Highly Sensitive, Selective, Flexible and Scalable Room-Temperature NO2 Gas Sensor Based on Hollow SnO2/ZnO Nanofibers
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
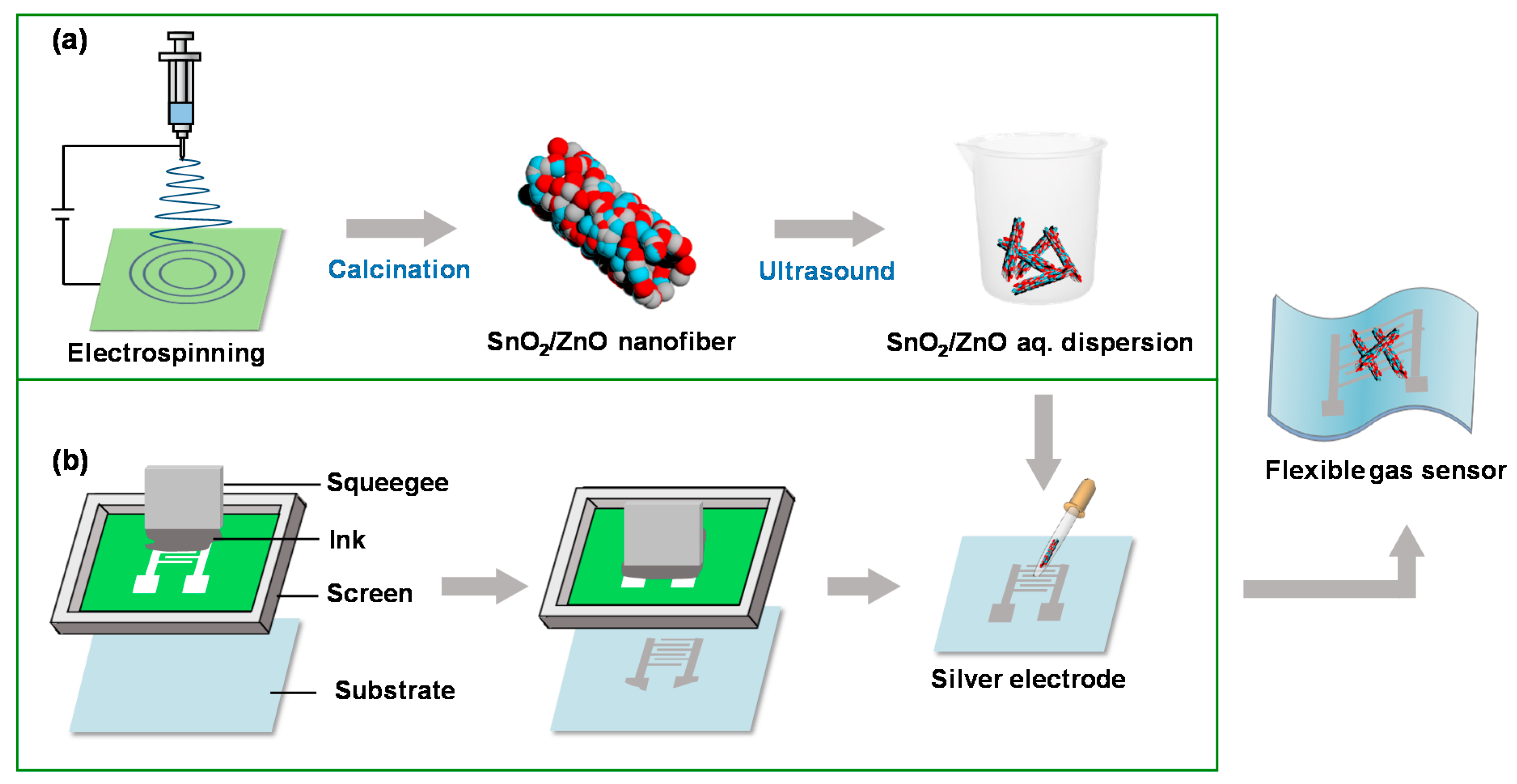
2.2. Synthesis of Hollow SnO2 and SnO2/ZnO Nanofibers
2.3. Fabrication of Flexible Patterned Electrodes
2.4. Preparation and Test of Gas Sensors
2.5. Characterizations
3. Results and Discussion
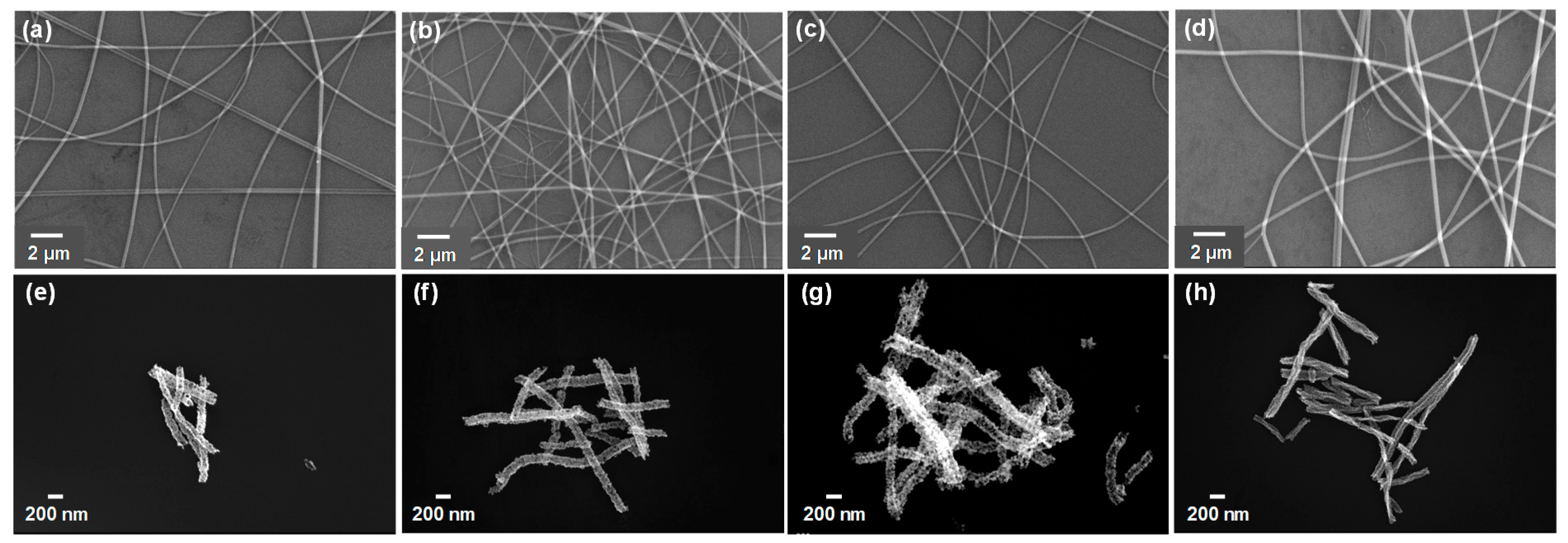
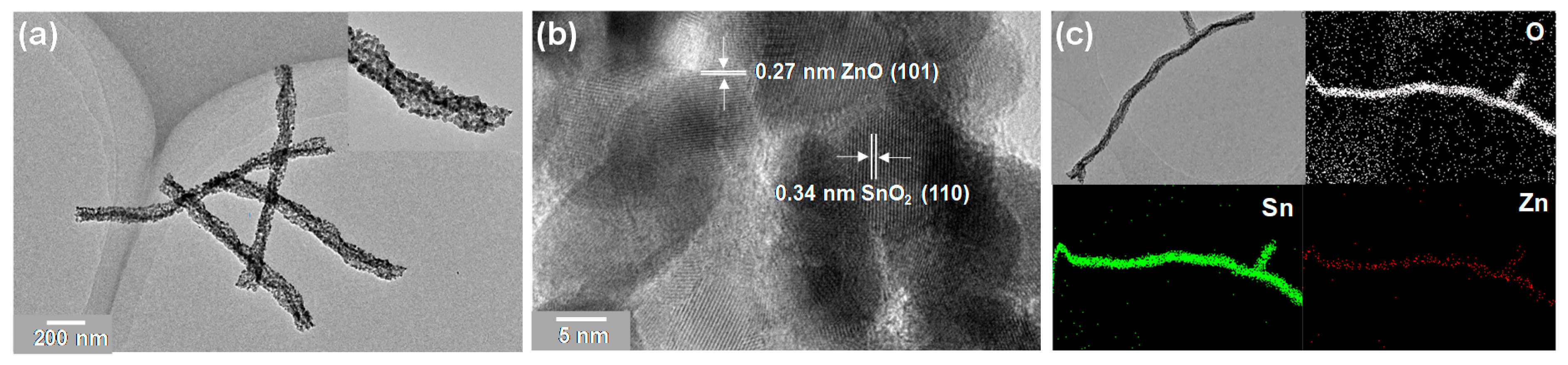
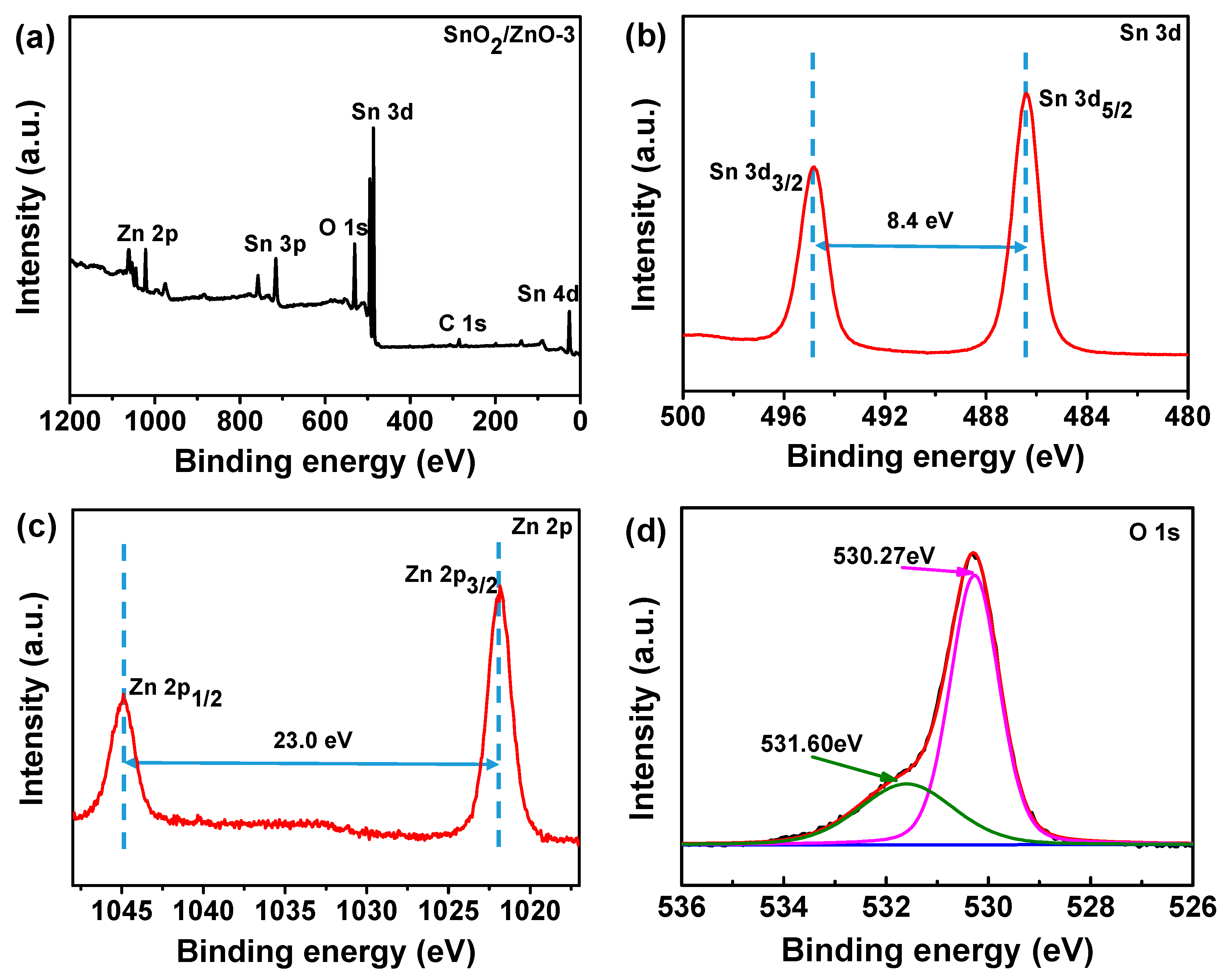
3.1. Characterizations of SnO2, SnO2/ZnO Nanofibers
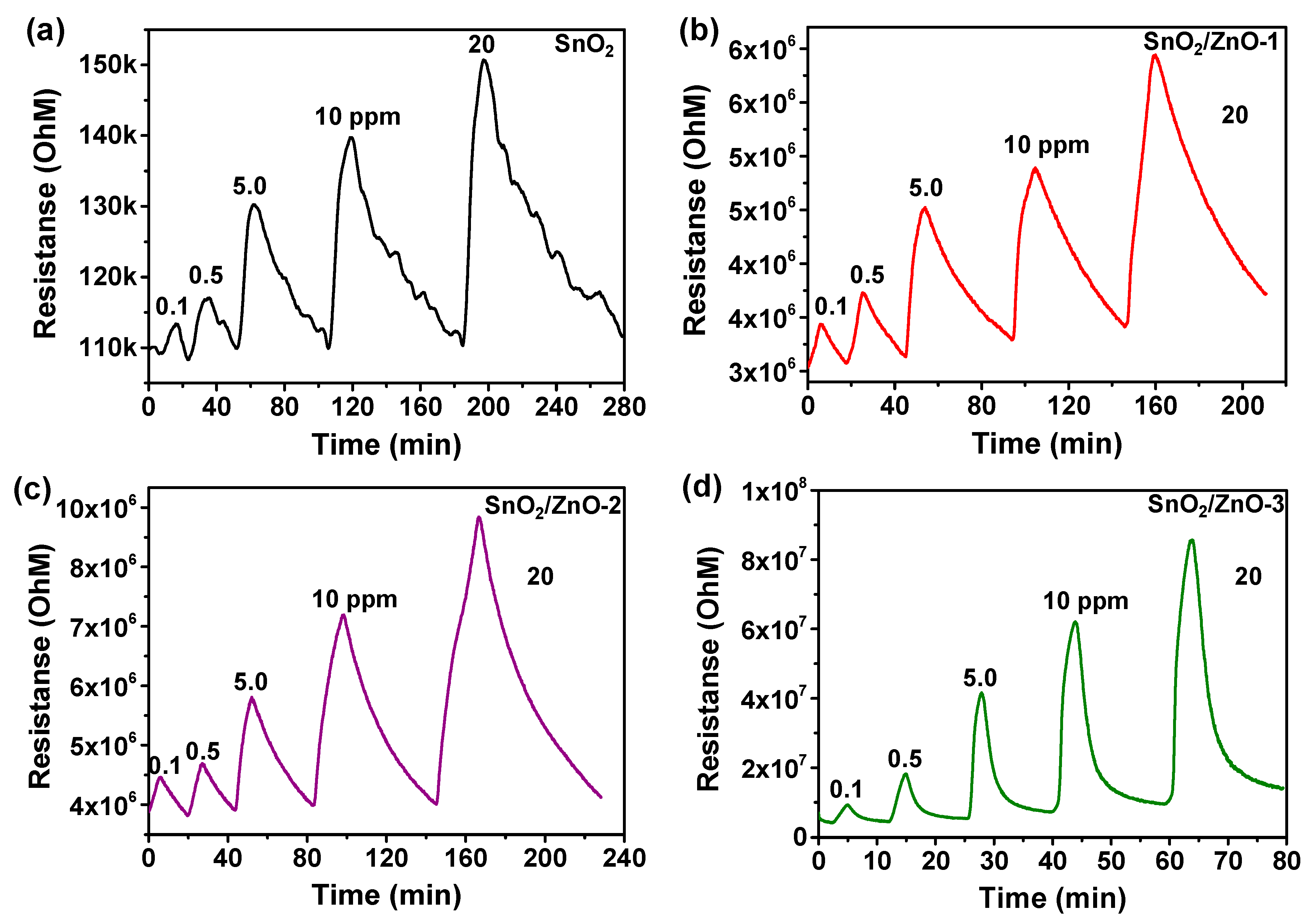
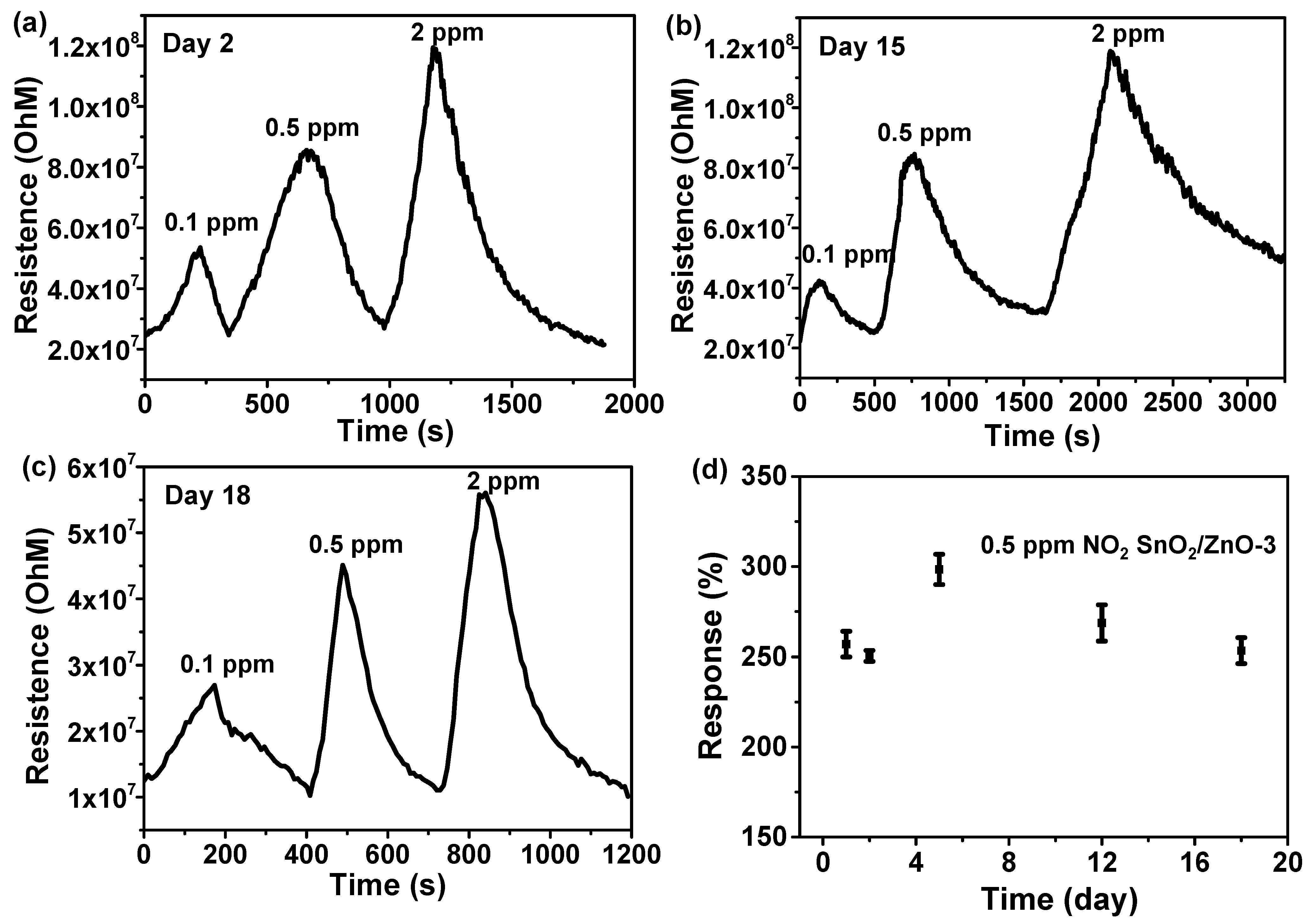
3.2. Gas Sensing Properties of SnO2, SnO2/ZnO Composite Nanofibers
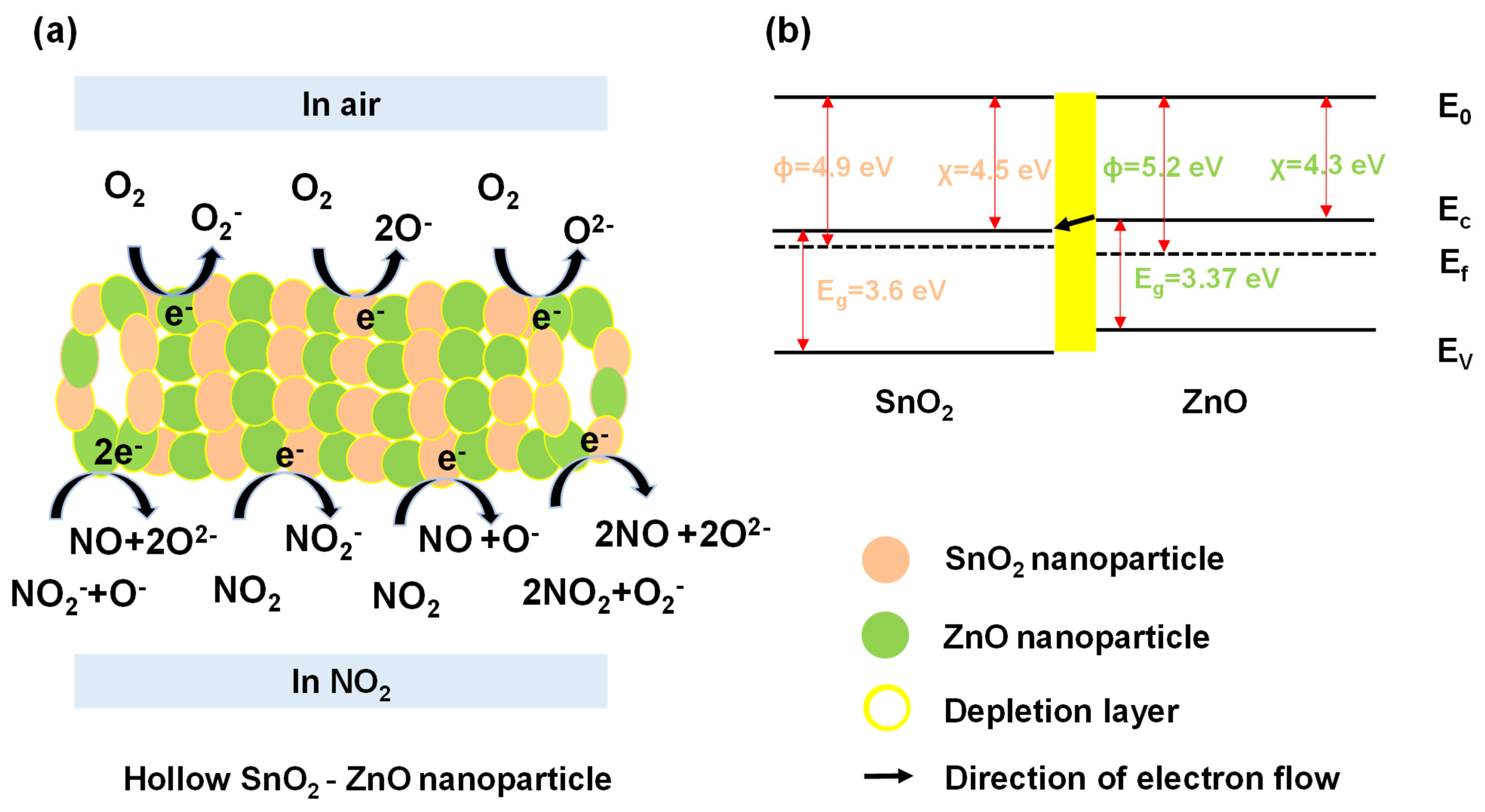
3.3. Sensing Mechanism
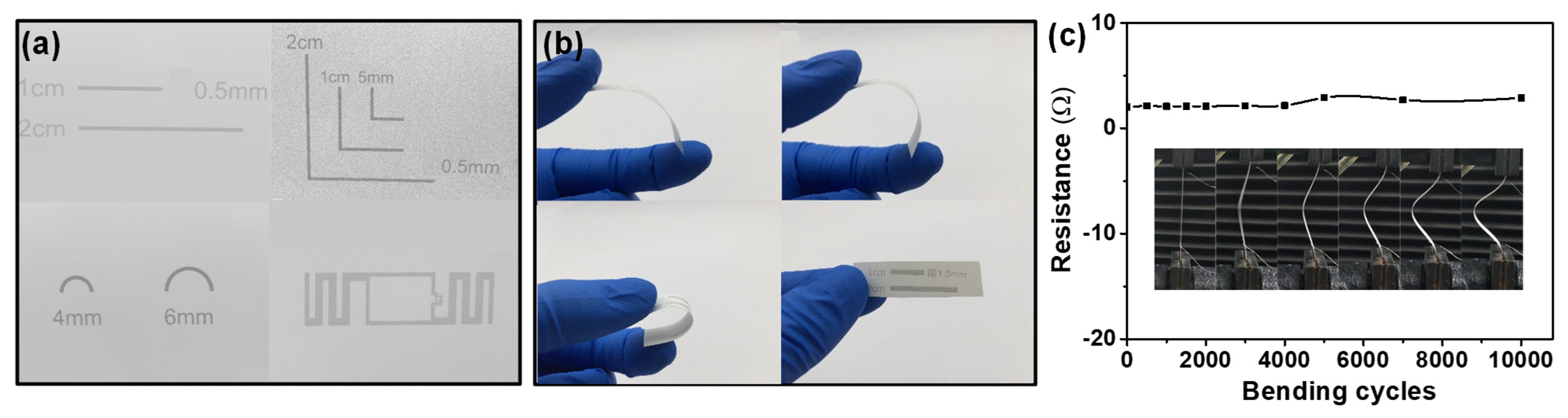
3.4. Integration and Gas Sensing Properties of Flexible Gas Sensors
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xiao, B.; Song, S.; Wang, P.; Zhao, Q.; Chuai, M.; Zhang, M. Promoting effects of Ag on In2O3 nanospheres of sub-ppb NO2 detection. Sens. Actuators B Chem. 2017, 241, 489–497. [Google Scholar] [CrossRef]
- Kabcum, S.; Kotchasak, N.; Channei, D.; Tuantranont, A.; Wisitsoraat, A.; Phanichphant, S.; Liewhiran, C. Highly sensitive and selective NO2 sensor based on Au-impregnated WO3 nanorods. Sens. Actuators B Chem. 2017, 252, 523–536. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, D.; Zhu, Q.; Wu, J.; Huang, Q.; Zhang, W.; Xie, C. Enhanced room temperature NO2 response of NiO-SnO2 nanocomposites induced by interface bonds at the p-n heterojunction. Phys. Chem. 2016, 18, 5386–5396. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, C.; Wang, Z.; Song, Z.; Zhou, X.; Han, N.; Chen, Y. Sputtered SnO2: NiO thin films on self-assembled Au nano-particle arrays for MEMS compatible NO2 gas sensors. Sens. Actuators B Chem. 2019, 278, 28–38. [Google Scholar] [CrossRef]
- Yuan, L.; Hu, M.; Wei, Y.; Ma, W. Enhanced NO2 sensing characteristics of Au modified porous silicon/thorn-sphere-like tung-sten oxide composites. Appl. Surf. Sci. 2016, 389, 824–834. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, J.; Fei, T.; Liu, S.; Zhang, T. SnO2 nanoparticles-reduced graphene oxide nanocomposites for NO2 sensing at low operating temperature. Sens. Actuators B Chem. 2014, 190, 472–478. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc oxide nanostructures for NO2 gas–sensor applications: A review. Nano-Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Liu, Z.; Li, C.; Tang, T.; Liu, X.; Han, S.; Lei, A.B.; Zhou, C. Detection of NO2 down to ppb Levels Using Individual and Multiple In2O3 Nanowire Devices. Nano Lett. 2004, 4, 1919–1924. [Google Scholar] [CrossRef]
- Ruiz, A.M.; Sakai, G.; Cornet, A.; Shimanoe, K.; Morante, J.R.; Yamazoe, N. Cr-doped TiO2 gas sensor for exhaust NO2 moni-toring. Sens. Actuators B Chem. 2003, 93, 509–518. [Google Scholar] [CrossRef]
- Bao, M.; Chen, Y.; Li, F.; Ma, J.; Lv, T.; Tang, Y.; Chen, L.; Xu, Z.; Wang, T. Plate-like p–n heterogeneous NiO/WO3 nanocomposites for high performance room temperature NO2 sensors. Nanoscale 2014, 6, 4063–4066. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Tomer, V.K.; Mishra, Y.K.; Lin, L. Functional gas sensing nanomaterials: A panoramic view. Appl. Phys. Rev. 2020, 7, 021301. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Kolmakov, A.; Lilach, Y.; Moskovits, M. Electronic control of chemistry and catalysis at the surface of an individual tin oxide nanowire. J. Phys. Chem. B 2005, 109, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.J.; Hwang, I.-S.; Park, J.-G.; Lee, J.-H. Novel fabrication of an SnO2nanowire gas sensor with high sensitivity. Nanotechnology 2008, 19, 095508. [Google Scholar] [CrossRef]
- Comini, E.; Cristalli, A.; Faglia, G.; Sberveglieri, G. Light enhanced gas sensing properties of indium oxide and tin dioxide sensors. Sens. Actuators B Chem. 2000, 65, 260–263. [Google Scholar] [CrossRef]
- Li, W.; Guo, J.; Cai, L.; Qi, W.; Sun, Y.; Xu, J.-L.; Sun, M.; Zhu, H.; Xiang, L.; Xie, D.; et al. UV light irradiation enhanced gas sensor selectivity of NO2 and SO2 using rGO functionalized with hollow SnO2 nanofibers. Sens. Actuators B Chem. 2019, 290, 443–452. [Google Scholar] [CrossRef]
- Choi, K.I.; Hübner, M.; Haensch, A.; Kim, H.J.; Weimar, U.; Barsan, N.; Lee, J.H. Ambivalent effect of Ni loading on gas sensing per-formance in SnO2 based gas sensor. Sens. Actuators B Chem. 2013, 183, 401–410. [Google Scholar] [CrossRef]
- Wan, G.X.; Ma, S.Y.; Li, X.B.; Li, F.M.; Bian, H.Q.; Zhang, L.P.; Li, W.Q. Synthesis and acetone sensing properties of Ce-doped ZnO nano-fibers. Mater. Lett. 2014, 114, 103–106. [Google Scholar] [CrossRef]
- Li, W.; Teng, C.; Sun, Y.; Cai, L.; Xu, J.L.; Sun, M.; Li, X.; Yang, X.; Xiang, L.; Xie, D.; et al. Sprayed, scalable, wearable, and portable NO2 sensor array using fully flexible AgNPs-All-Carbon nanostructures. ACS Appl. Mater. Inter. 2018, 10, 34485–34493. [Google Scholar] [CrossRef]
- Miao, Y.-E.; He, S.; Zhong, Y.; Yang, Z.; Tjiu, W.W.; Liu, T. A novel hydrogen peroxide sensor based on Ag/SnO2 composite nanotubes by electrospinning. Electrochimica Acta 2013, 99, 117–123. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, T.; Lian, G.; Yu, Q.; Luan, C.; Wang, Q.; Cui, D. Room temperature CO sensor fabricated from Pt-loaded SnO2 porous nanosolid. Sensors Actuators B Chem. 2013, 184, 33–39. [Google Scholar] [CrossRef]
- Park, S.; An, S.; Mun, Y.; Lee, C. UV-enhanced NO2 gas sensing properties of SnO2-core/ZnO-shell nanowires at room temperature. ACS Appl. Mater. Inter. 2013, 5, 4285–4292. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Gu, K.; Zhu, M.; Lu, Q.; Zhai, C.; Zhao, Q.; Yang, X.; Zhang, M. ZnO-SnO2 heterojunction nanobelts: Synthesis and ultraviolet light irradiation to improve the triethylamine sensing properties. Sens. Actuators B Chem. 2019, 279, 410–417. [Google Scholar] [CrossRef]
- Song, J.; Son, D.; Kim, J.; Yoo, Y.J.; Lee, G.J.; Wang, L.; Choi, M.K.; Yang, J.; Lee, M.; Do, K.; et al. Wearable force touch sensor array using a flexible and transparent electrode. Adv. Funct. Mater. 2017, 27, 1–9. [Google Scholar] [CrossRef]
- Uddin, A.I.; Yaqoob, U.; Phan, D.-T.; Chung, G.-S. A novel flexible acetylene gas sensor based on PI/PTFE-supported Ag-loaded vertical ZnO nanorods array. Sens. Actuators B Chem. 2016, 222, 536–543. [Google Scholar] [CrossRef]
- Secor, E.B.; Lim, S.; Zhang, H.; Frisbie, C.D.; Francis, L.F.; Hersam, M.C. Gravure printing of graphene for large-area flexible electronics. Adv. Mater. 2014, 26, 4533–4538. [Google Scholar] [CrossRef]
- Hyun, W.J.; Secor, E.B.; Rojas, G.A.; Hersam, M.C.; Francis, L.F.; Frisbie, C.D. All-printed, foldable organic thin-film transistors on glassine paper. Adv. Mater. 2015, 27, 7058–7064. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Wang, Y.; Wang, Y.; Zhu, B.; Xia, H.; Guo, X.; Zhang, S.; Huang, W.; Wu, S. NO2 sensing performance of SnO2 hol-low-sphere sensor. Sens. Actuators B Chem. 2009, 135, 610–617. [Google Scholar] [CrossRef]
- Ju, D.-X.; Xu, H.-Y.; Qiu, Z.-W.; Zhang, Z.-C.; Xu, Q.; Zhang, J.; Wang, J.-Q.; Cao, B.-Q. Near room temperature, fast-response, and highly sensitive triethylamine sensor assembled with au-loaded ZnO/SnO2 core–shell nanorods on flat alumina substrates. ACS Appl. Mater. Interfaces 2015, 7, 19163–19171. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, Q.; Wang, C.; Wei, Z.; Xu, L.; Gui, Y. Electrospun ZnO-SnO2 composite nanofibers and enhanced sensing properties to SF6 decomposition byproduct H2S. Front. Chem. 2018, 6, 540. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Xu, M.; Liu, L.; Ruan, X.; Yan, J.; Zhao, W.; Yun, J.; Wang, Y.; Qin, S.; Zhang, T. Novel SnO2@ZnO hierarchical nanostructures for highly sensitive and selective NO2 gas sensing. Sens. Actuators B Chem. 2018, 257, 714–727. [Google Scholar] [CrossRef]
- Fan, H.J.; Knez, M.; Scholz, R.; Hesse, D.; Nielsch, K.; Zacharias, M.; Gösele, U. Influence of surface diffusion on the formation of hollow nanostructures induced by the kirkendall effect: The basic concept. Nano Lett. 2007, 7, 993–997. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, Y.; Koh, H.J.; Jung, H.; Kim, J.S.; Yoo, H.W.; Kim, J.; Jung, H.T. Superior chemical sensing performance of black phosphorus: Comparison with MoS2 and graphene. Adv. Mater. 2016, 28, 7020–7028. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, D.; Zhao, S.; Wu, J.; Xu, K.; Zhu, Q.; Zhang, G.; Xie, C. Room temperature NO2 sensing: What advantage does the rGO–NiO nanocomposite have over pristine NiO? Phys. Chem. Chem. Phys. 2015, 17, 14903–14911. [Google Scholar] [CrossRef]
- Long, H.; Harley-Trochimczyk, A.; Pham, T.; Tang, Z.; Shi, T.; Zettl, A.; Carraro, C.; Worsley, M.A.; Maboudian, R. High surface area MoS2/Graphene hybrid aerogel for ultrasensitive NO2Detection. Adv. Funct. Mater. 2016, 26, 5158–5165. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Wang, J.; Xu, J.-L.; Li, X.; Xie, D.; Xiang, L.; Komarneni, S. Confined formation of ultrathin ZnO nanorods/reduced graphene oxide mesoporous nanocomposites for high-performance room-temperature NO2 sensors. ACS Appl. Mater. Interfaces 2016, 8, 35454–35463. [Google Scholar] [CrossRef]
- Shishiyanu, S.T.; Shishiyanu, T.S.; Lupan, O. Sensing characteristics of tin-doped ZnO thin films as NO2 gas sensor. Sens. Actuators B Chem. 2005, 107, 379–386. [Google Scholar] [CrossRef]
- Deng, S.; Tjoa, V.; Fan, H.M.; Tan, H.R.; Sayle, D.C.; Olivo, M.; Mhaisalkar, S.; Wei, J.; Sow, C.H. Reduced graphene oxide con-jugated Cu2O nanowire mesocrystals for high-performance NO2 gas sensor. J. Am. Chem. Soc. 2012, 134, 4905–4917. [Google Scholar] [CrossRef]
- Chen, N.; Li, X.; Wang, X.; Yu, J.; Wang, J.; Tang, Z.; Akbar, S. Enhanced room temperature sensing of Co3O4-intercalated reduced graphene oxide based gas sensors. Sens. Actuators B Chem. 2013, 188, 902–908. [Google Scholar] [CrossRef]
- Ou, J.Z.; Ge, W.; Carey, B.; Daeneke, T.; Rotbart, A.; Shan, W.; Wang, Y.; Fu, Z.; Chrimes, A.F.; Wlodarski, W.; et al. Physisorption-based charge transfer in two-dimensional SnS2 for selective and reversible NO2 gas sensing. ACS Nano. 2015, 9, 10313–10323. [Google Scholar] [CrossRef]
- Khan, H.; Zavabeti, A.; Wang, Y.; Harrison, C.J.; Carey, B.J.; Mohiuddin, M.; Chrimes, A.F.; De Castro, I.A.; Zhang, B.Y.; Sabri, Y.M.; et al. Quasi physisorptive two dimensional tungsten oxide nanosheets with ex-traordinary sensitivity and selectivity to NO2. Nanoscale 2017, 9, 19162–19175. [Google Scholar] [CrossRef]
- Li, W.; Ma, S.; Li, Y.; Yang, G.; Mao, Y.; Luo, J.; Gengzang, D.; Xu, X.; Yan, S. Enhanced ethanol sensing performance of hollow ZnO-SnO2 core–shell nanofibers. Sens. Actuators B Chem. 2015, 211, 392–402. [Google Scholar] [CrossRef]
- Huang, H.; Gong, H.; Chow, C.L.; Guo, J.; White, T.; Tse, M.S.; Tan, O.K. Low-temperature growth of SnO2 Nanorod arrays and tunable n-p-n sensing response of a ZnO/SnO2 Heterojunction for exclusive hydrogen sensors. Adv. Funct. Mater. 2011, 21, 2680–2686. [Google Scholar] [CrossRef]
- Rakshit, T.; Santra, S.; Manna, I.; Ray, S.K. Enhanced sensitivity and selectivity of brush-like SnO2 nanowire/ZnO nanorod heterostructure based sensors for volatile organic compounds. RSC Adv. 2014, 4, 36749–36756. [Google Scholar] [CrossRef]
- Luo, Y.; Dong, Z.; Chen, Y.; Zhang, Y.; Lu, Y.; Xia, T.; Wang, L.; Li, S.; Zhang, W.; Xiang, W.; et al. Self-powered NiO@ZnO-nanowire-heterojunction ultraviolet micro-photodetectors. Opt. Mater. Express 2019, 9, 2775–2784. [Google Scholar] [CrossRef]
- Dijith, K.S.; Pillai, S.; Surendran, K.P. Screen printed silver patterns on La0.5Sr0.5CoO3−δ—Epoxy composite as a strategy for many-fold increase in EMI shielding. Surf. Coat. Technol. 2017, 330, 34–41. [Google Scholar] [CrossRef]
- Hösel, M.; Søndergaard, R.R.; Angmo, D.; Krebs, F.C. Comparison of fast roll-to-roll flexographic, inkjet, flatbed, and rotary screen printing of metal back electrodes for polymer solar cells. Adv. Eng. Mater. 2013, 15, 995–1001. [Google Scholar] [CrossRef]
- Park, J.-H.; Park, M.-J.; Lee, J.-S. Dry writing of highly conductive electrodes on papers by using silver nanoparticle–graphene hybrid pencils. Nanoscale 2017, 9, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Juang, Y.-J.; Li, W.-S.; Chen, P.-S. Fabrication of microfluidic paper-based analytical devices by filtration-assisted screen printing. J. Taiwan Inst. Chem. Eng. 2017, 80, 71–75. [Google Scholar] [CrossRef]



| Samples | Sn (at.%) | Zn (at.%) | O (at.%) | C (at.%) | Specific Surface Area (m2/g) |
|---|---|---|---|---|---|
| SnO2 | 18.6 | - | 51.8 | 29.6 | 28.3 |
| SnO2/ZnO-1 | 23.9 | 1.3 | 58.4 | 16.4 | 31.2 |
| SnO2/ZnO-2 | 23.9 | 3.5 | 59.1 | 13.5 | 35.8 |
| SnO2/ZnO-3 | 22.9 | 8.2 | 55.6 | 13.3 | 38.7 |
| Materials | Method | °C | Response (%) | Concentration (ppm) | Response Time | Ref. |
|---|---|---|---|---|---|---|
| Au-WO3 | modified precipitation/impregnation | 250 | 836.6 | 5 | 64.2 s | [2] |
| Black Phosphorus | chemical exfoliation | RT | 80 | 1 | 200 s | [33] |
| rGO-NiO | hydrothermal method | RT | 100 | 15 | 300 s | [34] |
| MoS2/Graphene | annealing process | 100 | 12.5 | 0.5 | 10 min | [35] |
| rGO-ZnO | solution synthesis | RT | 119 | 1 | 2.4 min | [36] |
| Sn-doped ZnO | successive ionic layer adsorption | 150 | 10.5 | 1.5 | 20 min | [37] |
| SnO2/ZnO | electrospin | RT | 336.15 | 0.5 | 126 s | This work |
| SnO2/ZnO | thermal evaporation | RT | 239 | 1 | - | [21] |
| SnO2/ZnO | two–step hydrothermal | 150 | 0.2 | 5 ppb | 60 s | [30] |
| SnO2/rGO | hydrothermal treatment | 50 | 3.31 | 5 | 135 s | [6] |
| rGO-Cu2O | nonclassic crystallization | RT | 67.8 | 2 | 1000 s | [38] |
| rGO-Co3O4 | hydrothermal method | RT | 80 | 60 | 2 min | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Li, W.; Zhao, X.; Hu, H.; Wang, M.; Luo, Y.; Xie, D.; Zhang, Y.; Zhu, H. Highly Sensitive, Selective, Flexible and Scalable Room-Temperature NO2 Gas Sensor Based on Hollow SnO2/ZnO Nanofibers. Molecules 2021, 26, 6475. https://doi.org/10.3390/molecules26216475
Guo J, Li W, Zhao X, Hu H, Wang M, Luo Y, Xie D, Zhang Y, Zhu H. Highly Sensitive, Selective, Flexible and Scalable Room-Temperature NO2 Gas Sensor Based on Hollow SnO2/ZnO Nanofibers. Molecules. 2021; 26(21):6475. https://doi.org/10.3390/molecules26216475
Chicago/Turabian StyleGuo, Jiahui, Weiwei Li, Xuanliang Zhao, Haowen Hu, Min Wang, Yi Luo, Dan Xie, Yingjiu Zhang, and Hongwei Zhu. 2021. "Highly Sensitive, Selective, Flexible and Scalable Room-Temperature NO2 Gas Sensor Based on Hollow SnO2/ZnO Nanofibers" Molecules 26, no. 21: 6475. https://doi.org/10.3390/molecules26216475
APA StyleGuo, J., Li, W., Zhao, X., Hu, H., Wang, M., Luo, Y., Xie, D., Zhang, Y., & Zhu, H. (2021). Highly Sensitive, Selective, Flexible and Scalable Room-Temperature NO2 Gas Sensor Based on Hollow SnO2/ZnO Nanofibers. Molecules, 26(21), 6475. https://doi.org/10.3390/molecules26216475








