Polyphenols Targeting MAPK Mediated Oxidative Stress and Inflammation in Rheumatoid Arthritis
Abstract
:1. Introduction
- Regulation of cyclooxygenase-2 activity;
- Inhibition of eicosanoid-generating enzymes (phospholipase A2 and cyclooxygenase);
- Inhibition of NO release;
- Regulation of cytokines;
- Inhibition of NF-κB;
- Regulation of MAPK pathway [24].
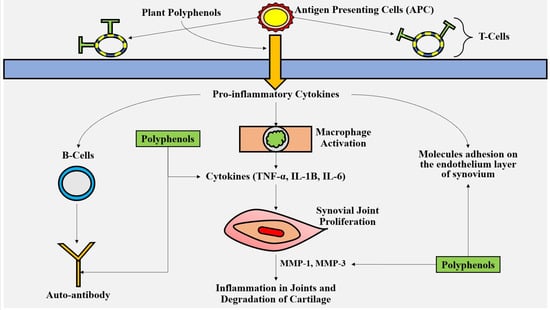
2. Pathogenesis of Rheumatoid Arthritis
3. Polyphenols and Rheumatoid Arthritis
3.1. Phenolic Acids
3.2. Stilbenes
3.3. Flavonoids
3.4. Other Compounds
4. Plant Polyphenols Targeting Oxidative Stress and Inflammation
4.1. Polyphenol’s Antioxidant Characteristics
4.2. Polyphenols and Their Interactions with Free Radicals
4.3. Enzyme Inhibition Included in Oxidation
5. Anti-Inflammatory Polyphenols
5.1. Polyphenols Have Modulatory Effects on the Cells Involved in Inflammations
5.2. Mechanism of Anti-Inflammatory Effects of Polyphenols
- Cucurmin suppresses NF-κB, reduces IL-1β and stimulates IL-6 and vascular endothelial growth factor (VEGF) by rheumatoid arthritis fibroblast-like synoviocytes (RA-FLS);
- Cucurmin stimulates IL-6 and VEGF by RA-FLS and induces the apoptosis of RA-FLS;
- RSV inhibits Th-17, B-cells and the MAPK signaling pathway and reduces IL-6 and IL-1;
- EGCG suppresses NF-κB and MAPK and inhibits osteoclast differentiation;
- Extra virgin olive oil polyphenol extract (oleocanthal, oleacein, ligstroside aglycone monoaldehyde) reduces TNF-α, IL-1β, IL-6 pro-inflammatory cytokines, COX-1 and NF-κB translocation [53];
- Quercetin alters phosphatidylinositol 3-kinase/protein kinase B signaling pathway and reduces IL-1 and IL-6 [103].
6. The Roles of Polyphenols in MAPK Pathway in Rheumatoid Arthritis
Epigallocatechin-3-Gallate, Magnolol, and Other Polyphenols’ Anti-Inflammatory Properties against RA, via MAPK Pathway
7. p53 Gene Mutation via Oxidizing Agents in RA
8. Future Directions and Conclusions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LOX | 12-15 lipoxygenases |
| AMPK | Activated protein kinase |
| AP1 | Activator protein 1 |
| AP-1 | Activator protein-1 |
| ACLT | Anterior cruciate ligament transection |
| ACPA | Anti-citrullinated protein antibodies |
| ACLT | Artesunate attenuates |
| BCL-2/Bax | B-cell lymphoma protein 2-associated X |
| BMMs | Bone marrow macrophages |
| JNK | c-Jun N-terminal kinases |
| CRP | C-reactive proteins |
| CAT | Catalase |
| CIA | Collagen induced arthritis |
| COX | Cyclooxygenase |
| DLN | Draining lymph node |
| EA | Ellagic acid |
| ESP-B4 | Eosinophil stimulation promoter -leukotriene B4 |
| EGCG | Epigallocatechin-3-gallate |
| ESR | Erythrocytes sedimentations rate |
| EVOO | Extra virgin olive oil |
| ERK | Extracellular signal-directed kinase |
| FA | Ferulic acid |
| FLSs | Fibroblast-like synoviocytes |
| GSH | Glutathione |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| GRO | Growth-regulated oncogene |
| HLA | Human leukocyte antigen |
| H2O2 | Hydrogen peroxide |
| HO2 | Hydroxyl radical |
| HT/PCy | Hydroxytyrosol and procyanidins |
| IgG1, IgG2a | Immunoglobulins G |
| IFN | Interferon |
| IL | Interleukin |
| LPS | lipopolysaccharide |
| MnSOD | Manganese-subordinates superoxide dismutase |
| MAPKKs | MAP kinase kinases |
| MMP | Matrix metalloproteinases |
| MAPK | Mitogen-activating protein kinase |
| MIA | Monosodium iodoacetate |
| MPO | Myeloperoxidase |
| NADPH | Nicotinamides adenine dinucleotide phosphates |
| NO | Nitric oxide |
| NO2 | Nitrogen dioxide |
| NF-κB | Nuclear factor kappa light chain enhancer of activated B cells |
| NFATC1 | Nuclear factor of activated T cells cytoplasmic-1 |
| OA | Osteoarthritis |
| OPG | Osteoprotegerin |
| p38 | a mitogen-activating protein kinase |
| PRRs | Pattern recognition receptor |
| OONO | Peroxynitrite |
| PIA | Pristane-induced arthritis |
| PGE2 | Prostaglandins E2 |
| PA | Punicalagin |
| AA | Reactive amyloids |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive oxygen species |
| RANKL | Receptor activator of nuclear factors kappa-B-ligand |
| RANTES | Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted |
| RSV | Resveratrol |
| RA | Rheumatoid arthritis |
| RA-FLS | Rheumatoid arthritis fibroblast-like synoviocytes |
| RF | Rheumatoid factor |
| RASF | Rheumatoid pain aggravation synovial fibroblasts |
| SSCP | Single-stranded conformation polymorphism |
| SOD | Superoxide dismutase |
| SJC-28 | Swollen 28-joint count |
| SF | Synovial fibroblast |
| TJC-28 | Tender 28-joint Count |
| TR | Thioredoxin reductase |
| TLR | Toll-like receptor |
| TGF-β | transforming growth factor beta |
| TwHF | Tripterygium wilfordii hook factor |
| TNF-α | Tumor necrosis factor |
| CII | Type II collagen |
| UV | Ultraviolet |
| UCOC | Uncarboxylated osteocalcin |
| VEGF | Vascular endothelial growth factor |
| XDH | Xanthine dehydrogenase |
| XO | Xanthine oxidase |
References
- Choy, E.H.; Panayi, G.S. Cytokine Pathways and Joint Inflammation in Rheumatoid Arthritis. N. Engl. J. Med. 2001, 344, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Lajas, C.; Abasolo, L.; Bellajdel, B.; Hernández-García, C.; Carmona, L.; Vargas, E.; Lázaro, P.; Jover, J.A. Costs and predictors of costs in rheumatoid arthritis: A prevalence-based study. Arthritis Rheum. 2003, 49, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Alam, F.; Solayman, M.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Dietary Phytochemicals: Natural Swords Combating Inflammation and Oxidation-Mediated Degenerative Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 5137431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, T.; Kameda, H. Overview of biotherapy in rheumatoid arthritis (RA). Arthritis Res. Ther. 2012, 14, O41. [Google Scholar] [CrossRef] [Green Version]
- Radu, A.-F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef]
- Deane, K.D.; Demoruelle, M.K.; Kelmenson, L.B.; Kuhn, K.A.; Norris, J.M.; Holers, V.M. Genetic and environmental risk factors for rheumatoid arthritis. Best Pr. Res. Clin. Rheumatol. 2017, 31, 3–18. [Google Scholar] [CrossRef]
- Ingegnoli, F.; Castelli, R.; Gualtierotti, R. Rheumatoid Factors: Clinical Applications. Dis. Markers 2013, 35, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-J.; Anzaghe, M.; Schülke, S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020, 9, 880. [Google Scholar] [CrossRef] [Green Version]
- Filippin, L.I.; Vercelino, R.; Marroni, N.P.; Xavier, R.M. Redox signalling and the inflammatory response in rheumatoid arthritis. Clin. Exp. Immunol. 2008, 152, 415–422. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Ind. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Aruoma, O.I.; Kaur, H.; Halliwell, B. Oxygen Free Radicals and Human Diseases. J. R. Soc. Health 1991, 111, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I. Mechanism of Action of Biological Antioxidants. Proc. Soc. Exp. Biol. Med. 1992, 200, 248–254. [Google Scholar] [CrossRef]
- Hitchon, C.A.; El-Gabalawy, H.S. Oxidation in rheumatoid arthritis. Arthritis Res. Ther. 2004, 6, 265–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.Z.; Gheita, T.A.; Kenawy, S.A.; Fahim, A.T.; El-Sorougy, I.M.; Abdou, M.S. Oxidative stress in systemic lupus erythematosus and rheumatoid arthritis patients: Relationship to disease manifestations and activity. Int. J. Rheum. Dis. 2011, 14, 325–331. [Google Scholar] [CrossRef]
- Conway, R.; Carey, J.J. Methotrexate and lung disease in rheumatoid arthritis. Panminerva Med. 2017, 59, 33–46. [Google Scholar] [CrossRef]
- Kalpakcioglu, B.; Senel, K. The interrelation of glutathione reductase, catalase, glutathione peroxidase, superoxide dismutase, and glucose-6-phosphate in the pathogenesis of rheumatoid arthritis. Clin. Rheumatol. 2007, 27, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Q.; Hadi, R.A.; Al-Rawi, Z.S.; Padron, V.A.; Stohs, S.J. The glutathione defense system in the pathogenesis of rheumatoid arthritis. J. Appl. Toxicol. 2001, 21, 69–73. [Google Scholar] [CrossRef]
- Gözel, N.; Çakirer, M.; Karataş, A.; Tuzcu, M.; Özdemir, F.A.; Dağli, A.F.; Şahin, K.; Koca, S.S. Sorafenib Reveals Anti-Arthritic Potentials in Collagen Induced Experimental Arthritis Model. Arch. Rheumatol. 2018, 33, 309–315. [Google Scholar] [CrossRef]
- Pacheco-Tena, C.; González-Chávez, S.A. The Danger Model Approach to the Pathogenesis of the Rheumatic Diseases. J. Immunol. Res. 2015, 2015, 506089. [Google Scholar] [CrossRef] [Green Version]
- Kundu, S.; Ghosh, P.; Datta, S.; Ghosh, A.; Chattopadhyay, S.; Chatterjee, M. Oxidative stress as a potential biomarker for determining disease activity in patients with Rheumatoid Arthritis. Free. Radic. Res. 2012, 46, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Pallag, A.; Bungau, S.; Tit, D.M.; Jurca, T.; Sirbu, V.; Honiges, A.; Horhogea, C. Comparative Study of Polyphenols, Flavonoids and Chlorophylls in Equisetum arvense L. Populations. Rev. Chim. 2016, 67, 530–533. [Google Scholar]
- Glevitzky, I.; Dumitrel, G.A.; Glevitzky, M.; Pasca, B.; Otřísal, P.; Bungau, S.; Cioca, G.; Pantis, C.; Popa, M. Statistical Analysis of the Relationship Between Antioxidant Activity and the Structure of Flavonoid Compounds. Rev. Chim. 2019, 70, 3103–3107. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Pecorari, M.; Villaño, D.; Testa, M.F.; Schmid, M.; Serafini, M. Biomarkers of antioxidant status following ingestion of green teas at different polyphenol concentrations and antioxidant capacity in human volunteers. Mol. Nutr. Food Res. 2010, 54, S278–S283. [Google Scholar] [CrossRef] [PubMed]
- Taysi, S.; Polat, F.; Gul, M.; Sari, R.A.; Bakan, E. Lipid peroxidation, some extracellular antioxidants, and antioxidant enzymes in serum of patients with rheumatoid arthritis. Rheumatol. Int. 2002, 21, 200–204. [Google Scholar] [CrossRef]
- Ueki, Y.; Eguchi, K.; Miyake, S.; Nagataki, S.; Tominaga, Y. Increment of CD8S6F1 cells in synovial fluid from patients with rheumatoid arthritis. Ann. Rheum. Dis. 1994, 53, 816–822. [Google Scholar] [CrossRef] [Green Version]
- Onur, Ö.; Akinci, A.S.; Akbıyık, F.; Ünsal, I. Elevated levels of nitrate in rheumatoid arthritis. Rheumatol. Int. 2001, 20, 154–158. [Google Scholar] [CrossRef]
- Pham, T.N.Q.; Brown, B.L.; Dobson, P.R.M.; Richardson, V.J. Protein kinase C-eta (PKC-η) is required for the development of inducible nitric oxide synthase (iNOS) positive phenotype in human monocytic cells. Nitric Oxide 2003, 9, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Jikimoto, T.; Nishikubo, Y.; Koshiba, M.; Kanagawa, S.; Morinobu, S.; Morinobu, A.; Saura, R.; Mizuno, K.; Kondo, S.; Toyokuni, S.; et al. Thioredoxin as a biomarker for oxidative stress in patients with rheumatoid arthritis. Mol. Immunol. 2002, 38, 765–772. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The Pathogenesis of Rheumatoid Arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [Green Version]
- Pašková, Ľ.; Kuncírová, V.; Poništ, S.; Mihálová, D.; Nosáľ, R.; Harmatha, J.; Hrádková, I.; Čavojský, T.; Bilka, F.; Šišková, K.; et al. Effect of N-Feruloylserotonin and Methotrexate on Severity of Experimental Arthritis and on Messenger RNA Expression of Key Proinflammatory Markers in Liver. J. Immunol. Res. 2016, 2016, 7509653. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.C.; Lee, C.; Kim, J.-Y.; Oh, H.M.; So, H.-S.; Lee, M.S.; Rho, M.C.; Oh, J. Chlorogenic Acid Inhibits Osteoclast Differentiation and Bone Resorption by Down-Regulation of Receptor Activator of Nuclear Factor Kappa-B Ligand-Induced Nuclear Factor of Activated T Cells c1 Expression. Biol. Pharm. Bull. 2013, 36, 1779–1786. [Google Scholar] [CrossRef] [Green Version]
- Neog, M.K.; Pragasam, S.J.; Krishnan, M.; Rasool, M. p-Coumaric acid, a dietary polyphenol ameliorates inflammation and curtails cartilage and bone erosion in the rheumatoid arthritis rat model. BioFactors 2017, 43, 698–717. [Google Scholar] [CrossRef] [PubMed]
- Thalhamer, T.; McGrath, M.A.; Harnett, M.M. MAPKs and their relevance to arthritis and inflammation. Rheumatology 2007, 47, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Fu, X.; Chen, X.; Li, Z.; Huang, Y.; Liang, C. Promising Therapeutic Targets for Treatment of Rheumatoid Arthritis. Front. Immunol. 2021, 12, 686155. [Google Scholar] [CrossRef] [PubMed]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxid. Med. Cell. Longev. 2016, 2016, 3128951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Song, X.; Cao, W.; Lu, J.; Wang, X.; Wang, G.; Wang, Z.; Chen, X. Autophagy and mitochondrial dysfunction in adjuvant-arthritis rats treatment with resveratrol. Sci. Rep. 2016, 6, 32928. [Google Scholar] [CrossRef] [PubMed]
- Khojah, H.M.; Ahmed, S.; Abdel-Rahman, M.S.; Elhakeim, E.H. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: A clinical study. Clin. Rheumatol. 2018, 37, 2035–2042. [Google Scholar] [CrossRef]
- Yang, G.; Chang, C.-C.; Yang, Y.; Yuan, L.; Xu, L.; Ho, C.-T.; Li, S. Resveratrol Alleviates Rheumatoid Arthritis via Reducing ROS and Inflammation, Inhibiting MAPK Signaling Pathways, and Suppressing Angiogenesis. J. Agric. Food Chem. 2018, 66, 12953–12960. [Google Scholar] [CrossRef]
- Noss, E.H.; Brenner, M.B. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol. Rev. 2008, 223, 252–270. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Hsu, L.-F.; Lee, C.-W.; Chiang, Y.-C.; Lee, M.-H.; How, J.-M.; Wu, C.-M.; Huang, C.-L.; Lee, I.-T. Resveratrol inhibits urban particulate matter-induced COX-2/PGE2 release in human fibroblast-like synoviocytes via the inhibition of activation of NADPH oxidase/ROS/NF-κB. Int. J. Biochem. Cell Biol. 2017, 88, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chen, J.-W.; Gao, J.-S.; Li, L.; Xie, X. Resveratrol inhibits TNF-α-induced IL-1β, MMP-3 production in human rheumatoid arthritis fibroblast-like synoviocytes via modulation of PI3kinase/Akt pathway. Rheumatol. Int. 2013, 33, 1829–1835. [Google Scholar] [CrossRef]
- Kim, K.; Vance, T.M.; Chun, O.K. Greater flavonoid intake is associated with improved CVD risk factors in US adults. Br. J. Nutr. 2016, 115, 1481–1488. [Google Scholar] [CrossRef] [Green Version]
- Mulvihill, E.E.; Burke, A.C.; Huff, M.W. Citrus Flavonoids as Regulators of Lipoprotein Metabolism and Atherosclerosis. Annu. Rev. Nutr. 2016, 36, 275–299. [Google Scholar] [CrossRef]
- Kometani, T.; Fukuda, T.; Kakuma, T.; Kawaguchi, K.; Tamura, W.; Kumazawa, Y.; Nagata, K. Effects of α-Glucosylhesperidin, a Bioactive Food Material, on Collagen-Induced Arthritis in Mice and Rheumatoid Arthritis in Humans. Immunopharmacol. Immunotoxicol. 2008, 30, 117–134. [Google Scholar] [CrossRef]
- He, Y.; Zhou, J.; Wang, Y.; Xiao, C.; Tong, Y.; Tang, J.C.-O.; Chan, A.S.; Lu, A. Anti-inflammatory and anti-oxidative effects of cherries on Freund’s adjuvant-induced arthritis in rats. Scand. J. Rheumatol. 2006, 35, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Pakozdi, A.; Koch, A.E. Regulation of interleukin-1β–induced chemokine production and matrix metalloproteinase 2 activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2006, 54, 2393–2401. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.-J.; Yoo, W.-H.; Han, M.-K.; Lee, Y.-R.; Kim, J.-S.; Lee, S.-I. Epigallocatechin-3-gallate suppresses TNF-α -induced production of MMP-1 and -3 in rheumatoid arthritis synovial fibroblasts. Rheumatol. Int. 2008, 29, 23–29. [Google Scholar] [CrossRef]
- Min, S.-Y.; Yan, M.; Kim, S.B.; Ravikumar, S.; Kwon, S.-R.; Vanarsa, K.; Kim, H.-Y.; Davis, L.S.; Mohan, C. Green Tea Epigallocatechin-3-Gallate Suppresses Autoimmune Arthritis Through Indoleamine-2,3-Dioxygenase Expressing Dendritic Cells and the Nuclear Factor, Erythroid 2-Like 2 Antioxidant Pathway. J. Inflamm. 2015, 12, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leichsenring, A.; Bäcker, I.; Furtmüller, P.G.; Obinger, C.; Lange, F.; Flemmig, J. Long-Term Effects of (−)-Epigallocatechin Gallate (EGCG) on Pristane-Induced Arthritis (PIA) in Female Dark Agouti Rats. PLoS ONE 2016, 11, e0152518. [Google Scholar] [CrossRef] [Green Version]
- Morinobu, A.; Biao, W.; Tanaka, S.; Horiuchi, M.; Jun, L.; Tsuji, G.; Sakai, Y.; Kurosaka, M.; Kumagai, S. (−)-Epigallocatechin-3-gallate suppresses osteoclast differentiation and ameliorates experimental arthritis in mice. Arthritis Rheum. 2008, 58, 2012–2018. [Google Scholar] [CrossRef]
- Rosillo, M.Á.; Alcaraz, M.J.; Sánchez-Hidalgo, M.; Fernández-Bolaños, J.G.; Alarcon-De-La-Lastra, C.; Ferrándiz, M.L. Anti-inflammatory and joint protective effects of extra-virgin olive-oil polyphenol extract in experimental arthritis. J. Nutr. Biochem. 2014, 25, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, G.; Al-Kahtani, M.A.; El-Sayed, W.M. Anti-inflammatory and Anti-oxidant Properties of Curcuma longa (Turmeric) Versus Zingiber officinale (Ginger) Rhizomes in Rat Adjuvant-Induced Arthritis. Inflammation 2011, 34, 291–301. [Google Scholar] [CrossRef]
- Kloesch, B.; Becker, T.; Dietersdorfer, E.; Kiener, H.; Steiner, G. Anti-inflammatory and apoptotic effects of the polyphenol curcumin on human fibroblast-like synoviocytes. Int. Immunopharmacol. 2013, 15, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Moon, D.-O.; Choi, I.-W.; Choi, B.T.; Nam, T.-J.; Rhu, C.-H.; Kwon, T.K.; Lee, W.H.; Kim, G.-Y.; Choi, Y.H. Curcumin induces apoptosis and inhibits prostaglandin E(2) production in synovial fibroblasts of patients with rheumatoid arthritis. Int. J. Mol. Med. 2007, 20, 365–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrotin, Y.; Mobasheri, A. Natural Products for Promoting Joint Health and Managing Osteoarthritis. Curr. Rheumatol. Rep. 2018, 20, 72. [Google Scholar] [CrossRef]
- Firestein, G.S.; Yeo, M.; Zvaifler, N.J. Apoptosis in rheumatoid arthritis synovium. J. Clin. Investig. 1995, 96, 1631–1638. [Google Scholar] [CrossRef] [Green Version]
- Oprea, O.; Apostol, L.; Bungau, S.B.; Cioca, G.; Samuel, A.D.; Badea, M.; Gaceu, L. Researches on the chemical composition and the rheological properties of wheat and grape epicarp flour mixes. Rev. Chim. 2018, 69, 70–75. [Google Scholar] [CrossRef]
- Oliviero, F.; Scanu, A.; Zamudio-Cuevas, Y.; Punzi, L.; Spinella, P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food Agric. 2018, 98, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Butein Activates Autophagy Through AMPK/TSC2/ULK1/mTOR Pathway to Inhibit IL-6 Expression in IL-1β Stimulated Human Chondrocytes. Cell. Physiol. Biochem. 2018, 49, 932–946. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Khan, N.M.; Haqqi, T.M. A standardized extract of Butea monosperma (Lam.) flowers suppresses the IL-1β-induced expression of IL-6 and matrix-metalloproteases by activating autophagy in human osteoarthritis chondrocytes. Biomed. Pharmacother. 2017, 96, 198–207. [Google Scholar] [CrossRef]
- Khan, N.M.; Haseeb, A.; Ansari, M.; Haqqi, T.M. A wogonin-rich-fraction of Scutellaria baicalensis root extract exerts chondroprotective effects by suppressing IL-1β-induced activation of AP-1 in human OA chondrocytes. Sci. Rep. 2017, 7, srep43789. [Google Scholar] [CrossRef] [Green Version]
- Haseeb, A.; Ansari, M.Y.; Haqqi, T.M. Harpagoside suppresses IL-6 expression in primary human osteoarthritis chondrocytes. J. Orthop. Res. 2017, 35, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Ucuncu, Y.; Celik, N.; Ozturk, C.; Turkoglu, M.; Cetin, N.; Kockara, N.; Sener, E.; Dundar, C.; Arslan, A.; Dogan, H.; et al. Chondroprotective effects of a new glucosamine combination in rats: Gene expression, biochemical and histopathological evaluation. Life Sci. 2015, 130, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, L.R.; Seo, J.H.; Kang, S. Curcumin and tetrahydrocurcumin both prevent osteoarthritis symptoms and decrease the expressions of pro-inflammatory cytokines in estrogen-deficient rats. Genes Nutr. 2016, 11, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakibaei, M.; Mobasheri, A.; Buhrmann, C. Curcumin synergizes with resveratrol to stimulate the MAPK signaling pathway in human articular chondrocytes in vitro. Genes Nutr. 2011, 6, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Csaki, C.; Mobasheri, A.; Shakibaei, M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1β-induced NF-κB-mediated inflammation and apoptosis. Arthritis Res. Ther. 2009, 11, R165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buhrmann, C.; Brockmueller, A.; Mueller, A.-L.; Shayan, P.; Shakibaei, M. Curcumin Attenuates Environment-Derived Osteoarthritis by Sox9/NF-kB Signaling Axis. Int. J. Mol. Sci. 2021, 22, 7645. [Google Scholar] [CrossRef]
- Zhang, Z.; Leong, D.J.; Xu, L.; He, Z.; Wang, A.; Navati, M.; Kim, S.J.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 2016, 18, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannuru, R.R.; Osani, M.C.; Al-Eid, F.; Wang, C. Efficacy of curcumin and Boswellia for knee osteoarthritis: Systematic review and meta-analysis. Semin. Arthritis Rheum. 2018, 48, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Coury, J.R.; Nixon, R.; Collins, M.; Schwartz, J.; Chahine, N.O.; Grande, D.A. Oral Administration of a Chemically Modified Curcumin, TRB-N0224, Reduced Inflammatory Cytokines and Cartilage Erosion in a Rabbit ACL Transection Injury Model. Cartilage 2021, 12, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Badavath, V.N.; Baysal, I.; Uçar, G.; Mondal, S.K.; Sinha, B.N.; Jayaprakash, V. Monoamine Oxidase Inhibitory Activity of Ferulic Acid Amides: Curcumin-Based Design and Synthesis. Arch. Pharm. 2016, 349, 9–19. [Google Scholar] [CrossRef]
- Chen, M.P.; Yang, S.H.; Chou, C.H.; Yang, K.C.; Wu, C.C.; Cheng, Y.H.; Lin, F.-H. The chondroprotective effects of ferulic acid on hydrogen peroxide-stimulated chondrocytes: Inhibition of hydrogen peroxide-induced pro-inflammatory cytokines and metalloproteinase gene expression at the mRNA level. Inflamm. Res. 2010, 59, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Frischholz, S.; Berberich, O.; Böck, T.; Meffert, R.H.; Blunk, T. Resveratrol counteracts IL-1β-mediated impairment of extracellular matrix deposition in 3D articular chondrocyte constructs. J. Tissue Eng. Regen. Med. 2020, 14, 897–908. [Google Scholar] [CrossRef]
- Wang, J.; Gao, J.-S.; Chen, J.-W.; Li, F.; Tian, J. Effect of resveratrol on cartilage protection and apoptosis inhibition in experimental osteoarthritis of rabbit. Rheumatol. Int. 2012, 32, 1541–1548. [Google Scholar] [CrossRef]
- Wei, Y.; Jia, J.; Jin, X.; Tong, W.; Tian, H. Resveratrol ameliorates inflammatory damage and protects against osteoarthritis in a rat model of osteoarthritis. Mol. Med. Rep. 2017, 17, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-C.; Hung, L.-F.; Wu, W.-L.; Chang, D.-M.; Huang, C.-Y.; Lai, J.-H.; Ho, L.-J. Chondroprotective effects and mechanisms of resveratrol in advanced glycation end products-stimulated chondrocytes. Arthritis Res. Ther. 2010, 12, R167. [Google Scholar] [CrossRef] [Green Version]
- Lei, M.; Wang, J.-G.; Xiao, D.-M.; Fan, M.; Wang, D.-P.; Xiong, J.-Y.; Chen, Y.; Ding, Y.; Liu, S.-L. Resveratrol inhibits interleukin 1β-mediated inducible nitric oxide synthase expression in articular chondrocytes by activating SIRT1 and thereby suppressing nuclear factor-κB activity. Eur. J. Pharmacol. 2012, 674, 73–79. [Google Scholar] [CrossRef]
- Ravalli, S.M.; Szychlinska, M.A.; Leonardi, R.M.; Musumeci, G. Recently highlighted nutraceuticals for preventive management of osteoarthritis. World J. Orthop. 2018, 9, 255–261. [Google Scholar] [CrossRef]
- Castrogiovanni, P.; Trovato, F.M.; Loreto, C.; Nsir, H.; Szychlinska, M.A.; Musumeci, G. Nutraceutical Supplements in the Management and Prevention of Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. [Google Scholar] [CrossRef]
- Cetrullo, S.; D’Adamo, S.; Guidotti, S.; Borzì, R.M.; Flamigni, F. Hydroxytyrosol prevents chondrocyte death under oxidative stress by inducing autophagy through sirtuin 1-dependent and -independent mechanisms. Biochim. Biophys. Acta BBA Gen. Subj. 2016, 1860, 1181–1191. [Google Scholar] [CrossRef]
- Musumeci, G.; Trovato, F.M.; Pichler, K.; Weinberg, A.M.; Loreto, C.; Castrogiovanni, P. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model: An in vivo and in vitro study on lubricin expression. J. Nutr. Biochem. 2013, 24, 2064–2075. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; Castrogiovanni, P.; Trovato, F.M.; Nsir, H.; Zarrouk, M.; Furno, D.L.; Di Rosa, M.; Imbesi, R.; Musumeci, G. Physical activity and Mediterranean diet based on olive tree phenolic compounds from two different geographical areas have protective effects on early osteoarthritis, muscle atrophy and hepatic steatosis. Eur. J. Nutr. 2019, 58, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Mével, E.; Merceron, C.; Vinatier, C.; Krisa, S.; Richard, T.; Masson, M.; Lesoeur, J.; Hivernaud, V.; Gauthier, O.; Abadie, J.; et al. Olive and grape seed extract prevents post-traumatic osteoarthritis damages and exhibits in vitro anti IL-1β activities before and after oral consumption. Sci. Rep. 2016, 6, 33527. [Google Scholar] [CrossRef] [Green Version]
- Horcajada, M.-N.; Sanchez, C.; Scalfo, F.M.; Drion, P.; Comblain, F.; Taralla, S.; Donneau, A.-F.; Offord, E.; Henrotin, Y. Oleuropein or rutin consumption decreases the spontaneous development of osteoarthritis in the Hartley guinea pig. Osteoarthr. Cartil. 2015, 23, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtar, N.; Haqqi, T.M. Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes. Arthritis Res. Ther. 2011, 13, R93. [Google Scholar] [CrossRef] [Green Version]
- Willcox, J.K.; Ash, S.L.; Catignani, G.L. Antioxidants and Prevention of Chronic Disease. Crit. Rev. Food Sci. Nutr. 2004, 44, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh, M.S.; Moats, S.M.; Miller, J.D. Acid bake-leach process for the treatment of enargite concentrates. Hydrometallurgy 2012, 119–120, 30–39. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Mishra, A.; Sharma, A.K.; Kumar, S.; Saxena, A.K.; Pandey, A.K. Bauhinia variegataLeaf Extracts Exhibit Considerable Antibacterial, Antioxidant, and Anticancer Activities. BioMed Res. Int. 2013, 2013, 915436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Pandey, A.K. Antioxidant and hepatoprotective activity of cinnamaldehyde against isoniazid induced hepatotoxicity. Free. Radic. Biol. Med. 2018, 120, S111. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, S.; Pandey, A.K. Scientific Validation of the Medicinal Efficacy ofTinospora cordifolia. Sci. World J. 2013, 2013, 292934. [Google Scholar] [CrossRef] [Green Version]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane Interactions: A Protective Role of Flavonoids at the Membrane Surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Cheon, B.S.; Kim, Y.H.; Son, K.S.; Chang, H.W.; Kang, S.S.; Kim, H.P. Effects of Prenylated Flavonoids and Biflavonoids on Lipopolysaccharide-Induced Nitric Oxide Production from the Mouse Macrophage Cell Line RAW 264.7. Planta Med. 2000, 66, 596–600. [Google Scholar] [CrossRef]
- Sarkar, A.; Bhaduri, A. Black Tea Is a Powerful Chemopreventor of Reactive Oxygen and Nitrogen Species: Comparison with Its Individual Catechin Constituents and Green Tea. Biochem. Biophys. Res. Commun. 2001, 284, 173–178. [Google Scholar] [CrossRef]
- Hong, J.; Smith, T.J.; Ho, C.-T.; August, D.A.; Yang, C.S. Effects of purified green and black tea polyphenols on cyclooxygenase- and lipoxygenase-dependent metabolism of arachidonic acid in human colon mucosa and colon tumor tissues. Biochem. Pharmacol. 2001, 62, 1175–1183. [Google Scholar] [CrossRef]
- Nagao, A.; Seki, M.; Kobayashi, H. Inhibition of Xanthine Oxidase by Flavonoids. Biosci. Biotechnol. Biochem. 1999, 63, 1787–1790. [Google Scholar] [CrossRef]
- Rotelli, A.E.; Guardia, T.; Juárez, A.O.; De La Rocha, N.E.; Pelzer, L. Comparative study of flavonoids in experimental models of inflammation. Pharmacol. Res. 2003, 48, 601–606. [Google Scholar] [CrossRef]
- Paradkar, P.N.; Blum, P.S.; Berhow, M.A.; Baumann, H.; Kuo, S.-M. Dietary isoflavones suppress endotoxin-induced inflammatory reaction in liver and intestine. Cancer Lett. 2004, 215, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.A.; Sefton, B.M. Protein tyrosine phosphorylation is induced in murine B lymphocytes in response to stimulation with anti-immunoglobulin. EMBO J. 1990, 9, 2125–2131. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Del Cornò, M.; Scazzocchio, B.; Masella, R.; Gessani, S. Regulation of Dendritic Cell Function by Dietary Polyphenols. Crit. Rev. Food Sci. Nutr. 2016, 56, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, S.; Kawai, K.; Tsuno, N.H.; Okaji, Y.; Asakage, M.; Tsuchiya, T.; Yamada, J.; Sunami, E.; Osada, T.; Kitayama, J.; et al. Epigallocatechin gallate affects human dendritic cell differentiation and maturation. J. Allergy Clin. Immunol. 2008, 121, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Lin, C.-H.; Hung, S.-K.; Chou, J.-H.; Chi, C.-W.; Fu, S.-L. Fisetin Inhibits Lipopolysaccharide-Induced Macrophage Activation and Dendritic Cell Maturation. J. Agric. Food Chem. 2010, 58, 10831–10839. [Google Scholar] [CrossRef] [PubMed]
- Dugo, L.; Belluomo, M.G.; Fanali, C.; Russo, M.; Cacciola, F.; Maccarrone, M.; Sardanelli, A.M. Effect of Cocoa Polyphenolic Extract on Macrophage Polarization from Proinflammatory M1 to Anti-Inflammatory M2 State. Oxid. Med. Cell. Longev. 2017, 2017, 6293740. [Google Scholar] [CrossRef]
- Hassanain, E.; Silverberg, J.I.; Norowitz, K.B.; Chice, S.; Wright, L.; Forgy, C.C.; Bluth, M.H.; Brody, N.; Joks, R.; Durkin, H.G.; et al. Green Tea (Camelia Sinensis) Suppresses B Cell Production Of IgE Without Inducing Apoptosis. J. Allergy Clin. Immunol. 2010, 125, AB12. [Google Scholar] [CrossRef]
- Sanbongi, C.; Suzuki, N.; Sakane, T. Polyphenols in Chocolate, Which Have Antioxidant Activity, Modulate Immune Functions in Humansin Vitro. Cell. Immunol. 1997, 177, 129–136. [Google Scholar] [CrossRef]
- Wong, C.P.; Nguyen, L.P.; Noh, S.K.; Bray, T.M.; Bruno, R.S.; Ho, E. Induction of regulatory T cells by green tea polyphenol EGCG. Immunol. Lett. 2011, 139, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, T.; Ishida, J.; Nakagawa, S.; Ogawara, H.; Watanabe, S.; Itoh, N.; Shibuya, M.; Fukami, Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987, 262, 5592–5595. [Google Scholar] [CrossRef]
- Tordera, M.; Ferrándiz, M.L.; Alcaraz, M.J. Influence of Anti-Inflammatory Flavonoids on Degranulation and Arachidonic Acid Release in Rat Neutrophils. Zeitschrift für Naturforschung C 1994, 49, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, R.; Varì, R.; Scazzocchio, B.; Filesi, C.; Santangelo, C.; Giovannini, C.; Matarrese, P.; D’Archivio, M.; Masella, R. Tyrosol, the major extra virgin olive oil compound, restored intracellular antioxidant defences in spite of its weak antioxidative effectiveness. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Young, M.R.; Bobe, G.; Colburn, N.H.; Milner, J.A. Bioactive Food Components, Inflammatory Targets, and Cancer Prevention. Cancer Prev. Res. 2009, 2, 200–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.-Y.; Hu, K.-Z.; Yin, Z.-S. Inhibition of the p38-MAPK signaling pathway suppresses the apoptosis and expression of proinflammatory cytokines in human osteoarthritis chondrocytes. Cytokine 2017, 90, 135–143. [Google Scholar] [CrossRef]
- Dong, C.; Davis, R.J.; Flavell, R.A. MAP Kinases in the Immune Response. Annu. Rev. Immunol. 2002, 20, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of MAPKs activation. J. Funct. Foods 2018, 43, 62–69. [Google Scholar] [CrossRef]
- Westra, J.; Limburg, P.C. p38 mitogen-activated protein kinase (MAPK) in rheumatoid arthritis. Mini-Rev. Med. Chem. 2006, 6, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, J.; Maier, R.V. Mitogen-activated protein kinase (MAPK). Crit. Care Med. 2005, 33, S417–S419. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, Z.-X.; Zhao, Y.; Brautigan, D.L.; Zhang, Z.-Y.; Lee, S.H.; Jin, J.B.; Song, J.; Min, M.K.; Park, D.S.; et al. The Specificity of Extracellular Signal-regulated Kinase 2 Dephosphorylation by Protein Phosphatases. J. Biol. Chem. 2002, 277, 31818–31825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schett, G.; Tohidast-Akrad, M.; Smolen, J.S.; Schmid, B.J.; Steiner, C.-W.; Bitzan, P.; Zenz, P.; Redlich, K.; Xu, Q.; Steiner, G. Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis Rheum. 2000, 43, 2501–2512. [Google Scholar] [CrossRef]
- Kotlyarov, A.; Neininger, A.; Schubert, C.; Eckert, R.; Birchmeier, C.; Volk, H.-D.; Gaestel, M. MAPKAP kinase 2 is essential for LPS-induced TNF-α biosynthesis. Nat. Cell Biol. 1999, 1, 94–97. [Google Scholar] [CrossRef]
- Han, Z.; Boyle, D.L.; Chang, L.; Bennett, B.; Karin, M.; Yang, L.; Manning, A.M.; Firestein, G.S. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J. Clin. Investig. 2001, 108, 73–81. [Google Scholar] [CrossRef]
- Murata, K.; Yoshitomi, H.; Furu, M.; Ishikawa, M.; Shibuya, H.; Ito, H.; Matsuda, S. MicroRNA-451 Down-Regulates Neutrophil Chemotaxis via p38 MAPK. Arthritis Rheumatol. 2014, 66, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Hammaker, D.R.; Boyle, D.L.; Chabaud-Riou, M.; Firestein, G.S. Regulation of c-Jun N-terminal kinase by MEKK-2 and mitogen-activated protein kinase kinase kinases in rheumatoid arthritis. J. Immunol. 2004, 172, 1612–1618. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.I.; Kim, H.J.; Kim, Y.-J.; Jeong, S.-I.; You, Y.-O. Tanshinone IIA inhibits LPS-induced NF-κB activation in RAW 264.7 cells: Possible involvement of the NIK–IKK, ERK1/2, p38 and JNK pathways. Eur. J. Pharmacol. 2006, 542, 1–7. [Google Scholar] [CrossRef]
- Ko, W.; Sohn, J.H.; Jang, J.-H.; Ahn, J.S.; Kang, D.G.; Lee, H.S.; Kim, J.-S.; Kim, Y.-C.; Oh, H. Inhibitory effects of alternaramide on inflammatory mediator expression through TLR4-MyD88-mediated inhibition of NF-κB and MAPK pathway signaling in lipopolysaccharide-stimulated RAW264.7 and BV2 cells. Chem. Interactions 2016, 244, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peng, F.; Xie, C.; Wu, W.; Han, X.; Chen, L. (E)-3-(3,4-Dimethoxyphenyl)-1-(5-hydroxy-2,2-dimethyl-2H-chromen-6-yl)prop-2-en-1-one ameliorates the collagen-arthritis via blocking ERK/JNK and NF-κB signaling pathway. Int. Immunopharmacol. 2013, 17, 1125–1133. [Google Scholar] [CrossRef]
- Ichikawa, D.; Matsui, A.; Imai, M.; Sonoda, Y.; Kasahara, T. Effect of Various Catechins on the IL-12p40 Production by Murine Peritoneal Macrophages and a Macrophage Cell Line, J774.1. Biol. Pharm. Bull. 2004, 27, 1353–1358. [Google Scholar] [CrossRef]
- Rasheed, Z.; Akhtar, N.; Haqqi, T.M. Pomegranate extract inhibits the interleukin-1β-induced activation of MKK-3, p38α-MAPK and transcription factor RUNX-2 in human osteoarthritis chondrocytes. Arthritis Res.Ther. 2010, 12, R195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Shu, Z.; Xing, N.; Xu, B.; Wang, C.; Sun, G.; Sun, X.; Kuang, H. A pure polysaccharide from Ephedra sinica treating on arthritis and inhibiting cytokines expression. Int. J. Biol. Macromol. 2016, 86, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Jean-Gilles, D.; Li, L.; Vaidyanathan, V.G.; King, R.; Cho, B.; Worthen, D.R.; Chichester, C.O.; Seeram, N.P. Inhibitory effects of polyphenol punicalagin on type-II collagen degradation in vitro and inflammation in vivo. Chem. Interactions 2013, 205, 90–99. [Google Scholar] [CrossRef]
- Wang, J.-H.; Shih, K.-S.; Liou, J.-P.; Wu, Y.-W.; Chang, A.S.-Y.; Wang, K.-L.; Tsai, C.-L.; Yang, C.-R. Anti-Arthritic Effects of Magnolol in Human Interleukin 1β-Stimulated Fibroblast-Like Synoviocytes and in a Rat Arthritis Model. PLoS ONE 2012, 7, e31368. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, B.; Zhang, N.; Liu, Z.; Liang, D.; Li, F.; Cao, Y.; Feng, X.; Zhang, X.; Yang, Z. Magnolol inhibits lipopolysaccharide-induced inflammatory response by interfering with TLR4 mediated NF-κB and MAPKs signaling pathways. J. Ethnopharmacol. 2013, 145, 193–199. [Google Scholar] [CrossRef]
- Kim, K.-S.; Lee, D.-S.; Bae, G.-S.; Park, S.-J.; Kang, D.-G.; Lee, H.-S.; Oh, H.; Kim, Y.-C. The inhibition of JNK MAPK and NF-κB signaling by tenuifoliside A isolated from Polygala tenuifolia in lipopolysaccharide-induced macrophages is associated with its anti-inflammatory effect. Eur. J. Pharmacol. 2013, 721, 267–276. [Google Scholar] [CrossRef]
- Baier, A.; Meineckel, I.; Gay, S.; Pap, T. Apoptosis in rheumatoid arthritis. Curr. Opin. Rheumatol. 2003, 15, 274–279. [Google Scholar] [CrossRef]
- Celli, A.; Que, F.G. Dysregulation of Apoptosis in the Cholangiopathies and Cholangiocarcinoma. Semin. Liver Dis. 1998, 18, 177–185. [Google Scholar] [CrossRef]
- Haanen, C.; Vermes, I. Apoptosis and Inflammation. Mediators Inflamm. 1995, 4, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S.; Echeverri, F.; Yeo, M.; Zvaifler, N.J.; Green, D.R. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc. Natl. Acad. Sci. USA 1997, 94, 10895–10900. [Google Scholar] [CrossRef] [Green Version]
- Rème, T.; Travaglio, A.; Gueydon, E.; Adla, L.; Jorgensen, C.; Sany, J. Mutations of the p53 tumour suppressor gene in erosive rheumatoid synovial tissue. Clin. Exp. Immunol. 1998, 111, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Ladiwala, U.; Li, H.; Antel, J.P.; Nalbantoglu, J. p53 Induction by Tumor Necrosis Factor-α and Involvement of p53 in Cell Death of Human Oligodendrocytes. J. Neurochem. 2002, 73, 605–611. [Google Scholar] [CrossRef]
- Leri, A.; Liu, Y.; Claudio, P.P.; Kajstura, J.; Wang, X.; Wang, S.; Kang, P.; Malhotra, A.; Anversa, P. Insulin-Like Growth Factor-1 Induces Mdm2 and Down-Regulates p53, Attenuating the Myocyte Renin-Angiotensin System and Stretch-Mediated Apoptosis. Am. J. Pathol. 1999, 154, 567–580. [Google Scholar] [CrossRef] [Green Version]
- Sheffield, V.C.; Beck, J.S.; Kwitek, A.E.; Sandstrom, D.W.; Stone, E.M. The Sensitivity of Single-Strand Conformation Polymorphism Analysis for the Detection of Single Base Substitutions. Genomics 1993, 16, 325–332. [Google Scholar] [CrossRef]
- Nygaard, G.; Firestein, G.S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 2020, 16, 316–333. [Google Scholar] [CrossRef]
- Kung, C.-P.; Murphy, M.E. The role of the p53 tumor suppressor in metabolism and diabetes. J. Endocrinol. 2016, 231, R61–R75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, H.; Sopko, N.A.; Campbell, J.D.; Liu, X.; Reinhardt, A.; Weyne, E.; Castiglione, F.; Albersen, M.; Hannan, J.L.; Bivalacqua, T.J. Increased Level of Tumor Necrosis Factor-Alpha (TNF-α) Leads to Downregulation of Nitrergic Neurons Following Bilateral Cavernous Nerve Injury and Modulates Penile Smooth Tone. J. Sex. Med. 2021, 18, 1181–1190. [Google Scholar] [CrossRef]
- Badea, M.; di Modugno, F.; Floroian, L.; Tit, D.M.; Restani, P.; Bungau, S.; Iovan, C.; Badea, G.E.; Aleya, L. Electrochemical strategies for gallic acid detection: Potential for application in clinical, food or environmental analyses. Sci. Total Environ. 2019, 672, 129–140. [Google Scholar] [CrossRef]
- Direito, R.; Rocha, J.; Sepodes, B.; Eduardo-Figueira, M. Phenolic Compounds Impact on Rheumatoid Arthritis, Inflammatory Bowel Disease and Microbiota Modulation. Pharmaceutics 2021, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Kwon, D.; Um, E.; Kim, B. Could Polyphenols Help in the Control of Rheumatoid Arthritis? Molecules 2019, 24, 1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christman, L.M.; Gu, L. Efficacy and mechanisms of dietary polyphenols in mitigating rheumatoid arthritis. J. Funct. Foods 2020, 71, 104003. [Google Scholar] [CrossRef]




| Polyphenols | Source | Experimental Setup | Actioning Mechanism | Ref. |
|---|---|---|---|---|
| Ferulic acid | apple, sugar beet, popcorn, grains, vegetables | Macrophage, monocytes, Rats | NFATc1, c-Fos, NF-κB, MMP | [32] |
| N-feruloyl serotonin | safflower seed | AA | CRP, LOX, TNF-α, iNOS, IL-1β | [33] |
| Resveratrol | red grapes, peanut, soy | CIA, FLS | COX-2, PGE2, NADPH oxidase, ROS, p38, MAPK, ERK1/2, NF-κB | [39] |
| Epigallocatechin-3-gallate | green tea, strawberries, blackberries | CIA rat | IL-6, TNF-α, IFN-γ | [129,130] |
| Gallic acid | cinnamon bark | AIA rat | TNF-α | [32,33,147] |
| EVOO polyphenol extract | EVOO, fruit of olea, olives | CIA | TNF-α, IL-1β, IL-6, PEG2, p38, JNK, p65 | [55] |
| Curcumin | turmeric rhizome | RA-FLS | IL-1β, IL-6, NF-κB, ERK1/2 | [70] |
| p-Coumaric acid | gnetum | AIA | TNF-α, IgG | [34] |
| Emodin | rhubarb, asian knotweed | Synovial membrane in human | MMP-1, MMP-9, NF-κB, MAPK | [57] |
| Hesperidin | soybean, sweet orange, tangerine | Wistar rat | GSH, SOD, catalase | [47,48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behl, T.; Upadhyay, T.; Singh, S.; Chigurupati, S.; Alsubayiel, A.M.; Mani, V.; Vargas-De-La-Cruz, C.; Uivarosan, D.; Bustea, C.; Sava, C.; et al. Polyphenols Targeting MAPK Mediated Oxidative Stress and Inflammation in Rheumatoid Arthritis. Molecules 2021, 26, 6570. https://doi.org/10.3390/molecules26216570
Behl T, Upadhyay T, Singh S, Chigurupati S, Alsubayiel AM, Mani V, Vargas-De-La-Cruz C, Uivarosan D, Bustea C, Sava C, et al. Polyphenols Targeting MAPK Mediated Oxidative Stress and Inflammation in Rheumatoid Arthritis. Molecules. 2021; 26(21):6570. https://doi.org/10.3390/molecules26216570
Chicago/Turabian StyleBehl, Tapan, Tanuj Upadhyay, Sukhbir Singh, Sridevi Chigurupati, Amal M. Alsubayiel, Vasudevan Mani, Celia Vargas-De-La-Cruz, Diana Uivarosan, Cristiana Bustea, Cristian Sava, and et al. 2021. "Polyphenols Targeting MAPK Mediated Oxidative Stress and Inflammation in Rheumatoid Arthritis" Molecules 26, no. 21: 6570. https://doi.org/10.3390/molecules26216570
APA StyleBehl, T., Upadhyay, T., Singh, S., Chigurupati, S., Alsubayiel, A. M., Mani, V., Vargas-De-La-Cruz, C., Uivarosan, D., Bustea, C., Sava, C., Stoicescu, M., Radu, A.-F., & Bungau, S. G. (2021). Polyphenols Targeting MAPK Mediated Oxidative Stress and Inflammation in Rheumatoid Arthritis. Molecules, 26(21), 6570. https://doi.org/10.3390/molecules26216570