Anti-Multiple Myeloma Potential of Secondary Metabolites from Hibiscus sabdariffa—Part 2
Abstract
:1. Introduction
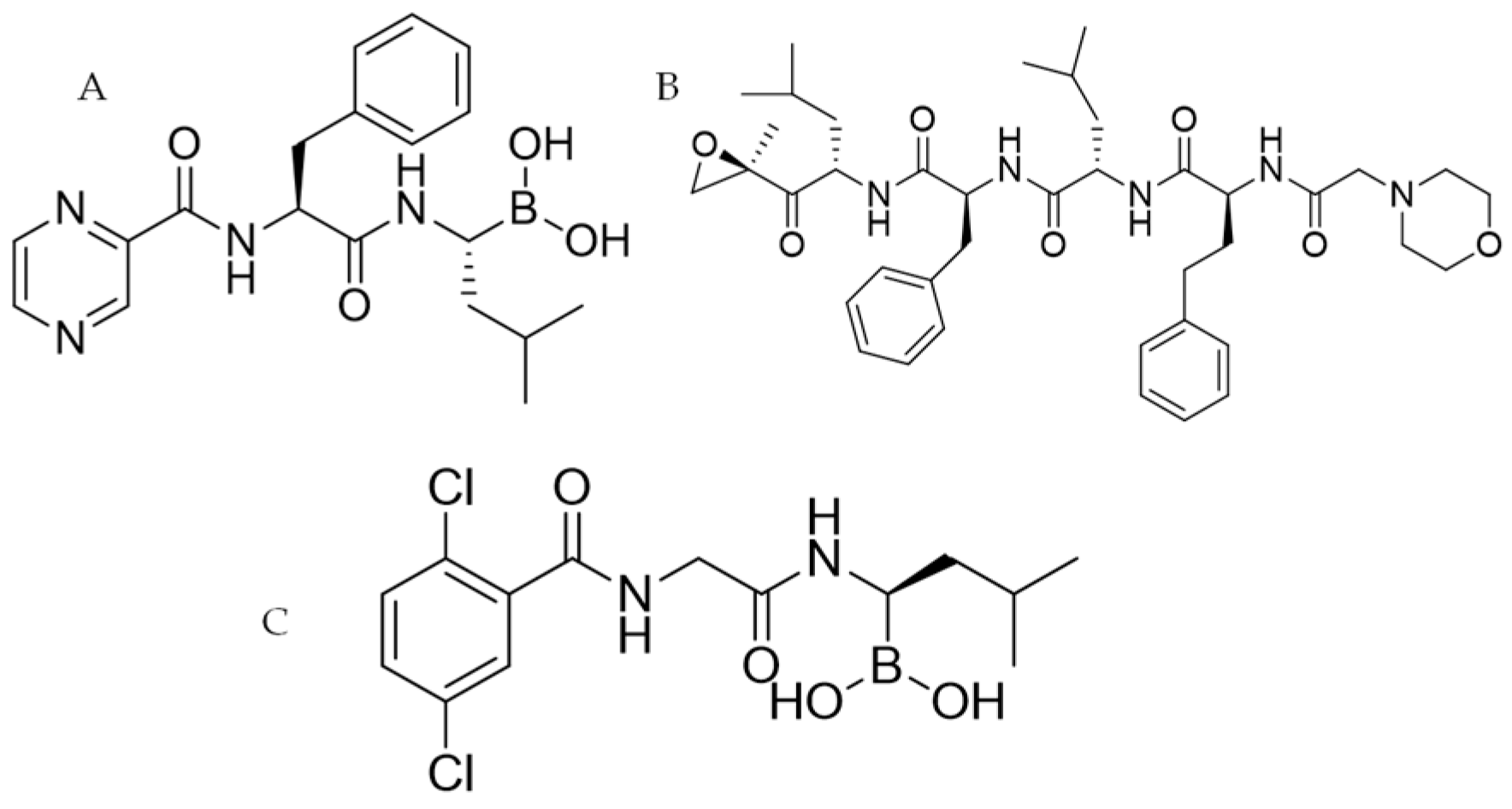
- Carfilzomib (Kyprolis®, CFZ: Figure 1B): this second-generation inhibitor belongs to the epoxyketone class and it has the same binding site as BTZ, but it delivers an irreversible effect. It has been approved to treat MM, but it is also studied for use against leukemia, amyloidosis, lymphoma and macroglobulinemia [10].
- Ixazomib (Ninlaro®: Figure 1C): belongs to the same class of drug as BTZ and has the same mechanism of action. Ixazomib also presents significant clinical advantages including an increased oral bioavailability. The anti-MM therapy with Ixazomib needs a combination with Dexamethasone and Lenalidomide [11].
2. Results and discussion
2.1. Molecular Modelling Studies
2.2. Extraction of H. sabdariffa
- HsEF was treated with polymer-supported carbonate (PS-carbonate) resin in methanol. After solvent removal, the resin was subjected to treatment with 0.1% HCl in methanol, filtrated and the solvent evaporated under a reduced pressure to obtain a simplified fraction. It was further purified by flash chromatography, thus obtaining Hib-ester (final yield = 0.22%) (Figure 2A).
- HsEF was subjected to a second liquid/liquid extraction using dichloromethane as the organic solvent and renewing the aqueous phase during the process. This second phase was then evaporated and chromatographed to obtain pure Hib-carbaldehyde, with a 1.1% yield (Figure 2B).
2.3. Cell Viability and Cell Death of Myeloma Cell Lines
2.4. Evaluation of Apoptosis
2.5. Evaluation of Autophagy Inhibition
2.6. Evaluation of Proteasome Inhibition
3. Materials and Methods
3.1. General
3.2. Molecular Modeling Analysis
3.3. Pan Assay Interference Compounds (PAINS) Evaluation
3.4. Extraction Procedure
3.4.1. Plant Material
3.4.2. Isolation of Hib-ester
3.4.3. Isolation of Hib-carbaldehyde
3.5. Biological Assays
3.5.1. Cell Cultures and Hibiscus sabdariffa
3.5.2. Trypan Blue Vital Count Assay
3.5.3. Annexin V Assay
3.5.4. Western Blotting
3.5.5. Acridine Orange Staining
3.5.6. Proteasome Activity Assay
3.5.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robak, P.; Drozdz, I.; Szemraj, J.; Robak, T. Drug resistance in multiple myeloma. Cancer Treat. Rev. 2018, 70, 199–208. [Google Scholar] [CrossRef]
- Gay, F.; Palumbo, A. Multiple myeloma: Management of adverse events. Med. Oncol. 2010, 27, 646–653. [Google Scholar] [CrossRef]
- Hameed, A.; Brady, J.J.; Dowling, P.; Clynes, M.; O’Gorman, P. Bone disease in multiple myeloma: Pathophysiology and management. Cancer Growth Metastasis 2014, 7, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visconti, R.J.; Kolaja, K.; Cottrell, J.J. A Functional Three-Dimensional Microphysiological Human Model of Myeloma Bone Disease. Bone Miner. Res. 2021, 36, 1914–1930. [Google Scholar] [CrossRef] [PubMed]
- Mukkamalla, S.K.R.; Malipeddi, D. Myeloma Bone Disease: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 6208. [Google Scholar] [CrossRef]
- Crawford, L.J.; Walker, B.; Irvine, A.E. Proteasome inhibitors in cancer therapy. J. Cell Commun. Signal. 2011, 5, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manasanch, E.E.; Orlowski, R.Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2017, 14, 417–433. [Google Scholar] [CrossRef]
- Chen, D.; Frezza, M.; Schmitt, S.; Kanwar, J.P.; Dou, Q. Bortezomib as the First Proteasome Inhibitor Anticancer Drug: Current Status and Future Perspectives. Curr. Cancer Drug Targets 2011, 11, 239–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, L.J.A.; Walker, B.; Ovaa, H.; Chauhan, D.; Anderson, K.C.; Morris, T.C.M.; Irvine, A.E. Comparative Selectivity and Specificity of the Proteasome Inhibitors BzLLLCOCHO, PS-341, and MG-132. Cancer Res. 2006, 66, 6379–6386. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.A.; Cohen, A.D. Experimental approaches in the treatment of multiple myeloma. Ther. Adv. Hematol. 2011, 2, 213–230. [Google Scholar] [CrossRef] [Green Version]
- Moreau, P.; Rajkumar, S.V. Multiple myeloma—Translation of trial results into reality. Lancet 2016, 388, 111–113. [Google Scholar] [CrossRef]
- Meregalli, C. An Overview of Bortezomib-Induced Neurotoxicity. Toxics 2015, 3, 294–303. [Google Scholar] [CrossRef]
- Sloot, W.; Glaser, N.; Hansen, A.; Hellmann, J.; Jaeckel, S.; Johannes, S.; Knippel, A.; Lai, V.; Onidi, M. Improved nonclinical safety profile of a novel, highly selective inhibitor of the immunoproteasome subunit LMP7 (M3258). Toxicol. Appl. Pharmacol. 2021, 429, 115695. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Wu, X.; Liu, X.; Zheng, X.; Deng, J.; Gong, Y. Comparison of monoclonal antibodies targeting CD38, SLAMF7 and PD-1/PD-L1 in combination with Bortezomib/Immunomodulators plus dexamethasone/prednisone for the treatment of multiple myeloma: An indirect-comparison Meta-analysis of randomised controlled trials. BMC Cancer 2021, 21, 994. [Google Scholar]
- Listro, R.; Rossino, G.; Della Volpe, S.; Stabile, R.; Boiocchi, M.; Malavasi, L.; Rossi, D.; Collina, S. Enantiomeric Resolution and Absolute Configuration of a Chiral δ-Lactam, Useful Intermediate for the Synthesis of Bioactive Compounds. Molecules 2020, 25, 6023. [Google Scholar] [CrossRef] [PubMed]
- Listro, R.; Stotani, S.; Rossino, G.; Rui, M.; Malacrida, A.; Cavaletti, G.; Cortesi, M.; Arienti, C.; Tesei, A.; Rossi, D.; et al. Exploring the RC-106 Chemical Space: Design and Synthesis of Novel (E)-1-(3-Arylbut-2-en-1-yl)-4-(Substituted) Piperazine Derivatives as Potential Anticancer Agents. Front. Chem. 2020, 8, 495. [Google Scholar] [CrossRef] [PubMed]
- Keyata, E.O.; Tola, Y.B.; Bultosa, G.; Forsido, S.F. Phytochemical contents, antioxidant activity and functional properties of Raphanus sativus L., Eruca sativa L. and Hibiscus sabdariffa L. growing in Ethiopia. Heliyon 2021, 7, e05939. [Google Scholar] [CrossRef]
- Malacrida, A.; Cavalloro, V.; Martino, E.; Cassetti, A.; Nicolini, G.; Rigolio, R.; Cavaletti, G.; Mannucci, B.; Vasile, F.; Di Giacomo, M.; et al. Anti-multiple myeloma potential of secondary metabolites from hibiscus sabdariffa. Molecules 2019, 24, 2500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrödinger Release 2018-1: Maestro Graphics User Interface; Schrödinger LLC.: New York, NY, USA, 2018.
- Vigani, B.; Rossi, S.; Gentile, M.; Sandri, G.; Bonferoni, M.C.; Cavalloro, V.; Martino, E.; Collina, S.; Ferrari, F. Development of a mucoadhesive and an in situ gelling formulation based on κ-carrageenan for application on oral mucosa and esophagus walls. II. Loading of a bioactive hydroalcoholic extract. Mar. Drugs 2019, 17, 153. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Cavalloro, V.; Marrubini, G.; Stabile, R.; Rossi, D.; Linciano, P.; Gheza, G.; Assini, S.; Martino, E.; Collina, S. Microwave-Assisted Extraction and HPLC-UV-CD Determination of (S)-usnic Acid in Cladonia foliacea. Molecules 2021, 26, 455. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1-2. [Google Scholar] [CrossRef]
- Shah, N.N.A.K.; Ong, M.C.; Supian, N.M.A.; Sulaiman, A. Ultrasound-assisted extraction and gaseous ozone as preservation method for Roselle (Hibiscus sabdariffa L.) fruit juice. Food Res. 2020, 4, 85–95. [Google Scholar] [CrossRef]
- Al-Sayed, H.M.; Hegab, S.A.; Youssef, M.A.; Khalafalla, M.Y.; Almaroai, Y.A.; Ding, Z.; Eissa, M.A. Evaluation of quality and growth of roselle (Hibiscus sabdariffa L.) as affected by bio-fertilizers. J. Plant Nutr. 2020, 43, 1025–1035. [Google Scholar] [CrossRef]
- Saturnino, C.; Barone, I.; Iacopetta, D.; Mariconda, A.; Sinicropi, M.S.; Rosano, C.; Campana, A.; Catalano, S.; Longo, P.; Andò, S. N-heterocyclic carbene complexes of silver and gold as novel tools against breast cancer progression. Future Med. Chem. 2016, 8, 2213–2229. [Google Scholar] [CrossRef]
- Mah, L.Y.; Ryan, K.M. Autophagy and Cancer. Cold Spring Harb. Perspect. Biol. 2012, 4, a008821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacopetta, D.; Grande, F.; Caruso, A.; Mordocco, R.A.; Plutino, M.R.; Scrivano, L.; Ceramella, J.; Muià, N.; Saturnino, C.; Puoci, F.; et al. New insights for the use of quercetin analogs in cancer treatment. Future Med. Chem. 2017, 9, 2011–2028. [Google Scholar] [CrossRef]
- Tanida, I. Autophagy basics. Microbiol. Immunol. 2011, 55, 1–11. [Google Scholar] [CrossRef]
- Funderburk, S.F.; Wang, Q.J.; Yue, Z. The Beclin 1–VPS34 complex–at the crossroads of autophagy and beyond. Trends Cell Biol. 2010, 20, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Dreiseitel, A.; Schreier, P.; Oehme, A.; Locher, S.; Rogler, G.; Piberger, H.; Hajak, G.; Sand, P.G. Inhibition of proteasome activity by anthocyanins and anthocyanidins. Biochem. Biophys. Res. Commun. 2008, 372, 57–61. [Google Scholar] [CrossRef]
- Ayoub, L.; Aissam, E.; Yassine, K.; Said, E.; Mohammed, E.M.; Souad, A. A specific QSAR model for proteasome inhibitors from Oleaeuropaea and Ficuscarica. Bioinformation 2018, 14, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Schrader, J.; Henneberg, F.; Mata, R.A.; Tittmann, K.; Schneider, T.R.; Stark, H.; Bourenkov, G.; Chari, A. The inhibition mechanism of human 20S proteasomes enables next-generation inhibitor design. Science 2016, 353, 594–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protein Preparation Wizard; Schrodinger, LLC.: New York, NY, USA; Prime, Schrodinger, LLC.: New York, NY, USA, 2018.
- Schrödinger Release 2018-1: Glide; Schrödinger, LLC.: New York, NY, USA, 2018.
- Schrödinger Release 2018-1: LigPrep; Schrödinger, LLC.: New York, NY, USA, 2018.
- Schrödinger Release 2018-1: MacroModel; Schrödinger, LLC.: New York, NY, USA, 2018.
- Sterling, T.; Irwin, J.J. ZINC 15–Ligand Discovery for Everyone. J. Chem. Inf. Model. 2015, 55, 2324–2337. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Yang, W.-K.; Kim, H.; Min, B.; Caturla, N.; Jones, J.; Park, Y.-C.; Lee, Y.-C.; Kim, S.-H. Metabolaid® Combination of Lemon Verbena and Hibiscus Flower Extract Prevents High-Fat Diet-Induced Obesity through AMP-Activated Protein Kinase Activation. Nutrients 2018, 10, 1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Compounds | Docking Score * |
|---|---|
| Hib-ester | −5.62 |
| Hib-carbaldehyde | −6.18 |
| RPMI 8226 | |||
| µg/mL | IC50 24 h | IC50 48 h | IC50 72 h |
| HsEF | >3000 | 2356 ± 418 | 1634 ± 115 |
| Hib-ester | 454 ± 57 | 319 ± 38 | 35 ± 2 |
| Hib-carbaldehyde | 208 ± 10 | 85 ± 8 | 38 ± 8 |
| U266.B1 | |||
| µg/mL | IC50 24 h | IC50 48 h | IC50 72 h |
| HsEF | >3000 | 2497 ± 88 | 1837 ± 134 |
| Hib-ester | 640 ± 37 | 387 ± 62 | 272 ± 55 |
| Hib-carbaldehyde | 460 ± 75 | 207 ± 27 | 115 ± 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malacrida, A.; Cavalloro, V.; Martino, E.; Costa, G.; Ambrosio, F.A.; Alcaro, S.; Rigolio, R.; Cassetti, A.; Miloso, M.; Collina, S. Anti-Multiple Myeloma Potential of Secondary Metabolites from Hibiscus sabdariffa—Part 2. Molecules 2021, 26, 6596. https://doi.org/10.3390/molecules26216596
Malacrida A, Cavalloro V, Martino E, Costa G, Ambrosio FA, Alcaro S, Rigolio R, Cassetti A, Miloso M, Collina S. Anti-Multiple Myeloma Potential of Secondary Metabolites from Hibiscus sabdariffa—Part 2. Molecules. 2021; 26(21):6596. https://doi.org/10.3390/molecules26216596
Chicago/Turabian StyleMalacrida, Alessio, Valeria Cavalloro, Emanuela Martino, Giosuè Costa, Francesca Alessandra Ambrosio, Stefano Alcaro, Roberta Rigolio, Arianna Cassetti, Mariarosaria Miloso, and Simona Collina. 2021. "Anti-Multiple Myeloma Potential of Secondary Metabolites from Hibiscus sabdariffa—Part 2" Molecules 26, no. 21: 6596. https://doi.org/10.3390/molecules26216596
APA StyleMalacrida, A., Cavalloro, V., Martino, E., Costa, G., Ambrosio, F. A., Alcaro, S., Rigolio, R., Cassetti, A., Miloso, M., & Collina, S. (2021). Anti-Multiple Myeloma Potential of Secondary Metabolites from Hibiscus sabdariffa—Part 2. Molecules, 26(21), 6596. https://doi.org/10.3390/molecules26216596









