| ZnO | 1 | 19.70 ± 2.35 b | 36.76 ± 2.11 a | 2254.67 ± 443.50 b,c,d | 252.42 ± 30.06 a,b,c | 0.81 ± 0.05 e,f | 0.22 ± 0.01 d,e | 40.07 ± 1.83 a,b |
| 5 | 17.93 ± 2.12 b,c | 38.63 ± 2.03 a | 2597.08 ± 455.25 b,c | 255.58 ± 31.63 a,b,c | 1.10 ± 0.04 c,d | 0.26 ± 0.01 b,c,d | 43.42 ± 1.55 a | |
| 10 | 16.80 ± 2.04 b,c | 41.86 ± 2.57 a | 2661.83 ± 349.44 b,c | 261.49 ± 21.18 a,b | 0.94 ± 0.07 d,e,f | 0.18 ± 0.01 e,f | 36.17 ± 1.83 b,c | |
| Fe3O4 | 1 | 17.69 ± 1.76 b,c | 40.30 ± 2.02 a | 2366.82 ± 391.22 b,c,d | 220.85 ± 27.78 b,c,d | 1.13 ± 0.08 c,d | 0.22 ± 0.02 d,e | 32.92 ± 1.70 c,d |
| 5 | 31.62 ± 2.98 a | 38.08 ± 1.93 a | 4854.29 ± 769.03 a | 343.31 ± 39.90 a | 0.72 ± 0.05 f | 0.14 ± 0.01 f | 23.67 ± 1.58 e | |
| 10 | 26.35 ± 3.85 a | 41.95 ± 1.73 a | 3116.72 ± 381.17 b | 271.61 ± 27.06 a,b | 0.83 ± 0.07 e,f | 0.18 ± 0.01 e,f | 23.21 ± 2.04 e |
| Compound | Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | CuO (mg/L) | ZnO (mg/L) | Fe3O4 (mg/L) | |||||||
| 1 | 5 | 10 | 1 | 5 | 10 | 1 | 5 | 10 | ||
| L. viridis | ||||||||||
| O-Caffeoylquinic acid | 81 ± 8 a | 92 ± 7 a | 83 ± 0 a | 80.6 ± 0.7 a | 97 ± 7 a | 101 ± 2 a | 100 ± 5 a | 83 ± 10 a | 83 ± 3 a | 91 ± 8 a |
| Caffeic acid hexoside isomer I | 1039 ± 80 b | 1229 ± 85 a,b | 583 ± 32 c | 1400 ± 71 a | 171 ± 126 d | 144 ± 1 d | 221 ± 7 d | 221 ± 13 d | 325 ± 27 d | 231 ± 13 d |
| Caffeic acid hexoside isomer II | 159 ± 3 b | 1866 ± 16 b | 1091 ± 29 c | 2269 ± 170 a | 258 ± 207 d,e | 212 ± 7 e | 331.1 ± 0.5 d,e | 341 ± 35 d,e | 564 ± 96 d | 369 ± 55 d,e |
| Fertaric acid | 227 ± 16 a | 119 ± 5 b | 70 ± 3 c | 233 ± 9 a | 52 ± 2 c,d | 27.6 ± 0.4 e | 23.0 ± 0.1 e | 38 ± 1 d,e | 50 ± 4 c,d | 35 ± 1 d,e |
| Caffeic acid | 147.2 ± 0.5 | <LOQ | <LOQ | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOQ | <LOD |
| Ferulic acid | 2996 ± 134 a | 2336 ± 173 b | 1328 ± 14 d | 1650 ± 29 c | 1247 ± 48 d | 291 ± 3 f | 499 ± 36 f | 827 ± 44 e | 1352 ± 8 d | 751 ± 16 e |
| Rosmarinic acid | 59874 ± 1040 b | 59183 ± 1429 b | 49922 ± 772 c | 64501 ± 2858 a | 27262 ± 47 e | 9450± 2 g | 20505 ± 250 f | 17086 ± 483 f | 32747 ± 1317 d | 19656 ± 587 f |
| Salvianolic acid A isomer I | 287 ± 24 c | 415 ± 24 b | 484 ± 23 a | 108 ± 1 e | 169 ± 6 d | 86 ± 2 e | 206 ± 8 d | 118.0 ± 0.4 e | 197 ± 4 d | 106 ± 3 e |
| Salvianolic acid A isomer II | <LOQ | 50 ± 8 | 52 ± 10 | <LOQ | <LOQ | <LOD | <LOQ | <LOD | <LOQ | <LOD |
| Salvianolic acid I | <LOQ | 49.0 ± 0.1 | 45.2 ± 0.2 | <LOQ | <LOQ | <LOQ | <LOD | <LOQ | <LOQ | <LOD |
| Salvianolic acid B | 7281 ± 534 c | 11035 ± 654 a | 9545 ± 449 b | 3673 ± 55 d | 3344 ± 63 d | 1181 ± 44 e | 1612 ± 34 e | 2093 ± 61 e | 4078 ± 2 d | 2072 ± 91 e |
| Total phenolic acids | 73522 ± 1818 a | 76374 ± 2371 a | 63203 ± 1253 b | 73916 ± 3196 a | 32599 ± 489 d | 11494 ± 51 f | 23498 ± 330 e | 20808 ± 651 e | 39396 ± 1433 c | 23311 ± 741 e |
| Apigenin | <LOQ | <LOQ | <LOQ | 63.78 ± 2.17 | <LOD | <LOD | <LOD | <LOD | <LOQ | <LOD |
| Herniarin | 519 ± 26 b | 733 ± 7 a | 521 ± 31 b | 142 ± 17 e | 259 ± 5 d | 71 ± 4 f | 238 ± 5 d | 147 ± 8 e | 361 ± 4 c | 177 ± 2 e |
| Total phenolic compounds | 74042 ± 1845 a | 77107 ± 2378 a | 63725 ± 1284b | 74122 ± 3214 a | 32858 ± 494 d | 11565 ± 55 f | 23735 ± 325 e | 20955 ± 659 e | 39758 ± 1437 c | 23489 ± 739 e |
| T. lotocephalus | ||||||||||
| O-Caffeoylquinic acid | <LOQ | 64 ± 5 | <LOQ | <LOQ | <LOQ | <LOQ | n.d. | <LOQ | <LOQ | <LOQ |
| Caffeic acid hexoside isomer I | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 77 ± 3 |
| Caffeic acid hexoside isomer II | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 74.2 ± 0.5 |
| Caffeic acid | <LOQ | <LOD | 91 ± 6 b | <LOD | <LOQ | <LOQ | 110 ± 9 b | 113 ± 3 b | <LOQ | 149 ± 3 a |
| Rosmarinic acid | 30326 ± 1453 e | 10413 ± 512 f | 31285 ± 3028 d,e | 15743 ± 1239 f | 31395 ± 697 d,e | 40635 ± 331 c,d | 64658 ± 6034 a | 55268 ± 5250 b | 46136 ± 851 b,c | 65998 ± 2478 a |
| Salvianolic acid A isomer I | 225 ± 5 e | 217 ± 8 e | 1387 ± 200 a | 755 ± 96 b,c | 203 ± 12 e | 207 ± 6 e | 234 ± 22 e | 561 ± 51 c,d | 434 ± 5 d,e | 891.2 ± 0.72 b |
| Salvianolic acid A isomer II | 57 ± 8 f,g | 55 ± 7 f,g | 303 ± 6 a | 158 ± 2 b | 56 ± 2 f,g | 50 ± 1 g | 70 ± 4 e,f | 96 ± 4 d | 75 ± 3 e | 123 ± 8 c |
| Salvianolic acid I | 116 ± 6 d | 113 ± 4 d | 666 ± 91 a | 339 ± 45 b | 129 ± 7 c,d | 119 ± 2 d | 182 ± 17 c,d | 238 ± 24 b,c | 178 ± 2 c,d | 294.6 ± 0.4 b |
| Salvianolic acid B | 53 ± 4 e | 45 ± 1 e | 240 ± 33 a | 146 ± 16 b,c | 52 ± 2 e | 77 ± 2 d,e | 69 ± 7 e | 132 ± 13 c | 111.7 ± 0.3 c,d | 184 ± 3 b |
| Salvianolic acid A isomer III | 78 ± 4 e,f | 45 ± 3 f | 156 ± 22 a,b | 75 ± 10 e,f | 91 ± 4 d,e | 120 ± 2 c,d | 182 ± 14 a | 170 ± 11 a,b | 77 ± 1 e,f | 144 ± 5 b,c |
| Total phenolic acids | 30855 ± 1481 e | 10952 ± 540 f | 34128 ± 3375 d,e | 17216 ± 1408 f | 31927 ± 719 d,e | 41208 ± 344 c,d | 65506 ± 6090 a,b | 56578 ± 5355 b | 47013 ± 839 c | 67936 ± 2461 a |
| Luteolin-7-O-glucuronide | 133 ± 2 d | 81 ± 4 e | 215 ± 21 b | 83 ± 8 e | 134 ± 6 d | 167 ± 6 c,d | 209 ± 23 b | 301 ± 20 a | 193.3 ± 0.6 b,c | 315 ± 3 a |
| Herniarin | 76 ± 6 e | <LOQ | 120 ± 16 c | 49 ± 4 f | 88 ± 2 d,e | 107 ± 3 c,d | 174 ± 3 b | 163 ± 9 b | 157 ± 3 b | 204 ± 12 a |
| Total phenolic compounds | 31064 ± 1488 e | 11033 ± 544 f | 34463 ± 3412 d,e | 17348 ± 1421 f | 32149 ± 724 d,e | 41483 ± 352 c,d | 65889 ± 6070 a,b | 57042 ± 5366 b | 47363 ± 842 c | 68456 ± 2469 a |
| Phenolic Compounds | Antioxidant Activity | Enzyme Inhibitory Activity | |||||
|---|---|---|---|---|---|---|---|
| ABTS | DPPH | FRAP | ORAC | Tyr | AChE | BChE | |
| L. viridis | |||||||
| O-Caffeoylquinic acid | −0.492 * | −0.519 * | −0.443 | −0.432 | −0.517 * | −0.177 | −0.542 * |
| Caffeic acid hexoside isomer I | 0.778 ** | 0.879 ** | 0.941 ** | 0.766 ** | 0.440 * | 0.475 * | 0.897 ** |
| Caffeic acid hexoside isomer II | 0.783 ** | 0.903 ** | 0.942 ** | 0.788 ** | 0.501 * | 0.515 * | 0.910 ** |
| Fertaric acid | 0.674 ** | 0.824 ** | 0.853 ** | 0.631 ** | 0.284 | 0.605 ** | 0.785 ** |
| Ferulic acid | 0.702 ** | 0.841 ** | 0.881 ** | 0.712 ** | 0.402 | 0.392 | 0.733 ** |
| Rosmarinic acid | 0.811 ** | 0.975 ** | 0.985 ** | 0.876 ** | 0.632 ** | 0.508 * | 0.927 ** |
| Salvianolic acid A isomer I | 0.576 ** | 0.613 ** | 0.591 ** | 0.669 ** | 0.655 ** | −0.025 | 0.552 * |
| Salvianolic acid B | 0.669 ** | 0.776 ** | 0.791 ** | 0.817 ** | 0.645 ** | 0.102 | 0.745 ** |
| Herniarin | 0.614 ** | 0.662 ** | 0.704 ** | 0.698 ** | 0.540 * | 0.026 | 0.577 ** |
| Total phenolic contents | 0.815 ** | 0.973 ** | 0.988 ** | 0.890 ** | 0.642 ** | 0.462 * | 0.926 ** |
| T. lotocephalus | |||||||
| Caffeic acid | 0.910 ** | 0.970 ** | 0.932 ** | 0.032 | 0.310 | −0.049 | −0.203 |
| Rosmarinic acid | 0.928 ** | 0.873 ** | 0.891 ** | 0.794 ** | 0.888 ** | −0.178 | 0.147 |
| Salvianolic acid A isomer I | 0.243 | 0.172 | 0.255 | 0.394 | 0.065 | 0.269 | 0.851 ** |
| Salvianolic acid A isomer II | 0.061 | −0.032 | 0.032 | 0.306 | −0.011 | 0.289 | 0.845 ** |
| Salvianolic acid I | 0.132 | 0.020 | 0.104 | 0.382 | 0.037 | 0.221 | 0.842 ** |
| Salvianolic acid B | 0.362 | 0.293 | 0.390 | 0.514 * | 0.214 | 0.232 | 0.828 ** |
| Salvianolic acid A isomer III | 0.770 ** | 0.591 ** | 0.650 ** | 0.875 ** | 0.739 ** | 0.035 | 0.436 |
| Luteolin-7-O-glucuronide | 0.914 ** | 0.858 ** | 0.887 ** | 0.795 ** | 0.693 ** | 0.214 | 0.401 |
| Herniarin | 0.901 ** | 0.864 ** | 0.851 ** | 0.745 ** | 0.793 ** | −0.055 | 0.289 |
| Total phenolic contents | 0.935 ** | 0.877 ** | 0.898 ** | 0.808 ** | 0.887 ** | −0.165 | 0.183 |
| Treatment | NP Concentration (mg/L) | TYR (mgKAE/gextract) | AChE (mgGE/gextract) | BChE (mgGE/gextract) |
|---|---|---|---|---|
| L. viridis | ||||
| Control | 0 | 13.27 ± 0.08 c | 4.43 ± 0.08 a | 9.74 ± 0.89 b |
| CuO | 1 | 14.90 ± 0.66 b,c | 3.44 ± 0.19 b | 10.71 ± 0.56 b |
| 5 | 17.49 ± 0.36 a | 3.65 ± 0.37 b | 10.26 ± 0.26 b | |
| 10 | 15.87 ± 0.36 a,b | 4.58 ± 0.26 a | 12.33 ± 0.31 a | |
| ZnO | 1 | 13.99 ± 0.47 c | 3.42 ± 0.15 b | 4.04 ± 0.21 d,e |
| 5 | 10.31 ± 0.34 d | 3.30 ± 0.21 b | 2.49 ± 0.51 f | |
| 10 | 13.35 ± 0.31 c | 3.34 ± 0.23 b | 2.96 ± 0.44 e,f | |
| Fe3O4 | 1 | 13.64 ± 0.41 c | 3.29 ± 0.19 b | 5.12 ± 0.52 c,d |
| 5 | 14.60 ± 0.66 b,c | 3.75 ± 0.19 b | 4.95 ± 0.23 c,d | |
| 10 | 13.39 ± 0.08 c | 3.63 ± 0.26 b | 6.13 ± 0.65 c | |
| T. lotocephalus | ||||
| Control | 0 | 13.18 ± 0.58 d,e | 3.28 ± 0.19 a,b,c | 5.00 ± 0.20 d |
| CuO | 1 | 11.46 ± 0.67 e | 3.25 ± 0.28 a,b,c | 5.32 ± 0.32 c,d |
| 5 | 14.51 ± 0.97 b,c,d | 3.44 ± 0.12 a,b | 8.75 ± 0.60 a | |
| 10 | 14.16 ± 0.31 c,d,e | 3.05 ± 0.12 b,c | 6.36 ± 0.28 c | |
| ZnO | 1 | 13.88 ± 0.60 c,d,e | 2.86 ± 0.21 c | 3.38 ± 0.30 e |
| 5 | 15.49 ± 0.70 a,b,c,d | 3.05 ± 0.11 b,c | 4.92 ± 0.29 d | |
| 10 | 18.36 ± 1.51 a | 2.90 ± 0.18 b,c | 6.51 ± 0.67 b,c | |
| Fe3O4 | 1 | 17.39 ± 0.74 a,b | 3.74 ± 0.13 a | 4.92 ± 0.61 d |
| 5 | 15.68 ± 1.36 a,b,c,d | 2.90 ± 0.11 b,c | 5.27 ± 0.09 c,d | |
| 10 | 16.81 ± 1.04 a,b,c | 3.22 ± 0.12 a,b,c | 7.70 ± 0.32 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, S.; Mansinhos, I.; Rodríguez-Solana, R.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Romano, A. Impact of Metallic Nanoparticles on In Vitro Culture, Phenolic Profile and Biological Activity of Two Mediterranean Lamiaceae Species: Lavandula viridis L’Hér and Thymus lotocephalus G. López and R. Morales. Molecules 2021, 26, 6427. https://doi.org/10.3390/molecules26216427
Gonçalves S, Mansinhos I, Rodríguez-Solana R, Pereira-Caro G, Moreno-Rojas JM, Romano A. Impact of Metallic Nanoparticles on In Vitro Culture, Phenolic Profile and Biological Activity of Two Mediterranean Lamiaceae Species: Lavandula viridis L’Hér and Thymus lotocephalus G. López and R. Morales. Molecules. 2021; 26(21):6427. https://doi.org/10.3390/molecules26216427
Chicago/Turabian StyleGonçalves, Sandra, Inês Mansinhos, Raquel Rodríguez-Solana, Gema Pereira-Caro, José Manuel Moreno-Rojas, and Anabela Romano. 2021. "Impact of Metallic Nanoparticles on In Vitro Culture, Phenolic Profile and Biological Activity of Two Mediterranean Lamiaceae Species: Lavandula viridis L’Hér and Thymus lotocephalus G. López and R. Morales" Molecules 26, no. 21: 6427. https://doi.org/10.3390/molecules26216427
APA StyleGonçalves, S., Mansinhos, I., Rodríguez-Solana, R., Pereira-Caro, G., Moreno-Rojas, J. M., & Romano, A. (2021). Impact of Metallic Nanoparticles on In Vitro Culture, Phenolic Profile and Biological Activity of Two Mediterranean Lamiaceae Species: Lavandula viridis L’Hér and Thymus lotocephalus G. López and R. Morales. Molecules, 26(21), 6427. https://doi.org/10.3390/molecules26216427










