Review: Radionuclide Molecular Imaging Targeting HER2 in Breast Cancer with a Focus on Molecular Probes into Clinical Trials and Small Peptides
Abstract
:1. Introduction
2. Radionuclides
3. Radiolabeled HER2-Targeted Monoclonal Antibodies
4. Radiolabeled HER2-Targeted Antibody Fragments
5. Radiolabeled HER2-Targeted Nanobodies
6. Radiolabeled HER2-Targeted Affibodies
7. Radiolabeled HER2-Targeted Peptides
7.1. KCCYSL-Based Peptides
7.2. LTVSPWY-Based Peptides
7.3. The Peptide AHNP
7.4. The Peptides H6F and H10F
7.5. Other Peptides
8. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Fragomeni, S.M.; Sciallis, A.; Jeruss, J.S. Molecular subtypes and local-regional control of breast cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Denkert, C.; Liedtke, C.; Tutt, A.; von Minckwitz, G. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet 2017, 389, 2430–2442. [Google Scholar] [CrossRef] [Green Version]
- Hanna, W.M.; Slodkowska, E.; Lu, F.I.; Nafisi, H.; Nofech-Mozes, S. Comparative analysis of human epidermal growth factor receptor 2 testing in breast cancer according to 2007 and 2013 american society of clinical oncology/college of American pathologists guideline recommendations. J. Clin. Oncol. 2017, 35, 3039–3045. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Press, M.F.; Bernstein, L.; Thomas, P.A.; Meisner, L.F.; Zhou, J.Y.; Ma, Y.; Hung, G.; Robinson, R.A.; Harris, C.; El-Naggar, A.; et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: Poor prognosis in node-negative breast carcinomas. J. Clin. Oncol. 1997, 15, 2894–2904. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Steeg, P.S.; Price, J.E.; Krishnamurthy, S.; Mani, S.A.; Reuben, J.; Cristofanilli, M.; Dontu, G.; Bidaut, L.; Valero, V.; et al. Breast cancer metastasis: Challenges and opportunities. Cancer Res. 2009, 69, 4951–4953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, D.Y.; Bang, Y.J. HER2-targeted therapies—A role beyond breast cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Santinelli, A.; Pisa, E.; Stramazzotti, D.; Fabris, G. HER-2 status discrepancy between primary breast cancer and metastatic sites. Impact on target therapy. Int. J. Cancer 2008, 122, 999–1004. [Google Scholar] [CrossRef]
- Ligthart, S.T.; Bidard, F.C.; Decraene, C.; Bachelot, T.; Delaloge, S.; Brain, E.; Campone, M.; Viens, P.; Pierga, J.Y.; Terstappen, L.W. Unbiased quantitative assessment of Her-2 expression of circulating tumor cells in patients with metastatic and non-metastatic breast cancer. Ann. Oncol. 2013, 24, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Schrijver, W.A.M.E.; Suijkerbuijk, K.P.M.; van Gils, C.H.; van der Wall, E.; Moelans, C.B.; van Diest, P.J. Receptor conversion in distant breast cancer metastases: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2018, 110, 568–580. [Google Scholar] [CrossRef] [Green Version]
- Niikura, N.; Liu, J.; Hayashi, N.; Mittendorf, E.A.; Gong, Y.; Palla, S.L.; Tokuda, Y.; Gonzalez-Angulo, A.M.; Hortobagyi, G.N.; Ueno, N.T. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J. Clin. Oncol. 2012, 30, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Van Poznak, C.; Somerfield, M.R.; Bast, R.C.; Cristofanilli, M.; Goetz, M.P.; Gonzalez-Angulo, A.M.; Hicks, D.G.; Hill, E.G.; Liu, M.C.; Lucas, W.; et al. Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2015, 33, 2695–2704. [Google Scholar] [CrossRef]
- Tolmachev, V. Imaging of HER-2 overexpression in tumors for guiding therapy. Curr. Pharm. Des. 2008, 14, 2999–3019. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Liu, C.; Guo, X.; Xu, Y.; Gong, J.; Qi, C.; Zhang, X.; Yang, M.; Zhu, H.; Shen, L.; et al. Impact of 68Ga-NOTA-MAL-MZHER2 PET imaging in advanced gastric cancer patients and therapeutic response monitoring. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 161–175. [Google Scholar] [CrossRef]
- Mankoff, D.A.; Edmonds, C.E.; Farwell, M.D.; Pryma, D.A. Development of companion diagnostics. Semin. Nucl. Med. 2016, 46, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massicano, A.V.F.; Marquez-Nostra, B.V.; Lapi, S.E. Targeting HER2 in nuclear medicine for imaging and therapy. Mol. Imaging 2018, 17, 1536012117745386. [Google Scholar] [CrossRef]
- Zanzonico, P. Principles of nuclear medicine imaging: Planar, SPECT, PET, multi-modality, and autoradiography systems. Radiat. Res. 2012, 177, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Rahmim, A.; Zaidi, H. PET versus SPECT: Strengths, limitations and challenges. Nucl. Med. Commun. 2008, 29, 193–207. [Google Scholar] [CrossRef] [Green Version]
- Perik, P.J.; Lub-De Hooge, M.N.; Gietema, J.A.; van der Graaf, W.T.; de Korte, M.A.; Jonkman, S.; Kosterink, J.G.; van Veldhuisen, D.J.; Sleijfer, D.T.; Jager, P.L.; et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. 2006, 24, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhou, N.; Chen, Z.; Liu, T.; Xu, X.; Lei, X.; Shen, L.; Gao, J.; Yang, Z.; Zhu, H. Construction of 124I-trastuzumab for noninvasive PET imaging of HER2 expression: From patient-derived xenograft models to gastric cancer patients. Gastric Cancer 2020, 23, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Kurihara, H.; Yonemori, K.; Tsuda, H.; Suzuki, J.; Kono, Y.; Honda, N.; Kodaira, M.; Yamamoto, H.; Yunokawa, M.; et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J. Nucl. Med. 2013, 54, 1869–1875. [Google Scholar] [CrossRef] [Green Version]
- Dijkers, E.C.; Kosterink, J.G.; Rademaker, A.P.; Perk, L.R.; van Dongen, G.A.; Bart, J.; de Jong, J.R.; de Vries, E.G.; Lub-de Hooge, M.N. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J. Nucl. Med. 2009, 50, 974–981. [Google Scholar] [CrossRef] [Green Version]
- Behr, T.M.; Béhé, M.; Wörmann, B. Trastuzumab and breast cancer. N. Engl. J. Med. 2001, 345, 995–996. [Google Scholar]
- Ulaner, G.A.; Hyman, D.M.; Ross, D.S.; Corben, A.; Chandarlapaty, S.; Goldfarb, S.; McArthur, H.; Erinjeri, J.P.; Solomon, S.B.; Kolb, H.; et al. Detection of HER2-positive metastases in patients with HER2-negative primary breast cancer using 89Zr-Trastuzumab PET/CT. J. Nucl. Med. 2016, 57, 1523–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulaner, G.A.; Hyman, D.M.; Lyashchenko, S.K.; Lewis, J.S.; Carrasquillo, J.A. 89Zr-Trastuzumab PET/CT for detection of human epidermal growth factor receptor 2-positive metastases in patients with human epidermal growth factor receptor 2-negative primary breast cancer. Clin. Nucl. Med. 2017, 42, 912–917. [Google Scholar] [CrossRef]
- O’Donoghue, J.A.; Lewis, J.S.; Pandit-Taskar, N.; Fleming, S.E.; Schöder, H.; Larson, S.M.; Beylergil, V.; Ruan, S.; Lyashchenko, S.K.; Zanzonico, P.B.; et al. Pharmacokinetics, biodistribution, and radiation dosimetry for 89Zr-trastuzumab in patients with esophagogastric cancer. J. Nucl. Med. 2018, 59, 161–166. [Google Scholar] [CrossRef] [Green Version]
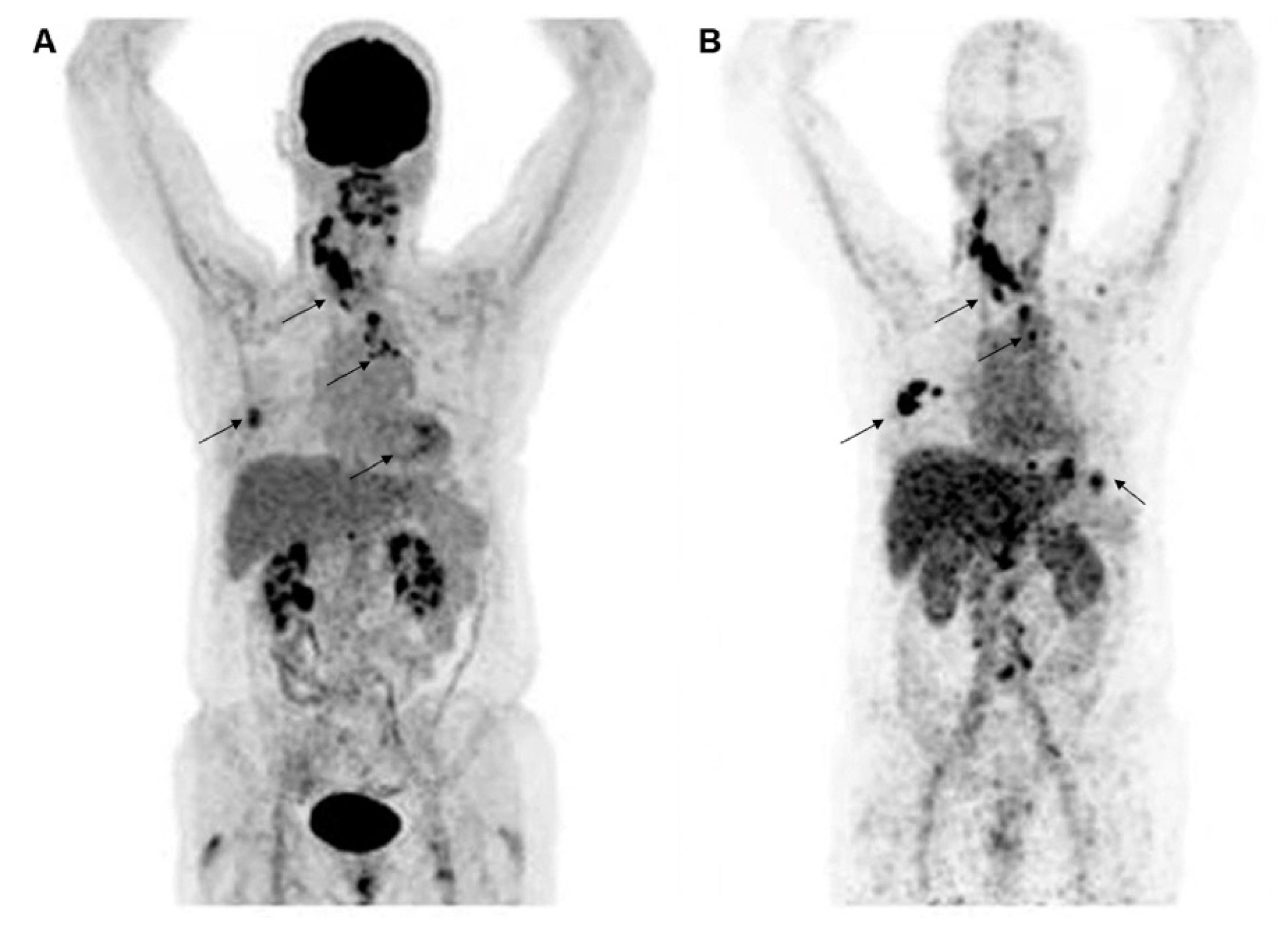
- Bensch, F.; Brouwers, A.H.; Lub-de Hooge, M.N.; de Jong, J.R.; van der Vegt, B.; Sleijfer, S.; de Vries, E.G.E.; Schröder, C.P. 89Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2300–2306. [Google Scholar] [CrossRef] [Green Version]
- Gebhart, G.; Lamberts, L.E.; Wimana, Z.; Garcia, C.; Emonts, P.; Ameye, L.; Stroobants, S.; Huizing, M.; Aftimos, P.; Tol, J.; et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): The ZEPHIR trial. Ann. Oncol. 2016, 27, 619–624. [Google Scholar] [CrossRef]
- Mortimer, J.E.; Bading, J.R.; Park, J.M.; Frankel, P.H.; Carroll, M.I.; Tran, T.T.; Poku, E.K.; Rockne, R.C.; Raubitschek, A.A.; Shively, J.E.; et al. Tumor Uptake of 64Cu-DOTA-trastuzumab in patients with metastatic breast cancer. J. Nucl. Med. 2018, 59, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Dijkers, E.C.; Oude Munnink, T.H.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; de Jong, J.R.; van Dongen, G.A.; Schröder, C.P.; Lub-de Hooge, M.N.; de Vries, E.G. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef]
- Mortimer, J.E.; Bading, J.R.; Colcher, D.M.; Conti, P.S.; Frankel, P.H.; Carroll, M.I.; Tong, S.; Poku, E.; Miles, J.K.; Shively, J.E.; et al. Functional imaging of human epidermal growth factor receptor 2-positive metastatic breast cancer using (64)Cu-DOTA-trastuzumab PET. J. Nucl. Med. 2014, 55, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Im, H.J.; Sun, H.; Valdovinos, H.F.; England, C.G.; Ehlerding, E.B.; Nickles, R.J.; Lee, D.S.; Cho, S.Y.; Huang, P.; et al. Radiolabeled pertuzumab for imaging of human epidermal growth factor receptor 2 expression in ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1296–1305. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Carrasquillo, J.A.; Riedl, C.C.; Yeh, R.; Hatzoglou, V.; Ross, D.S.; Jhaveri, K.; Chandarlapaty, S.; Hyman, D.M.; Zeglis, B.M.; et al. Identification of HER2-positive metastases in patients with HER2-negative primary breast cancer by using HER2-targeted 89Zr-pertuzumab PET/CT. Radiology 2020, 296, 370–378. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Lyashchenko, S.K.; Riedl, C.; Ruan, S.; Zanzonico, P.B.; Lake, D.; Jhaveri, K.; Zeglis, B.; Lewis, J.S.; O'Donoghue, J.A. First-in-human human epidermal growth factor receptor 2-targeted imaging using 89Zr-Pertuzumab PET/CT: Dosimetry and clinical application in patients with breast cancer. J. Nucl. Med. 2018, 59, 900–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, M.; Tolmachev, V.; Andersson, K.; Gedda, L.; Sandström, M.; Carlsson, J. [(177)Lu]pertuzumab: Experimental studies on targeting of HER-2 positive tumour cells. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 1457–1462. [Google Scholar] [CrossRef]
- McLarty, K.; Cornelissen, B.; Cai, Z.; Scollard, D.A.; Costantini, D.L.; Done, S.J.; Reilly, R.M. Micro-SPECT/CT with 111In-DTPA-pertuzumab sensitively detects trastuzumab-mediated HER2 downregulation and tumor response in athymic mice bearing MDA-MB-361 human breast cancer xenografts. J. Nucl. Med. 2009, 50, 1340–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquez, B.V.; Ikotun, O.F.; Zheleznyak, A.; Wright, B.; Hari-Raj, A.; Pierce, R.A.; Lapi, S.E. Evaluation of (89)Zr-pertuzumab in Breast cancer xenografts. Mol. Pharm. 2014, 11, 3988–3995. [Google Scholar] [CrossRef] [Green Version]
- Smith-Jones, P.M.; Solit, D.B.; Akhurst, T.; Afroze, F.; Rosen, N.; Larson, S.M. Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat. Biotechnol. 2004, 22, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Oude Munnink, T.H.; de Vries, E.G.; Vedelaar, S.R.; Timmer-Bosscha, H.; Schröder, C.P.; Brouwers, A.H.; Lub-de Hooge, M.N. Lapatinib and 17AAG reduce 89Zr-trastuzumab-F(ab’)2 uptake in SKBR3 tumor xenografts. Mol. Pharm. 2012, 9, 2995–3002. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.; Chan, C.; Reilly, R.M. Development and preclinical studies of 64Cu-NOTA-pertuzumab F(ab∲)2 for imaging changes in tumor HER2 expression associated with response to trastuzumab by PET/CT. MAbs 2017, 9, 154–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beylergil, V.; Morris, P.G.; Smith-Jones, P.M.; Modi, S.; Solit, D.; Hudis, C.A.; Lu, Y.; O’Donoghue, J.; Lyashchenko, S.K.; Carrasquillo, J.A.; et al. Pilot study of 68Ga-DOTA-F(ab’)2-trastuzumab in patients with breast cancer. Nucl. Med. Commun. 2013, 34, 1157–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debie, P.; Lafont, C.; Defrise, M.; Hansen, I.; van Willigen, D.M.; van Leeuwen, F.W.B.; Gijsbers, R.; D’Huyvetter, M.; Devoogdt, N.; Lahoutte, T.; et al. Size and affinity kinetics of nanobodies influence targeting and penetration of solid tumours. J. Control. Release 2020, 317, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.; Vaneycken, I.; D’huyvetter, M.; Heemskerk, J.; Keyaerts, M.; Vincke, C.; Devoogdt, N.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 Nanobodies for iPET imaging of HER2 receptor expression in cancer. J. Nucl. Med. 2013, 54, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P.; et al. Phase I Study of 68Ga-HER2-Nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J. Nucl. Med. 2016, 57, 27–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaneycken, I.; Devoogdt, N.; Van Gassen, N.; Vincke, C.; Xavier, C.; Wernery, U.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. FASEB J. 2011, 25, 2433–2446. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Chitneni, S.K.; Devoogdt, N.; Zalutsky, M.R.; Vaidyanathan, G. Fluorine-18 labeling of an anti-HER2 VHH using a residualizing prosthetic group via a strain-promoted click reaction: Chemistry and preliminary evaluation. Bioorg. Med. Chem. 2018, 26, 1939–1949. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; McDougald, D.; Devoogdt, N.; Zalutsky, M.R.; Vaidyanathan, G. Labeling single domain antibody fragments with fluorine-18 using 2,3,5,6-tetrafluorophenyl 6-[18F]Fluoronicotinate resulting in high tumor-to-kidney ratios. Mol. Pharm. 2019, 16, 214–226. [Google Scholar] [CrossRef] [PubMed]
- D’Huyvetter, M.; De Vos, J.; Xavier, C.; Pruszynski, M.; Sterckx, Y.G.J.; Massa, S.; Raes, G.; Caveliers, V.; Zalutsky, M.R.; Lahoutte, T.; et al. 131I-labeled Anti-HER2 Camelid sdAb as a theranostic tool in cancer treatment. Clin. Cancer Res. 2017, 23, 6616–6628. [Google Scholar] [CrossRef] [Green Version]
- D’Huyvetter, M.; Vincke, C.; Xavier, C.; Aerts, A.; Impens, N.; Baatout, S.; De Raeve, H.; Muyldermans, S.; Caveliers, V.; Devoogdt, N.; et al. Targeted radionuclide therapy with A 177Lu-labeled anti-HER2 nanobody. Theranostics 2014, 4, 708–720. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Shields, A.F.; Siegel, B.A.; Miller, K.D.; Krop, I.; Ma, C.X.; LoRusso, P.M.; Munster, P.N.; Campbell, K.; Gaddy, D.F.; et al. 64Cu-MM-302 positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin. Cancer Res. 2017, 23, 4190–4202. [Google Scholar] [CrossRef] [Green Version]
- Ståhl, S.; Gräslund, T.; Eriksson Karlström, A.; Frejd, F.Y.; Nygren, P.Å.; Löfblom, J. Affibody molecules in biotechnological and medical applications. Trends. Biotechnol. 2017, 35, 691–712. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, V.; Orlova, A.; Nilsson, F.Y.; Feldwisch, J.; Wennborg, A.; Abrahmsén, L. Affibody molecules: Potential for in vivo imaging of molecular targets for cancer therapy. Expert. Opin. Biol. Ther. 2007, 7, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, V.; Nilsson, F.Y.; Widström, C.; Andersson, K.; Rosik, D.; Gedda, L.; Wennborg, A.; Orlova, A. 111In-benzyl-DTPA-ZHER2:342, an affibody-based conjugate for in vivo imaging of HER2 expression in malignant tumors. J. Nucl. Med. 2006, 47, 846–853. [Google Scholar]
- Kramer-Marek, G.; Bernardo, M.; Kiesewetter, D.O.; Bagci, U.; Kuban, M.; Aras, O.; Zielinski, R.; Seidel, J.; Choyke, P.; Capala, J. PET of HER2-positive pulmonary metastases with 18F-ZHER2:342 affibody in a murine model of breast cancer: Comparison with 18F-FDG. J. Nucl. Med. 2012, 53, 939–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honarvar, H.; Westerlund, K.; Altai, M.; Sandström, M.; Orlova, A.; Tolmachev, V.; Karlström, A.E. Feasibility of affibody molecule-based PNA-mediated radionuclide pretargeting of malignant tumors. Theranostics 2016, 6, 93–103. [Google Scholar] [CrossRef]
- Baum, R.P.; Prasad, V.; Müller, D.; Schuchardt, C.; Orlova, A.; Wennborg, A.; Tolmachev, V.; Feldwisch, J. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J. Nucl. Med. 2010, 51, 892–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sörensen, J.; Sandberg, D.; Sandström, M.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Åström, G.; Lubberink, M.; Garske-Román, U.; Carlsson, J.; et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J. Nucl. Med. 2014, 55, 730–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandström, M.; Lindskog, K.; Velikyan, I.; Wennborg, A.; Feldwisch, J.; Sandberg, D.; Tolmachev, V.; Orlova, A.; Sörensen, J.; Carlsson, J.; et al. Biodistribution and radiation dosimetry of the Anti-HER2 affibody molecule 68Ga-ABY-025 in breast cancer patients. J. Nucl. Med. 2016, 57, 867–871. [Google Scholar] [CrossRef] [Green Version]
- Sörensen, J.; Velikyan, I.; Sandberg, D.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Orlova, A.; Sandström, M.; Lubberink, M.; Olofsson, H.; et al. Measuring HER2-receptor expression in metastatic breast cancer using [68Ga]ABY-025 Affibody PET/CT. Theranostics 2016, 6, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Glaser, M.; Iveson, P.; Hoppmann, S.; Indrevoll, B.; Wilson, A.; Arukwe, J.; Danikas, A.; Bhalla, R.; Hiscock, D. Three methods for 18F labeling of the HER2-binding affibody molecule Z(HER2:2891) including preclinical assessment. J. Nucl. Med. 2013, 54, 1981–1988. [Google Scholar] [CrossRef] [Green Version]
- Trousil, S.; Hoppmann, S.; Nguyen, Q.D.; Kaliszczak, M.; Tomasi, G.; Iveson, P.; Hiscock, D.; Aboagye, E.O. Positron emission tomography imaging with 18F-labeled ZHER2:2891 affibody for detection of HER2 expression and pharmacodynamic response to HER2-modulating therapies. Clin. Cancer Res. 2014, 20, 1632–1643. [Google Scholar] [CrossRef] [Green Version]
- Garousi, J.; Lindbo, S.; Nilvebrant, J.; Åstrand, M.; Buijs, J.; Sandström, M.; Honarvar, H.; Orlova, A.; Tolmachev, V.; Hober, S. ADAPT, a novel scaffold protein-based probe for radionuclide imaging of molecular targets that are expressed in disseminated cancers. Cancer Res. 2015, 75, 4364–4371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Witting, E.; Garousi, J.; Lindbo, S.; Vorobyeva, A.; Altai, M.; Oroujeni, M.; Mitran, B.; Orlova, A.; Hober, S.; Tolmachev, V. Selection of the optimal macrocyclic chelators for labeling with 111In and 68Ga improves contrast of HER2 imaging using engineered scaffold protein ADAPT6. Eur. J. Pharm. Biopharm. 2019, 140, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Bragina, O.; von Witting, E.; Garousi, J.; Zelchan, R.; Sandström, M.; Orlova, A.; Medvedeva, A.; Doroshenko, A.; Vorobyeva, A.; Lindbo, S.; et al. Phase I Study of 99mTc-ADAPT6, a scaffold protein-based probe for visualization of HER2 expression in breast cancer. J. Nucl. Med. 2021, 62, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Maecke, H.R.; Okarvi, S.M. Radiolabeled peptides: Valuable tools for the detection and treatment of cancer. Theranostics 2012, 2, 481–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karasseva, N.G.; Glinsky, V.V.; Chen, N.X.; Komatireddy, R.; Quinn, T.P. Identification and characterization of peptides that bind human ErbB-2 selected from a bacteriophage display library. J. Protein Chem. 2002, 21, 287–296. [Google Scholar] [CrossRef]
- Kumar, S.R.; Gallazzi, F.A.; Ferdani, R.; Anderson, C.J.; Quinn, T.P.; Deutscher, S.L. In vitro and in vivo evaluation of 64Cu-radiolabeled KCCYSL peptides for targeting epidermal growth factor receptor-2 in breast carcinomas. Cancer Biother. Radiopharm. 2010, 25, 693–703. [Google Scholar] [CrossRef] [Green Version]
- Larimer, B.M.; Thomas, W.D.; Smith, G.P.; Deutscher, S.L. Affinity maturation of an ERBB2-targeted SPECT imaging peptide by in vivo phage display. Mol. Imaging Biol. 2014, 16, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Shadidi, M.; Sioud, M. Identification of novel carrier peptides for the specific delivery of therapeutics into cancer cells. FASEB J. 2003, 17, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Shahsavari, S.; Shaghaghi, Z.; Abedi, S.M.; Hosseinimehr, S.J. Evaluation of 99mTc-HYNIC-(ser)3-LTVPWY peptide for glioblastoma imaging. Int. J. Radiat. Biol. 2020, 96, 502–509. [Google Scholar] [CrossRef]
- Aligholikhamseh, N.; Ahmadpour, S.; Khodadust, F.; Abedi, S.M.; Hosseinimehr, S.J. 99mTc-HYNIC-(Ser)3-LTVPWY peptide bearing tricine as co-ligand for targeting and imaging of HER2 overexpression tumor. Radiochim. Acta 2018, 106, 601–609. [Google Scholar] [CrossRef]
- Park, B.W.; Zhang, H.T.; Wu, C.; Berezov, A.; Zhang, X.; Dua, R.; Wang, Q.; Kao, G.; O'Rourke, D.M.; Greene, M.I.; et al. Rationally designed anti-HER2/neu peptide mimetic disables P185HER2/neu tyrosine kinases in vitro and in vivo. Nat. Biotechnol. 2000, 18, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.R.; Quinn, T.P.; Deutscher, S.L. Evaluation of an 111In-radiolabeled peptide as a targeting and imaging agent for ErbB-2 receptor expressing breast carcinomas. Clin. Cancer Res. 2007, 13, 6070–6079. [Google Scholar] [CrossRef] [Green Version]
- Sabahnoo, H.; Noaparast, Z.; Abedi, S.M.; Hosseinimehr, S.J. New small 99mTc-labeled peptides for HER2 receptor imaging. Eur. J. Med. Chem. 2017, 127, 1012–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodadust, F.; Ahmadpour, S.; Aligholikhamseh, N.; Abedi, S.M.; Hosseinimehr, S.J. An improved 99mTc-HYNIC-(Ser)3-LTVSPWY peptide with EDDA/tricine as co-ligands for targeting and imaging of HER2 overexpression tumor. Eur. J. Med. Chem. 2018, 144, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Biabani Ardakani, J.; Akhlaghi, M.; Nikkholgh, B.; Hosseinimehr, S.J. Targeting and imaging of HER2 overexpression tumor with a new peptide-based 68Ga-PET radiotracer. Bioorg. Chem. 2021, 106, 104474. [Google Scholar] [CrossRef]
- Guan, S.S.; Wu, C.T.; Chiu, C.Y.; Luo, T.Y.; Wu, J.Y.; Liao, T.Z.; Liu, S.H. Polyethylene glycol-conjugated HER2-targeted peptides as a nuclear imaging probe for HER2-overexpressed gastric cancer detection in vivo. J. Transl. Med. 2018, 16, 168. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, Y.; Wang, Z.; Jia, B.; Hu, Z.; Dong, C.; Wang, F. SPECT/CT Imaging of the Novel HER2-targeted peptide probe 99mTc-HYNIC-H6F in breast cancer mouse models. J. Nucl. Med. 2017, 58, 821–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Li, L.; Wang, Z.; Shi, J.; Hu, Z.; Gao, S.; Miao, W.; Ma, Q.; Dong, C.; Wang, F. Imaging and monitoring HER2 expression in breast cancer during trastuzumab therapy with a peptide probe 99mTc-HYNIC-H10F. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2613–2623. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, W.; Bu, X.; Wei, Z.; Geng, L.; Wu, Y.; Dong, C.; Li, L.; Zhang, D.; Yang, S.; et al. Microarray based screening of peptide nano probes for HER2 positive tumor. Anal. Chem. 2015, 87, 8367–8372. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Luo, C.; Yang, G.; Gao, H.; Wang, Y.; Li, X.; Zhao, H.; Luo, Q.; Ma, X.; Shi, J.; et al. Developing PEGylated reversed D-Peptide as a Novel HER2-targeted SPECT imaging probe for breast cancer detection. Bioconjug. Chem. 2020, 31, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.; Verdoliva, V.; Saviano, M. Peptide ligands specifically targeting HER2 Receptor and the role played by a synthetic model system of the receptor extracellular domain: Hypothesized future perspectives. J. Med. Chem. 2020, 63, 15333–15343. [Google Scholar] [CrossRef] [PubMed]
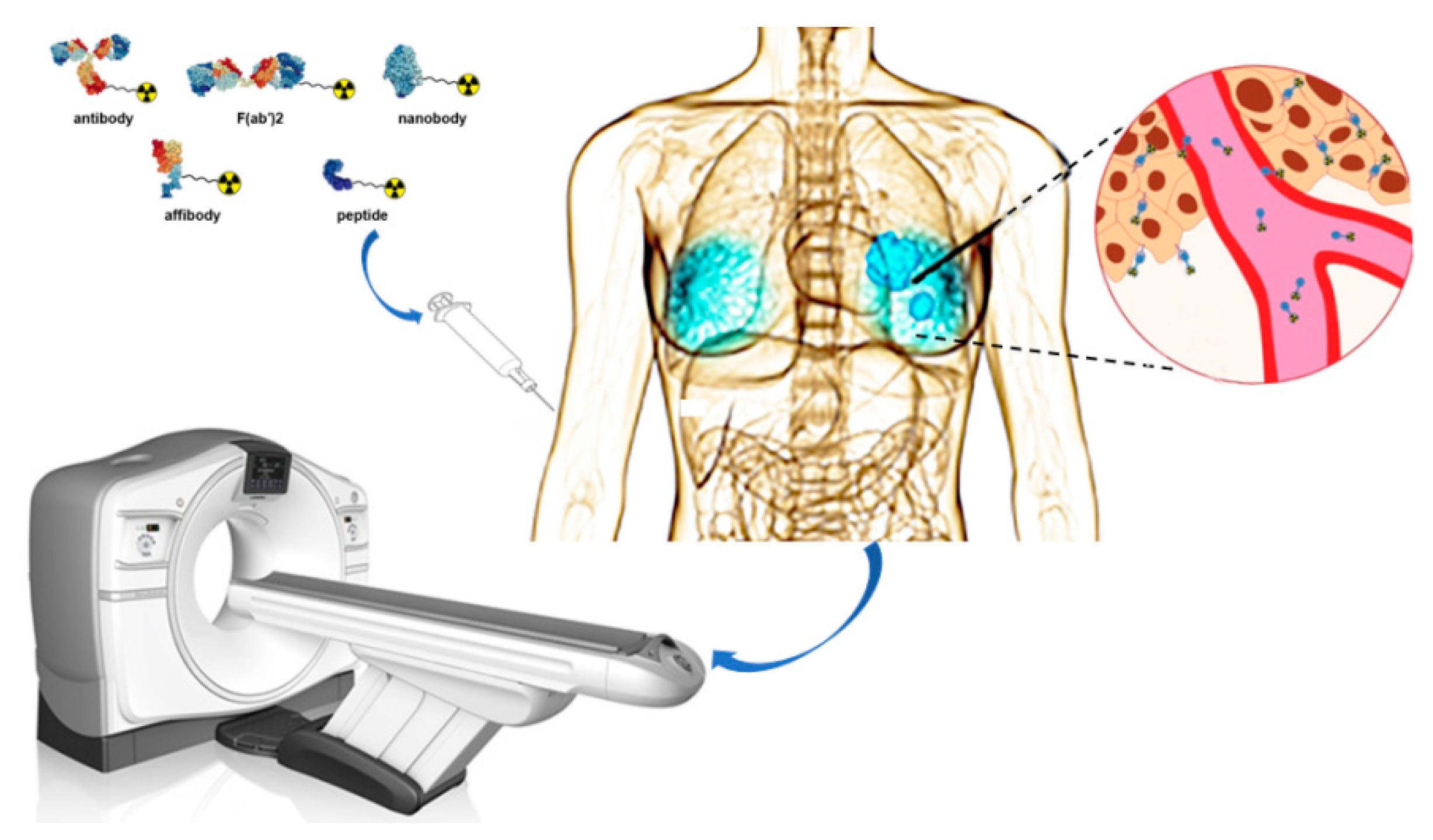
| Nuclide | T1/2 | Production Method |
|---|---|---|
| Nuclides for SPECT imaging | ||
| 99mTc | 6.01 h | 99Mo/99mTc Generator |
| 123I | 13.3 h | Cyclotron |
| 111In | 2.8 days | Cyclotron |
| Nuclides for PET imaging | ||
| 13N | 9.97 min | Cyclotron |
| 11C | 20.4 min | Cyclotron |
| 68Ga | 67.6 min | 68Ge/68Ga Generator |
| 18F | 109.8 min | Cyclotron |
| 64Cu | 12.7 h | Cyclotron |
| 89Zr | 78.4 h | Cyclotron |
| 124I | 100 h | Cyclotron |
| Monoclonal Antibodies | Nuclide/Chelator or Linker | Modality | Condition or Disease | Phase |
|---|---|---|---|---|
| Trastuzumab | 89Zr-Df | PET | HER2-Positive Solid Tumor | Phase II NCT04757090 |
| Trastuzumab | 89Zr | PET | Metastatic Breast Cancer | Phase II NCT01832051 |
| Trastuzumab | 89Zr-Df | PET/MRI | Breast Cancer | Early Phase I NCT03321045 |
| Trastuzumab | 89Zr-DFO | PET | Esophagogastric Cancer | NCT02023996 |
| Trastuzumab | 64Cu-DOTA | PET | HER2 Positive Breast Carcinoma | Phase II NCT02827877 |
| Trastuzumab | 64Cu | PET | HER2+ Metastatic Breast Cancer | Phase I NCT00605397 |
| Trastuzumab | 111In-DTPA | SPECT | Breast Cancer Prostate Cancer Lung Cancer Colon Cancer | Early Phase I NCT01445054 |
| Pertuzumab | 89Zr-DFO | PET | HER2-Positive cancer | Phase I NCT03109977 |
| Pertuzumab | 89Zr-SS 89Zr | PET | HER-2 Positive Malignant Carcinoma of Breast HER2-Positive Metastatic Breast Cancer | Phase I NCT04692831 |
| Pertuzumab | 111In | SPECT | Breast Cancer | Phase I NCT01805908 |
| Nanobodies | Nuclide/Chelator or Linker | Modality | Condition or Disease | Phase |
|---|---|---|---|---|
| 2Rs15d | 68Ga-NOTA | PET | Breast Carcinoma Brain Metastasis of Breast Carcinoma | Phase II NCT03331601, NCT03924466 |
| 2Rs15d | 131I | SPECT | Breast Cancer | Phase I NCT02683083 |
| NM-02 | 99mTc | SPECT | Breast Cancer | Early Phase I NCT04040686 |
| MM-302 | 64Cu | PET | Advanced HER2+ Cancers with Brain Mets | Early Phase I NCT02735798 |
| Affibodies | Nuclide/Chelator or Linker | Modality | Condition or Disease | Phase |
|---|---|---|---|---|
| ABY-025 | 68Ga | PET | HER2-Positive Breast Cancer, Breast Cancer | Phase I/II NCT02095210 NCT01858116 |
| ABY-025 | 111In | SPECT | Breast Cancer | Phase I/II NCT01216033 |
| ABH2 | 99mTc | SPECT | Breast Cancer | Early Phase I NCT03546478 |
| HPark2 | 99mTc | SPECT | Breast Cancer | Early Phase I NCT04267900 |
| GE-226 | 18F | PET | Breast Cancer | NCT03827317 |
| ADAPT6 | 99mTc | SPECT | Breast Cancer | NCT03991260 |
| Peptide | Labeling Strategy | Modality | Kd (nM) | Reference |
|---|---|---|---|---|
| KCCYSL | 111In-DOTA-GSG | SPECT | 295 ± 56 | [64] |
| MEGPSKCCYSLALASH | 111In-DOTA | SPECT | 236 ± 83 | [67] |
| GTKSKCCYSLRRSS | 111In-DOTA | SPECT | 289 ± 13 | [67] |
| CGGGLTVSPWY | 99mTc | SPECT | 4.3 ± 0.8 | [69] |
| CSSSLTVSPWY | 99mTc | SPECT | 33.9 ± 9.7 | [69] |
| SSSLTVPWY | 99mTc-HYNIC | SPECT | 2.6 ± 0.5 | [70] |
| SSSLTVPWY | 99mTc-HYNIC-EDDA/tricine | SPECT | 3.3 ± 1.0 | [71] |
| SSSLTVPWY | 68Ga-DOTA | PET | 2.5 ± 0.6 | [72] |
| FCGDFYACYMDV | 111In-DTPA-peptide-PEG | SPECT | 300 | [73] |
| H6F | 99mTc-HYNIC | SPECT | 7.48 ± 3.26 | [74] |
| H10F | 99mTc-HYNIC | SPECT | NA | [75] |
| A9 | 111In-DTPA | SPECT | 4.9/103 | [76] |
| Molecular Probes | Advantage | Shortcoming |
|---|---|---|
| Monoclonal antibodies | Identify HER2-positive lesions | Low blood clearance, low sensitivity, terrible tumor specificity, and non-specific uptake, higher radiation dose |
| Antibody fragments | Imaging capabilities within 24 h | Low tumor uptake, low lesion detection rate |
| Nanobodies | Low molecular weight, high stability, nanomole level affinity, better tumor penetration | Higher kidney background, unsuitable for evaluation of her2-positive primary breast lesions |
| Affibodies | Different binding sites from monoclonal antibodies, picomole level affinity | Higher liver and kidney background |
| Peptides | Potentially quicker circulation time, deeper tissue penetration, non-immunogenicity, ease of preparation | Low tumor uptake and tumor-to-organ ratios |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, S.; Li, J.; Yu, Y.; Chen, Z.; Yang, Y.; Zhu, L.; Sang, S.; Deng, S. Review: Radionuclide Molecular Imaging Targeting HER2 in Breast Cancer with a Focus on Molecular Probes into Clinical Trials and Small Peptides. Molecules 2021, 26, 6482. https://doi.org/10.3390/molecules26216482
Ge S, Li J, Yu Y, Chen Z, Yang Y, Zhu L, Sang S, Deng S. Review: Radionuclide Molecular Imaging Targeting HER2 in Breast Cancer with a Focus on Molecular Probes into Clinical Trials and Small Peptides. Molecules. 2021; 26(21):6482. https://doi.org/10.3390/molecules26216482
Chicago/Turabian StyleGe, Shushan, Jihui Li, Yu Yu, Zhengguo Chen, Yi Yang, Liqing Zhu, Shibiao Sang, and Shengming Deng. 2021. "Review: Radionuclide Molecular Imaging Targeting HER2 in Breast Cancer with a Focus on Molecular Probes into Clinical Trials and Small Peptides" Molecules 26, no. 21: 6482. https://doi.org/10.3390/molecules26216482
APA StyleGe, S., Li, J., Yu, Y., Chen, Z., Yang, Y., Zhu, L., Sang, S., & Deng, S. (2021). Review: Radionuclide Molecular Imaging Targeting HER2 in Breast Cancer with a Focus on Molecular Probes into Clinical Trials and Small Peptides. Molecules, 26(21), 6482. https://doi.org/10.3390/molecules26216482







