1H NMR Combined with Multivariate Statistics for Discrimination of Female and Male Flower Buds of Populus tomentosa
Abstract
:1. Introduction
2. Results
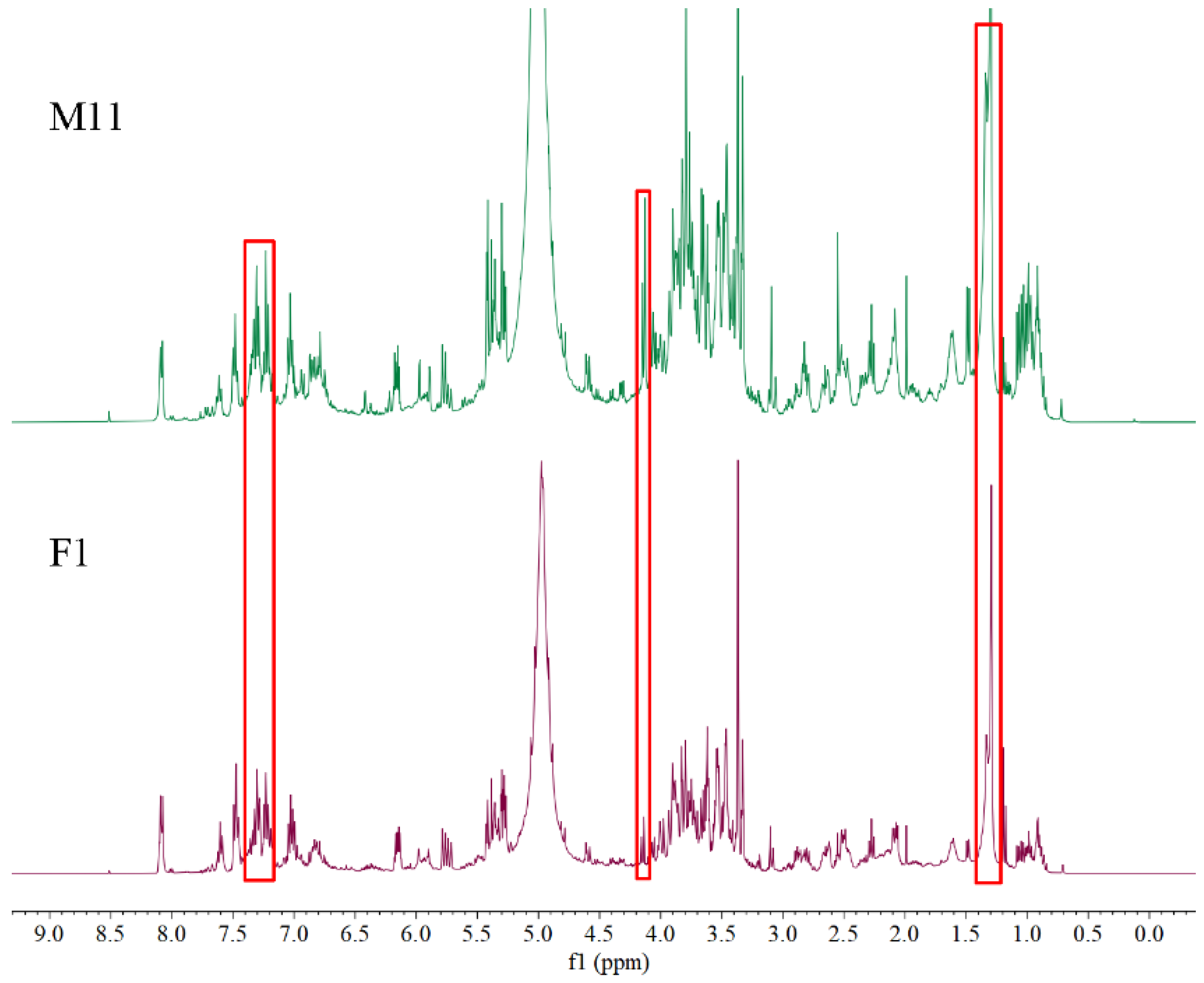
2.1. Visual Inspection of 1H NMR Spectra
2.2. Compound Assignment
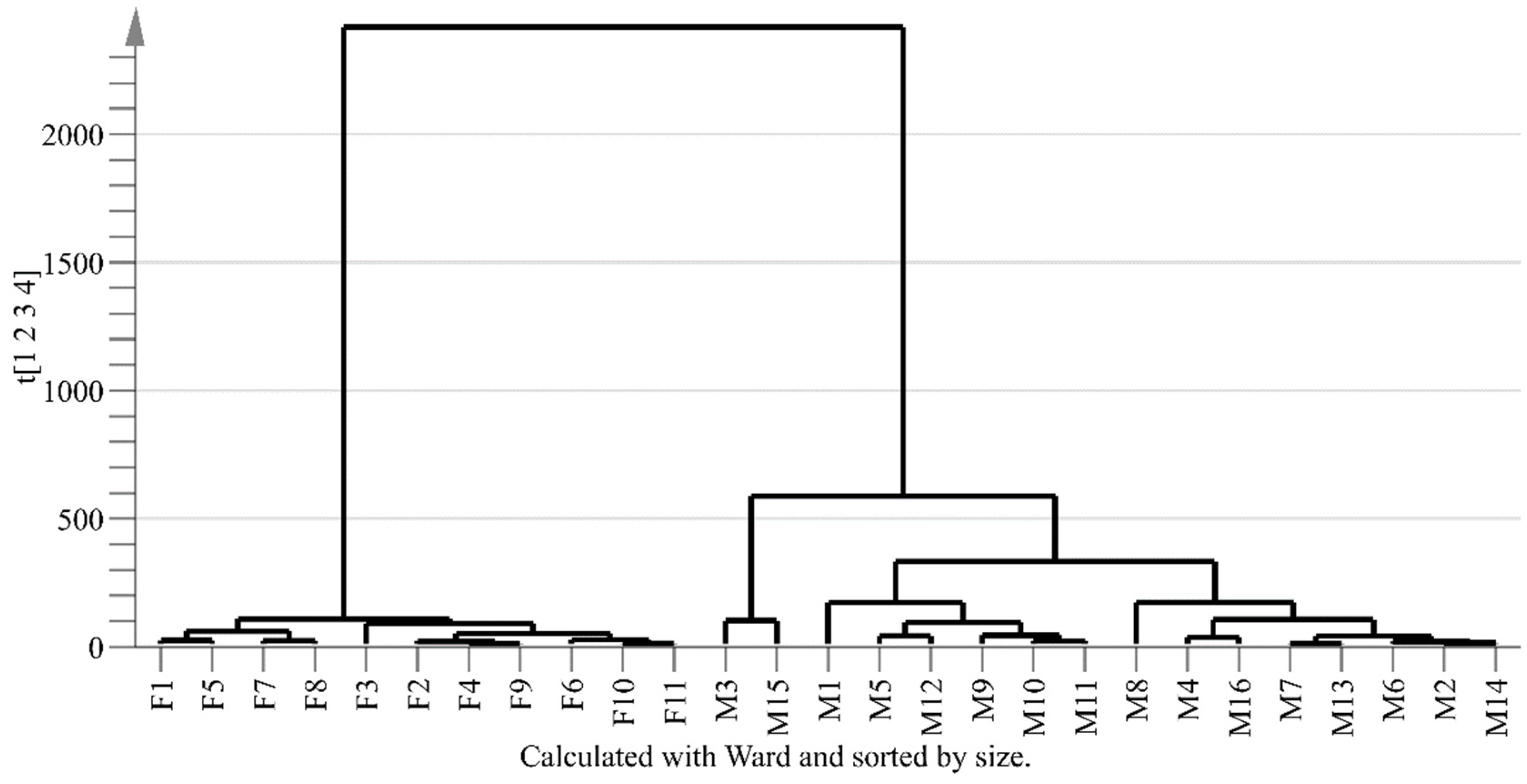
2.3. PCA and HCA
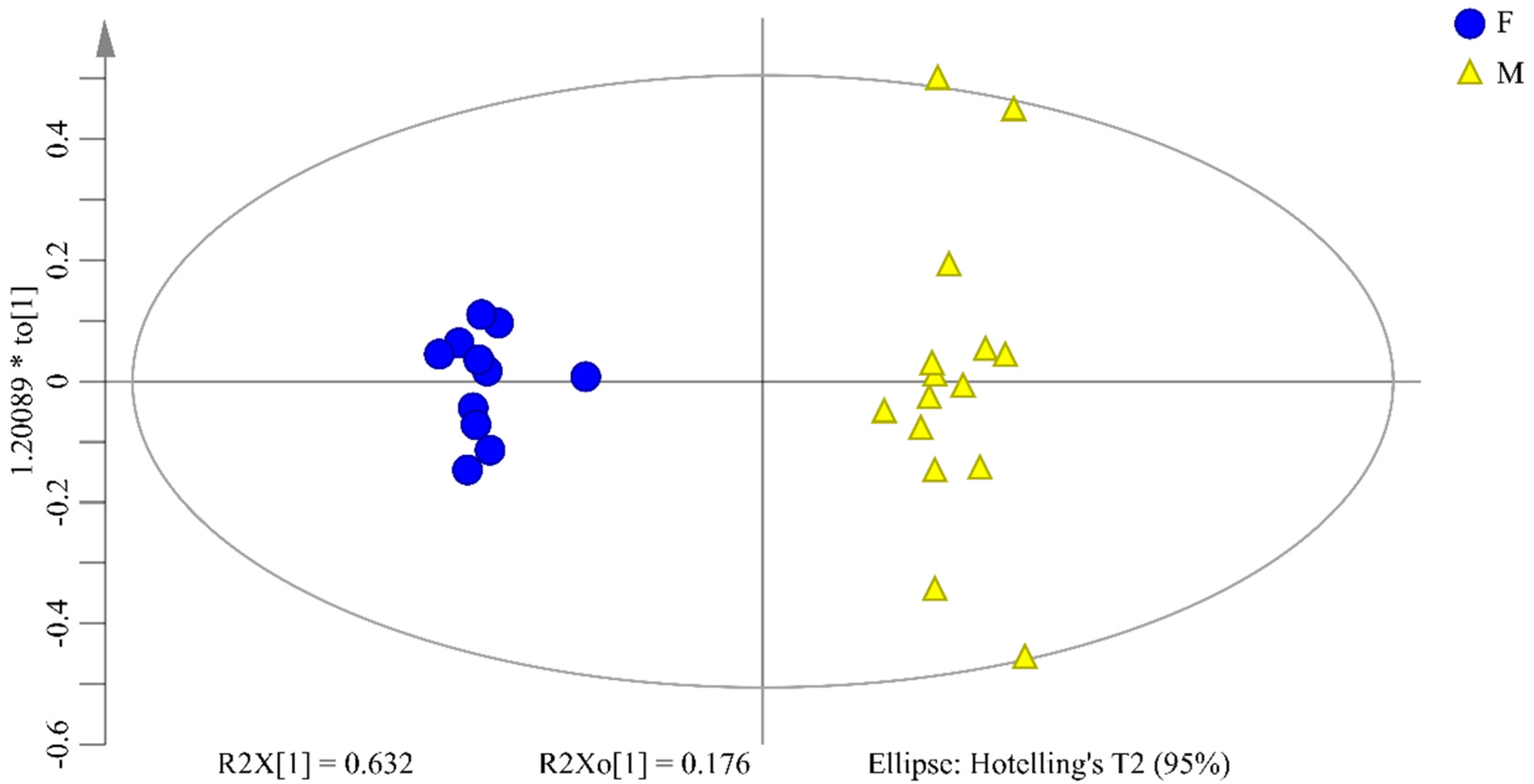
2.4. OPLS-DA
3. Materials and Methods
3.1. Reagents
3.2. Apparatus
3.3. Sample Collection
3.4. Sample Preparation
3.5. 1H NMR Measurement
3.6. Data Processing
3.6.1. Spectra Pre-Treatment
3.6.2. Statistical Analysis
3.7. Chemical Compound Assignment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Munné-Bosch, S. Sex ratios in dioecious plants in the framework of global change. Environ. Exp. Bot. 2015, 109, 99–102. [Google Scholar] [CrossRef]
- The Pharmacopoeia Commission of the People’s Republic of China. The Pharmacopoeia of the People’s Republic of China (Part 1); Revised Edition; China Medical Science and Technology Press: Beijing, China, 2020; pp. 57–329. [Google Scholar]
- Xi, B.Y.; Wang, Y.; Jia, L.M.; Bloomberg, M.; Li, G.D.; Di, N. Characteristics of fine root system and water uptake in a triploid Populus tomentosa plantation in the North China Plain: Implications for irrigation water management. Agric. Water Manag. 2013, 117, 83–92. [Google Scholar] [CrossRef]
- Jiao, M.; Zhou, W.Q.; Zheng, Z.; Wang, J.; Qian, Y.G. Patch size of trees affects its cooling effectiveness: A perspective from shading and transpiration processes. Agric. For. Meteorol. 2017, 247, 293–299. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, X.P.; Zhao, C.G. Nonlinear prediction model of noise reduction by greenbelts. Urban For. Urban Green. 2015, 14, 282–285. [Google Scholar] [CrossRef]
- Qin, Z.; Li, Z.D.; Cheng, F.Y.; Chen, J.F.; Liang, B. Influence of canopy structural characteristics on cooling and humidifying effects of Populus tomentosa community on calm sunny summer days. Landsc. Urban Plan. 2014, 127, 75–82. [Google Scholar] [CrossRef]
- Wang, X.; Hong, M.M.; Huang, Z.; Zhao, Y.F.; Ou, Y.S.; Jia, H.X.; Li, J. Biomechanical properties of plant root systems and their ability to stabilize slopes in geohazard-prone regions. Soil Tillage Res. 2019, 189, 148–157. [Google Scholar] [CrossRef]
- The Pharmacopoeia Commission of the People’s Republic of China. The Pharmacopoeia of the People’s Republic of China (Part 1); People’s Medical Publishing House Press: Beijing, China, 1977; p. 287. [Google Scholar]
- Xu, Q.Q.; Shen, Z.Q.; Wang, Y.B.; Guo, S.J.; Li, F.; Wang, Y.P.; Zhou, C.F. Anti-diarrhoeal and anti-microbial activity of Flos populi (male inflorescence of Populus tomentosa Carrière) aqueous extracts. J. Ethnopharmacol. 2013, 148, 640–646. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Wang, Y.B.; Guo, S.J.; Shen, Z.Q.; Wang, Y.P.; Yang, L.M. Anti-inflammatory and analgesic activity of aqueous extract of Flos populi. J. Ethnopharmacol. 2014, 152, 540–545. [Google Scholar] [CrossRef]
- Liu, H.P.; Chao, Z.M.; Wu, X.Y.; Tan, Z.G.; Wang, C.; Sun, W. Chemical constituents contained in Populus tomentosa. China J. Chin. Mater. Med. 2012, 37, 1422–1425. [Google Scholar]
- Xu, L.; Liu, H.P.; Ma, Y.C.; Wu, C.; Li, R.Q.; Chao, Z.M. Isomeric phenolic glycosides from Populus tomentosa. Rec. Nat. Prod. 2018, 13, 97–103. [Google Scholar] [CrossRef]
- Wu, C.; Xu, B.; Li, Z.J.; Song, P.P.; Chao, Z.M. Gender discrimination of Populus tomentosa barks by HPLC fingerprint combined with multivariate statistics. Plant Direct 2021, 5, e00311. [Google Scholar] [CrossRef]
- Xu, L.; Liu, H.P.; Ma, Y.C.; Wu, C.; Li, R.Q.; Chao, Z.M. Comparative study of volatile components from male and female flower buds of Populus tomentosa by HS-SPME-GC-MS. Nat. Prod. Res. 2019, 33, 2105–2108. [Google Scholar] [CrossRef]
- Silva, R.; Pereira, T.C.S.; Souza, A.R.; Ribeiro, P.R. 1H NMR-based metabolite profiling for biomarker identification. Clin. Chim. Acta 2020, 502, 269–279. [Google Scholar] [CrossRef]
- Brennan, L. NMR-based metabolomics: From sample preparation to applications in nutrition research. Prog. Nucl. Mag. Res. Spectrosc. 2014, 83, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ammerlaan, W.; Trezzi, J.P.; Mathay, C.; Hiller, K.; Betsou, F. Method validation for preparing urine samples for downstream proteomic and metabolomic applications. Biopreserv. Biobank. 2014, 12, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Hanganu, A.; Chira, N. When detection of dairy food fraud fails: An alternative approach through proton nuclear magnetic resonance spectroscopy. J. Dairy Sci. 2021, 104, 8454–8466. [Google Scholar] [CrossRef]
- Sun, L.L.; Wang, M.; Ren, X.L.; Jiang, M.M.; Deng, Y.R. Rapid authentication and differentiation of herbal medicine using 1H NMR fingerprints coupled with chemometrics. J. Pharm. Biomed. 2018, 160, 323–329. [Google Scholar] [CrossRef]
- Fan, S.X.; Zhong, Q.D.; Fauhl-Hassek, C.; Pfister, M.K.H.; Horn, B.; Huang, Z.B. Classification of Chinese wine varieties using 1H NMR spectroscopy combined with multivariate statistical analysis. Food Control 2018, 88, 113–122. [Google Scholar] [CrossRef]
- Barba, I.; Andrés, M.; Picón, I.; Aguade-Bruix, S.; Garcia-Dorado, D. Sex differences in the 1H NMR metabolic profile of serum in cardiovascular risk patients. Sci. Rep. 2019, 9, 2380–2387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawat, A.; Misra, G.; Saxena, M.; Tripathi, S.; Dubey, D.; Saxena, S.; Aggarwal, A.; Gupta, V.; Khan, M.Y.; Prakash, A. 1H NMR based serum metabolic profiling reveals differentiating biomarkers in patients with diabetes and diabetes-related complication. Diabetes Metab. Syndr. 2018, 13, 290–298. [Google Scholar] [CrossRef]
- Sobolev, A.P.; Thomas, F.; Donarski, J.; Ingallina, C.; Circi, S.; Cesare Marincola, F.; Capitani, D.; Mannina, L. Use of NMR applications to tackle future food fraud issues. Trends Food Sci. Technol. 2019, 91, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Jović, O.; Pičuljan, K.; Hrenar, T.; Smolić, T.; Primožič, I. 1H NMR adulteration study of hempseed oil with full chemometric approach on large variable data. Chemom. Intell. Lab. 2019, 185, 41–46. [Google Scholar] [CrossRef]
- Cusano, E.; Cagliani, L.R.; Consonni, R.; Simonato, B.; Zapparoli, G. NMR-based metabolic profiling of different yeast fermented apple juices. LWT 2020, 118, 108771. [Google Scholar] [CrossRef]
- Kock, F.V.C.; Rocha, T.C.; Araújo, G.M.; Simões, F.R.; Colnago, L.A.; Barbosa, L.L. Time-domain NMR: A novel analytical method to quantify adulteration of ethanol fuel with methanol. Fuel 2019, 258, 116158. [Google Scholar] [CrossRef]
- Ma, T.; Li, M.; Li, J.; Liu, S.; Wang, A. Chemical constituents from leaves of Populus tomentosa. Chin. Tradit. Herb. Drugs 1987, 18, 9–11. [Google Scholar]
- Hou, Y.; Li, B.; Zhang, G.; Li, M.; Miu, X.; Cui, H.; Xia, Z.; Tian, Y.; Liu, S.; Chen, L.; et al. Isolation and identification of chemical constituents of Populus tomentosa male inflorescence II. J. Int. Pharm. Res. 2018, 24, 77–81. [Google Scholar]
- Lin, M.; Li, S. Studies on the chemical constituents of Populus tomentosa carr. Acta Pharm. Sin. 1993, 28, 437–441. [Google Scholar]
- Hou, Y.; Zhang, G.; Cui, H.; Tian, Y.; Liu, S.; Chen, L.; Li, B.; Dong, J. Chemical constituents from the male anthotaxy of Populus tomentosa carr. China J. Exp. Tradit. Med. Form. 2017, 44, 1131–1136. [Google Scholar]
- Zhao, S.J.; Tian, J.S.; Tai, G.; Gao, X.X.; Liu, H.L.; Du, G.H.; Liu, X.J.; Qin, X.M. 1H NMR-based metabolomics revealed the protective effects of Guilingji on the testicular dysfunction of aging rats. J. Ethnopharmacol. 2019, 238, 111839. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.Z.; Lei, Z.H.; Qin, X.M.; Li, Z.Y. NMR based metabolomic comparison of the antitussive and expectorant effect of Farfarae Flos collected at different stages. J. Pharm. Biomed. 2018, 150, 377–385. [Google Scholar] [CrossRef]
- Li, T.; Su, C.; Li, C.; Si, M. Identification of Rhodiola quadrifida and Rhodiola crenulata based on NMR fingerprint and chemical pattern recognition method. Chin. Tradit. Herb. Drugs 2018, 49, 3918–3925. [Google Scholar]
- Winning, H.; Roldán-Marín, E.; Dragsted, L.O.; Viereck, N.; Poulsen, M.; Sánchez-Moreno, C.; Cano, M.P.; Engelsen, S.B. An exploratory NMR nutri-metabonomic investigation reveals dimethyl sulfone as a dietary biomarker for onion intake. Analyst 2009, 134, 2344. [Google Scholar] [CrossRef] [PubMed]
- Kemsley, E.K.; Defernez, M.; Marini, F. Multivariate statistics: Considerations and confidences in food authenticity problems. Food Control 2019, 105, 102–112. [Google Scholar] [CrossRef]
- He, S.S.; Wang, Y.L.; Xie, J.H.; Gao, H.; Li, X.J.; Huang, Z.B. 1H NMR-based metabolomic study of the effects of flavonoids on citrinin production by Monascus. Food Res. Int. 2020, 137, 109532. [Google Scholar] [CrossRef]
- Adnan, T.M.T.; Tanjim, M.M.; Adnan, M.A. Fast, scalable and geo-distributed PCA for big data analytics. Inform. Syst. 2021, 98, 101710. [Google Scholar] [CrossRef]
- Zhu, M.T.; Shi, T.; Chen, Y.; Luo, S.H.; Leng, T.; Wang, Y.L.; Guo, C.; Xie, M.Y. Prediction of fatty acid composition in camellia oil by 1H NMR combined with PLS regression. Food Chem. 2019, 279, 339–346. [Google Scholar] [CrossRef]
- Bylesjö, M.; Rantalainen, M.; Cloarec, O.; Nicholson, J.K.; Holmes, E.; Trygg, J. OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification. J. Chemometr. 2006, 20, 341–351. [Google Scholar] [CrossRef]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol. Biosyst. 2015, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Zeng, X.; Peng, W.; Wu, Z.; Su, W.W. Study on the discrimination between Citri Reticulatae Pericarpium Varieties based on HS-SPME-GC-MS combined with multivariate statistical analyses. Molecules 2018, 23, 1235. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Kim, S.M.; Yee, S.M.; Kim, E.M.; Lee, E.H.; Choi, H.R.; Lee, Y.S.; Yang, W.K.; Kim, H.Y.; Kim, K.H.; et al. Daucosterol suppresses dextran sulfate sodium (DSS)-induced colitis in mice. Int. Immunopharmacol. 2019, 72, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.D.; Wang, W.J.; Zhang, J.N.; Wang, T.; Liu, M.; Yang, M.; Sun, Z.H.; Li, X.; Li, Y.D. Antimicrobial and antibiofilm activities of ursolic acid against carbapenem-resistant Klebsiella pneumoniae. J. Antibiot. 2020, 73, 382–391. [Google Scholar] [CrossRef]
- Kong, L.B.; Li, S.S.; Liao, Q.J.; Zhang, Y.N.; Sun, R.; Zhu, X.D.; Zhang, Q.H.; Wang, J.; Wu, X.Y.; Fang, X.N.; et al. Oleanolic acid and ursolic acid: Novel hepatitis C virus antivirals that inhibit NS5B activity. Antivir. Res. 2013, 98, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Tan, Z.J.; Hu, Y.P.; Shu, Y.J.; Bao, R.F.; Jiang, L.; Wu, X.S.; Li, M.L.; Ding, Q.; Wang, X.A.; et al. Ursolic acid induces cell cycle arrest and apoptosis of gallbladder carcinoma cells. Cancer Cell Int. 2014, 14, 96–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Q.X.; Han, Y.T.; Gao, H.; Tian, R.; Li, P.; Wang, C.B. Ursolic acid induced anti-proliferation effects in rat primary vascular smooth muscle cells is associated with inhibition of microRNA-21 and subsequent PTEN/PI3K. Eur. J. Pharmacol. 2016, 781, 69–75. [Google Scholar] [CrossRef]
- Wu, X.; Chao, Z.; Wang, C.; Sun, W.; Zhang, G. Extraction and crystal structure of β-sitosterol. Chin. J. Struct. Chem. 2014, 33, 801–806. [Google Scholar] [CrossRef]
- Popov, S.A.; Kozlova, L.P.; Kornaukhova, L.M.; Shpatov, A. Simple and efficient process for large scale preparation of betulonic acid from birch bark extracts. Ind. Crops Prod. 2016, 92, 197–200. [Google Scholar] [CrossRef]



| No. | Compound Name | δH (Multiplicity, Coupling Constant, Protons) | References |
|---|---|---|---|
| 1 | Benzoic acid | 7.60 (t, 7.6, 1H), 7.48 (t, 7.6, 2H) | [11,27] |
| 2 | Sakuranetin | 6.94 (d, 8.0, 2H) | Standard and [11] |
| 3 | Rhamnocitrin | 8.20 (d, 9.2, 2H) | [11] |
| 4 | Salicyltremuloidin | 7.35 (t, 8.0, 2H), 7.25 (d, 8.0, 1H) | [11] |
| 5 | Daucosterol | 5.27(d, 4.8, 1H), 0.96 (s, 3H) | [11,28,29] |
| 6 | Tremuloidin | 7.30 (d, 7.6, 1H) | [11] |
| 7 | Isograndidentatin A | 6.84 (d, 8.4, 2H), 4.51 (d, 8.0, 1H) | Standard and [11] |
| 8 | Siebolside B | 5.53 (d, 13.2, 1H) | Standard and [11] |
| 9 | Sakuranin | 3.79 (s, 3H) | [11] |
| 10 | Micranthoside | 7.34 (d, 7.6, 2H) | [11] |
| 11 | Sucrose | 5.42 (d, 3.6, 1H), 4.13 (d, 8.4, 1H) | [11] |
| 12 | β-Sitosterol | 1.01 (s, 3H), 0.93 (d, 6.4, 3H), 0.81 (d, 8.4, 3H) | [11] |
| 13 | Salicin | 7.19 (d, 7.2, 1H) | [11,27,29] |
| 14 | Eriodictyol | 6.83 (s, 1H), 6.78 (s, 2H) | [28] |
| 15 | Myricetin | 7.23 (s, 2H) | [28] |
| 16 | Dihydromyricetin | 6.41 (s, 2H), 5.94 (d, 2.0, 1H) | [28] |
| 17 | Protocatechuic acid | 7.33 (d, 1.6, 1H) | [28] |
| 18 | Caffeic acid | 7.31 (d, 15.2, 1H) | [28] |
| 19 | 2-Acetyl-1,3-dicaffeoylglycerol | 2.06 (s, 3H) | [28] |
| 20 | Quercetin | 6.82 (d, 8.8, 1H) | [28] |
| 21 | Betulonic acid | 1.72 (s, 3H), 1.11 (s, 3H) | [30] |
| 22 | Chrysoeriol | 7.57 (d, 2.0, 1H) | [30] |
| 23 | Pinocembrin | 7.49(d, 8.0, 2H), 5.97 (d, 2.4, 1H) | [30] |
| 24 | Dillenetin | 7.14 (d, 8.8, 1H) | [30] |
| 25 | Naringenin | 7.32 (d, 8.0, 2H) | [30] |
| 26 | Isosakuranetin | 6.97 (d, 8.8, 2H) | [30] |
| 27 | Apigenin | 8.00 (d, 9.2, 2H) | [30] |
| 28 | Kaempferol | 6.42 (d, 2.0, 1H), 6.22 (d, 2.0, 1H) | [30] |
| 29 | 3,3′,4,4′-Tetrahydroxybiphenyl | 6.81 (d, 2.0, 2H) | [30] |
| 30 | Ursolic acid | 1.03 (s, 3H) | [30] |
| No. | Collection Location | Longitude and Latitude |
|---|---|---|
| F1~F5 | Qing Long Hutong | 39°56′53.74″ N, 116°25′24.82″ E |
| F6~F8 | Min’an Community | 39°56′35.12″ N, 116°25′49.62″ E |
| F9~F11 | Lishuiqiao South | 40°13′14.77″ N, 116°13′52.61″ E |
| M1~M2 | Bei’erhuan Road | 39°56′56.79″ N, 116°25′20.40″ E |
| M3~M10 | Tongjiao Temple | 40°13′14.77″ N, 116°13′52.61″ E |
| M11~M16 | Min’an Street | 39°56′40.67″ N, 116°25′48.47″ E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Wu, C.; Li, Z.; Song, P.; Chao, Z. 1H NMR Combined with Multivariate Statistics for Discrimination of Female and Male Flower Buds of Populus tomentosa. Molecules 2021, 26, 6458. https://doi.org/10.3390/molecules26216458
Xu B, Wu C, Li Z, Song P, Chao Z. 1H NMR Combined with Multivariate Statistics for Discrimination of Female and Male Flower Buds of Populus tomentosa. Molecules. 2021; 26(21):6458. https://doi.org/10.3390/molecules26216458
Chicago/Turabian StyleXu, Bo, Cui Wu, Zhuojun Li, Pingping Song, and Zhimao Chao. 2021. "1H NMR Combined with Multivariate Statistics for Discrimination of Female and Male Flower Buds of Populus tomentosa" Molecules 26, no. 21: 6458. https://doi.org/10.3390/molecules26216458
APA StyleXu, B., Wu, C., Li, Z., Song, P., & Chao, Z. (2021). 1H NMR Combined with Multivariate Statistics for Discrimination of Female and Male Flower Buds of Populus tomentosa. Molecules, 26(21), 6458. https://doi.org/10.3390/molecules26216458






