Abstract
In the presence of Cs2CO3, the first simple, efficient, and one-pot procedure for the synthesis of 3,5-diaryl pyridines via a variety of aromatic terminal alkynes with benzamides as the nitrogen source in sulfolane is described. The formation of pyridine derivatives accompanies the outcome of 1,3-diaryl propenes, which are also useful intermediates in organic synthesis. Thus, pyridine ring results from a formal [2+2+1+1] cyclocondensation of three alkynes with benzamides, and one of the alkynes provides one carbon, whilst benzamides provide a nitrogen source only. A new transformation of alkynes as well as new utility of benzamide are found in this work.
1. Introduction
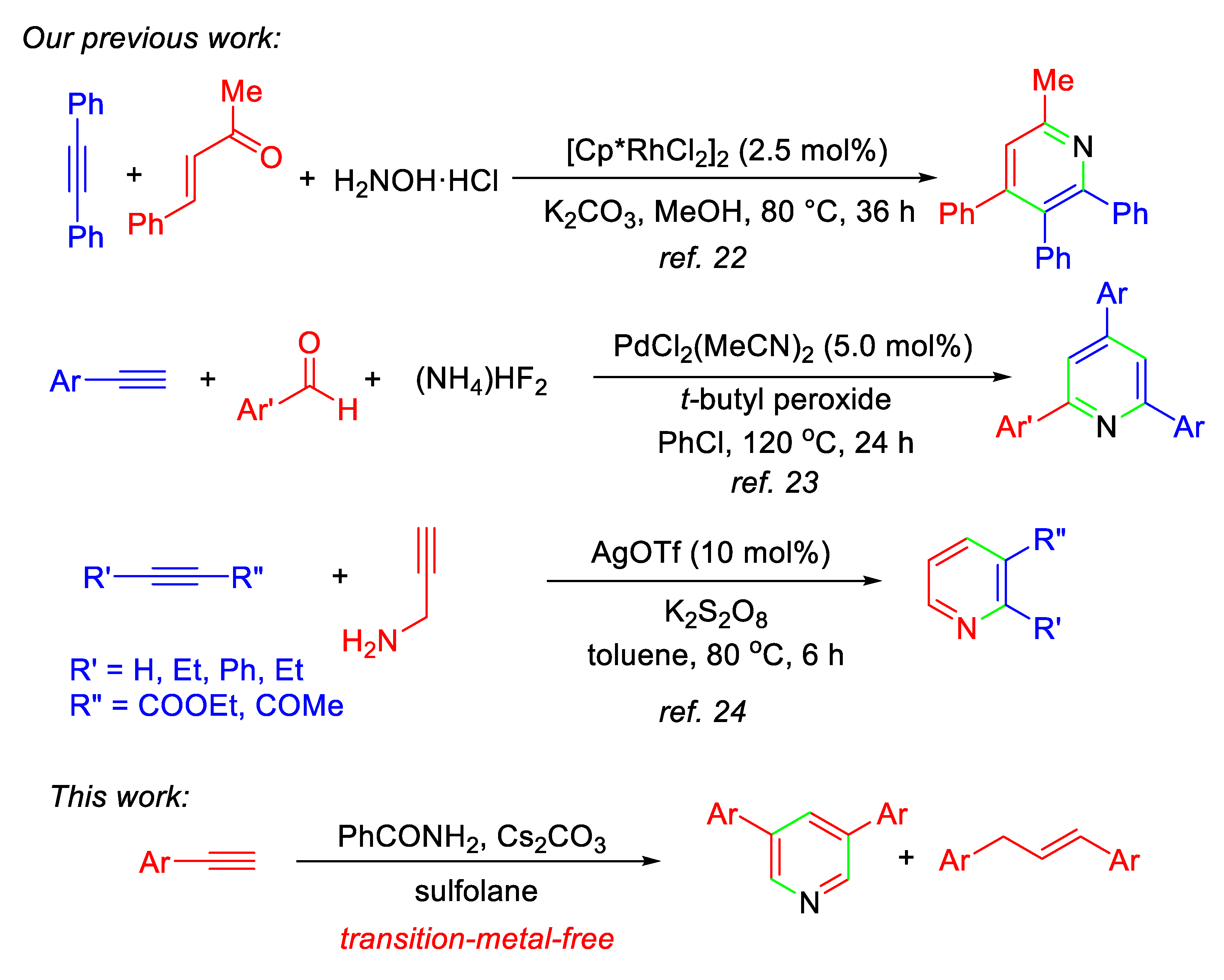
Pyridine derivatives are one of the most important and fundamental six-membered nitrogen-heterocyclic compounds, and pyridine ring is the core structure not only in pharmaceutical compounds [1,2,3,4], but also in natural products [5,6,7]. Therefore, development of synthetic methods for constructing pyridine ring is an interesting and enduring research topic in organic chemistry [5,8,9,10,11,12,13,14,15,16,17]. Among them, alkyne annulation with nitrogen-containing substrates has been well-documented [10,11,12,13,14,17]. We are interested in the development of alkyne annulation protocols for the synthesis of carbo- and heterocyclic compounds in a one-step procedure [18,19,20,21], as well as in the construction of pyridine ring starting from alkynes catalyzed by transition-metal complexes (Scheme 1) [22,23,24]. Encouraged by our recent success in the development of base-promoted formation of C-N and C-O bonds and their applications in the synthesis of heterocyclic compounds [25,26,27,28,29,30], in this paper we report a new protocol for the one-pot formation of 3,5-diaryl pyridines from aromatic terminal alkynes and benzamide promoted by Cs2CO3 in sulfolane, although the synthetic methods of 3,5-diaryl pyridines without use of alkyne have been recently reported [31,32,33]. In this procedure, benzamides are firstly used as the nitrogen source to provide nitrogen atom only, and the reactions also produce 1,3-diarylpropenes as the by-product.
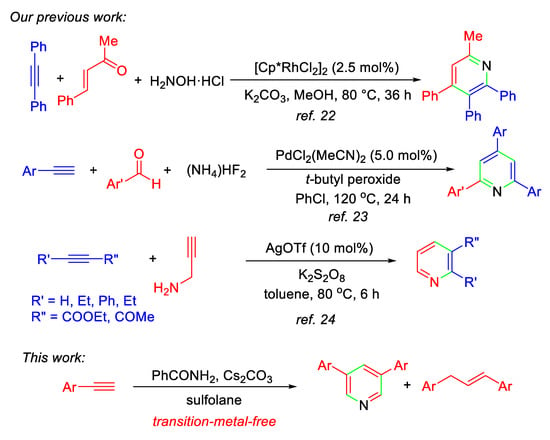
Scheme 1.
One-pot formation of pyridines by alkyne annulation reported by Hua’s group.
2. Results and Discussion
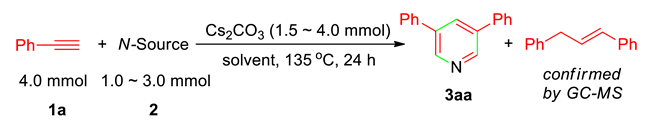
Our initial purposes are to develop a base-promoted cyclocondensation of alkynes with benzamide to form a nitrogen-heterocyclic compound. When the mixture of phenyl acetylene (1a, 2.0 mmol), benzamide (2a, 1.0 mmol), and KOtBu (2.0 mmol) in DMSO (2.0 mL) was heated at 135 °C for 24 h, the analyses of the reaction mixture by GC-MS disclosed the formation of 3,5-diphenylpyridine (3aa) and 1,3-diphenylpropene in trace amount. We are very interested in the formation of 3,5-disubstituted pyridines, since there has never been a report on the synthesis of 3,5-disubstituted pyridines in one-pot manner starting from alkynes; in addition, it is also a new transformation of alkynes. Therefore, we decided to optimize the reaction conditions to establish and provide an efficient synthetic method for access to 3,5-disubstituted pyridines by this new type of alkyne annulation protocol. On the basis of the formation of 3aa and the structure of the by-product, it can be confirmed that 3aa formation requires 4.0 equivalents of 1a as shown in Scheme 1. Therefore, the optimizing reaction conditions were performed with the use of 4.0 mmol of 1a under different conditions.
As shown in Table 1, in DMSO, the reactions of 1a (4.0 mmol) with benzamide (2a, 2.0 mmol) in the presence of 4.0 mmol of KOtBu, KOH, and K2CO3 resulted in a trace amount of 3aa formation, confirmed by GCMS of the reaction mixtures (entries 1–3). In the case of Cs2CO3 used, delightedly, 3aa could be isolated from the reaction mixture in 20% yield (the yield was based on the total amount of 1a used, entry 4). The structure of 3aa was confirmed by its NMR spectroscopic data, and the formation of the pyridine ring was unambiguously confirmed by its x-ray diffraction studies (3,5-Diphenyl pyridine (3aa) is a known compound, the structure was confirmed by its spectroscopic data and x-ray diffrac-tion studies. See Supplementary File and CCDC Number 2112477). The utility of other solvents, such as THF, 1,4-dioxane, DMF, and DMAc (N,N-dimethylacetamide) gave the trace amount and low yield of 3aa (entries 5–8). In addition, sulfolane (tetramethylene sulfone, or 2,3,4,5-tetrahydrothiophen-1,1-dioxide, undried) is a highly stable and versatile dipolar aprotic laboratory and industrial solvent commonly used in organic synthesis to drastically enhance the rate and selectivity [34], and this solvent is expected to have superior solubility for alkali metal salts [35]. Thus, we repeated the reactions in sulfolane with the use of different amounts of 2a; Cs2CO3, the desired 3aa, was obtained in low to good yields (entries 9–12). Among them, the best reaction conditions of 2a (1.0 mmol) and Cs2CO3 (2.5 mmol) produced 3aa in 77% yield (based on half the amount of 1a used) (entry 11). In this case, the by-product of 1,3-diphenyl propene, which has highly potential application and is not easily available by traditional organic synthesis, was isolated in a comparable yield (75%, based on half the amount of 1a used). (Their 1H, 13C NMR spectroscopic data and/or GC-MS are reported in Supplementary File), accompanied with the formation of benzoic acid (confirmed by GCMS after work-up by the addition of water, it is not isolated) (see in Supplementary File). With the use of 1.0 mmol of Cs2CO3, repeating the reactions in DMSO, DMF, formamide, and DMAc again gave either a low yield of 3aa, or no product at all (entries 13–16). If 2a was replaced by NH4OAc or acetamide as the nitrogen source, no desired product was formed (entries 17–18). Acetamide does not work, which is maybe due to being more basic than benzamide [36]. In addition, no product formation was observed in the absence of a base (entry 19).

Table 1.
Optimizing reaction conditions for 3,5-diphenylpyridine (3aa) formation a.

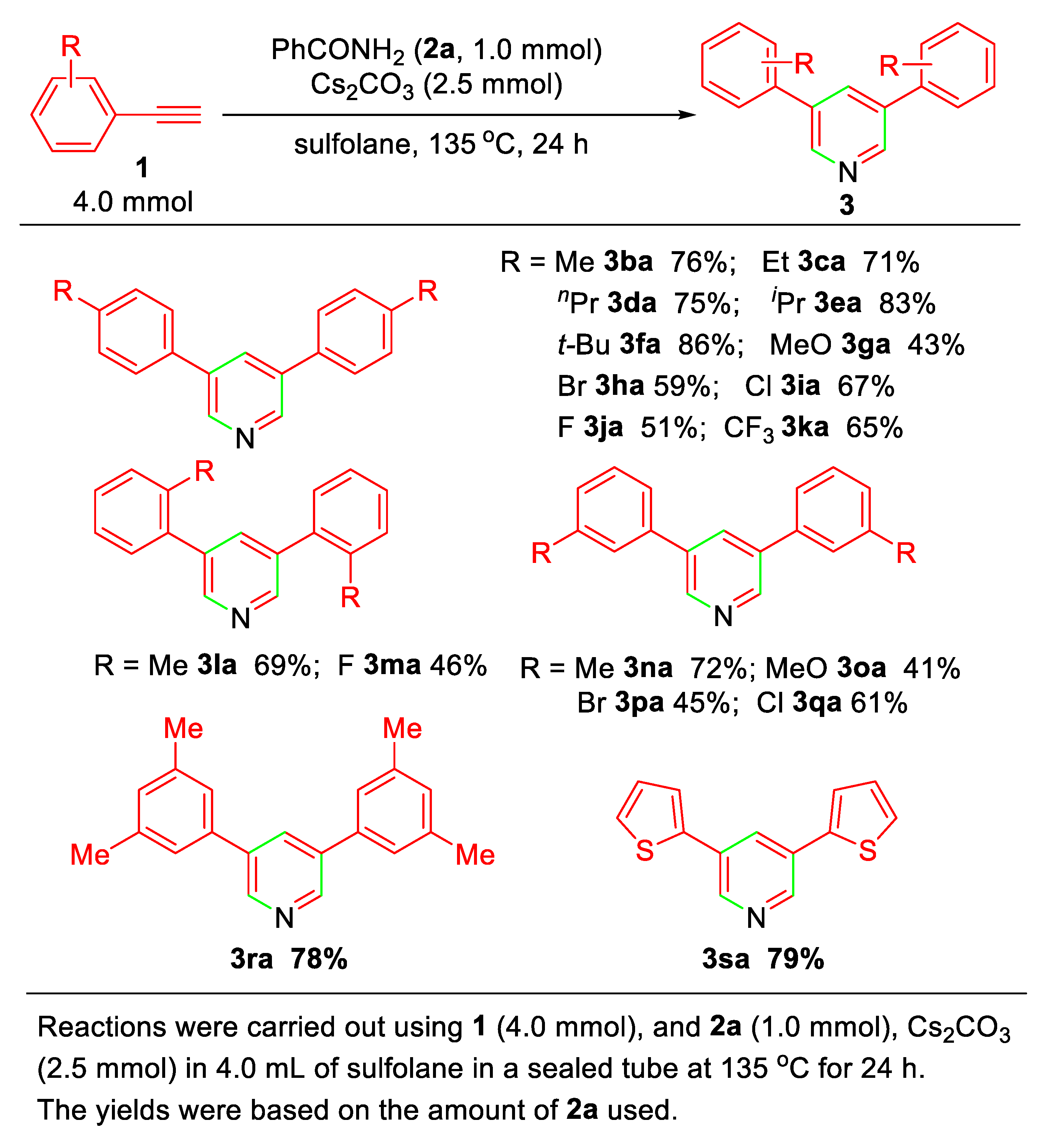
Under the optimized reaction conditions (entry 11 of Table 1), the substrate scope of the present alkyne annulation with 2a affording 3,5-disubstituted pyridines was then investigated. As concluded in Scheme 2, aromatic terminal alkynes bearing electron-donating (alkyl group) and electron-withdrawing substituents (Cl, F, CF3) could undergo the annulation to give the corresponding pyridines 3ba~3sa in moderate to good yields, but an apparent dependence of the electronic effects of the substituents in aromatic terminal alkynes was observed. Thus, alkynes 1b~1f, 1l, 1n, and 1r bearing electron-donating groups (R = Me, Et, nPr, iPr, and t-Bu) at the para-, ortho-, or meta-position show the higher reactivity compared to the halogen-substituted alkynes (R = Br, Cl, F, 1h~1k, 1m, 1p~1q).
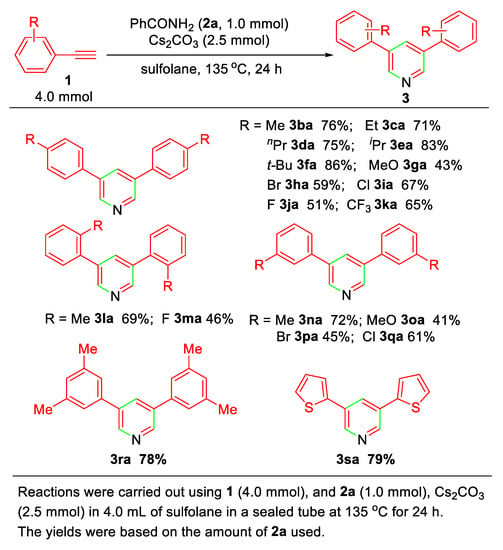
Scheme 2.
Substrate scope of pyridine synthesis.
The present annulation was also applicable to heteroaromatic terminal alkynes. For example, subjecting 2-ethynylthiophene (1s) to the standard reaction conditions afforded 3,5-di(thiophen-2-yl)pyridine (3sa) in 79% yield.
It should be noted that under the standard reaction conditions, two exceptions of the substituent effect were observed. The electron-rich para-methoxyphenylacetylenes (1g) and meta-methoxyphenylacetylenes (1o) show comparatively low reactivity to give the corresponding pyridines in 43% and 41% yields, respectively. For that reason, the considerable amount of enamine derivatives that resulted from the addition reaction of 1g or 1o with 2a under basic conditions was detected in the reaction mixtures by GCMS, which is due to the strong electron-donating methoxy group that suppresses the formation of amide anion for further transformation (vide infra).
In addition, it is notable that either aliphatic terminal alkynes or internal alkynes, as well as 1-ethynyl-4-nitrobenzene used as the substrates, resulted in no formation of pyridine derivatives at all.
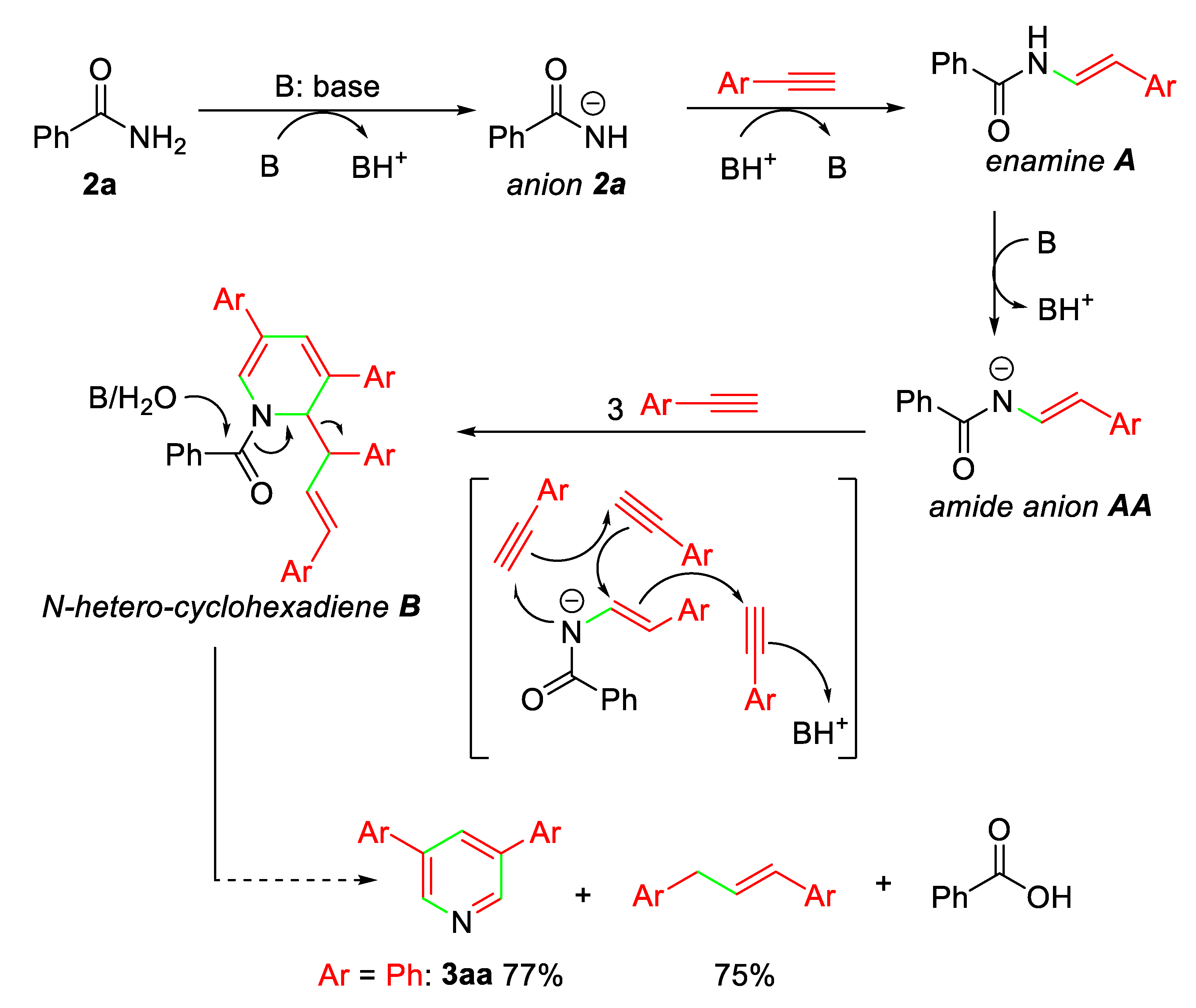
On the basis of the 3,5-diaryl pyridine and 1,3-diaryl propene formation, a proposed mechanism for the present base-promoted annulation of alkynes with benzamides to form pyridine ring is depicted in Scheme 3. It involves the common addition reaction of benzamides to terminal alkyne promoted by the base to give enamine intermediate A, which forms amide anion AA and then undergoes cycloaddition with three alkynes to afford nitrogen-heterocyclohexadiene intermediate B. Under basic conditions and with a small amount of water (wet solvent), intermediate B is considered to be converted into 3,5-diaryl pyridine and 1,3-diaryl propene via cleavage of C-N and C-C bonds.
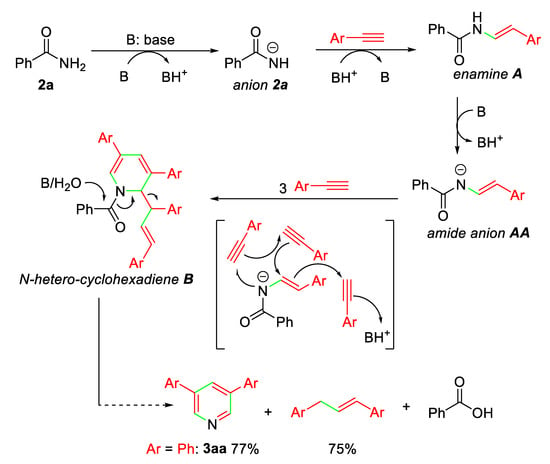
Scheme 3.
Possible mechanism for pyridine formation.
According to the proposed mechanism, the formation of 3aa and 1,3-diphenyl propene in the comparable yields (77% vs 75%) is easily understood. Also, the low reactivity of electron-rich para-methoxyphenylacetylenes (1g) and meta-methoxyphenylacetylenes (1o) is reasonably due to the strong electron-donating methoxy group that decreases the formation of amide anion AA (Ar = para-/meta-MeOC6H4) for further transformation.
Since acetamide is more basic than benzamide [36], when it was used as the nitrogen source, 3aa could not form due to the difficult formation of anion 2a (Table 1, entry 18).
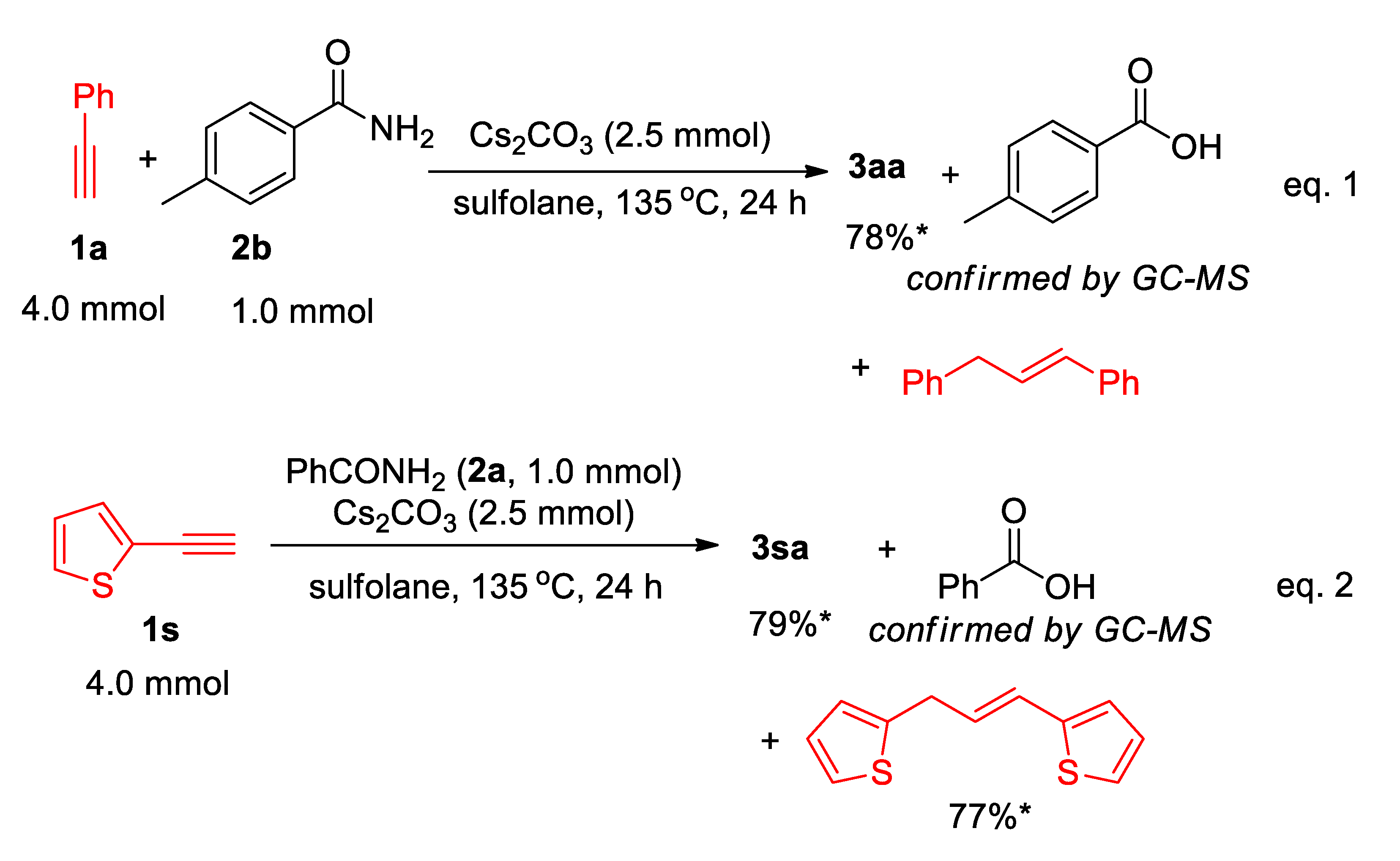
In addition, the reactions of 1a with para-methylbenzamide (2b) gave 3aa (78% yield), para-methylbenzoic acid (see in Supplementary File), and 1,3-diphenylpropene, which confirm again that the benzamide 2b is used as the nitrogen source only (Scheme 4 (eq.1)). Moreover, in the formation of 3sa (Scheme 2), the corresponding (E)-2,2′-(prop-1-ene-1,3-diyl)dithiophene was isolated in 77% yield (see in Supplementary File), and the yield is also comparable to the yield of 3sa (79%) (Scheme 4 (eq.2)).
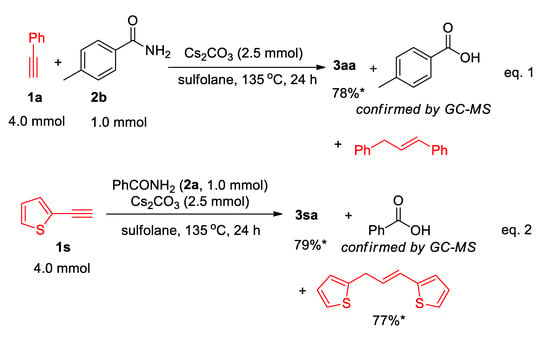
Scheme 4.
Isolation and confirmation of by-product. *: the yields were calculated on the basis of the half amount of alkyne used.
3. Materials and Methods
3.1. General Methods
All commercial reagents are analytically pure and used directly without further purification. Nuclear magnetic resonance (NMR) spectra were recorded on an ECA-400 spectrometer (JEOL, Tokyo, Japan) using CDCl3 as solvent at 298 K. 1H NMR (400 MHz) chemical shifts (δ) were referenced to internal standard TMS (for 1H, δ = 0.00 ppm). 13C NMR (100 MHz) chemical shifts were referenced to internal solvent CDCl3 (for 13C, δ = 77.16 ppm). Mass spectra (MS) were obtained on a GC-MS-QP2010S (Shimadzu, Tokyo, Japan) with a PEG-25M column, and the high-resolution mass spectra (HRMS) with electron spray ionization (ESI) were obtained with a micrOTOF-Q spectrometer (Agilent, California, CA, USA).
3.2. Typical Experimental Procedure for the Synthesis of 3,5-Diphenyl Pyridine (3aa)
A mixture of phenylacetylene (1a, 408.1 mg, 4.0 mmol), benzamide (2a, 121.5 mg, 1.0 mmol), and Cs2CO3 (815.1 mg, 2.5 mmol) in sulfolane (4.0 mL) in a 25 mL screw-capped thick-walled Pyrex tube was stirred at 135 °C for 24 h in an oil bath. After the reaction mixture was cooled to room temperature, it was poured into a solvent mixture of water (50.0 mL) and ethyl acetate (25.0 mL), and the two phases were then separated. The aqueous layer was extracted with ethyl acetate (3 × 15.0 mL), and the combined organic extracts were dried over anhydrous Mg2SO4. After removing the solvent under reduced pressure, the residue was purified by column chromatography on silica gel with petroleum ether/ethyl acetate (gradient mixture ratio from 100:0 to 90:10) as eluent to afford 3aa as a white solid (177.9 mg, 77%). (E)-1,3-diphenylpropene was isolated in 75% yield.
The structural characterization data for all the products are reported in the Supplementary Materials.
4. Conclusions
In summary, the present work provides a simple and efficient method for the synthesis of 3,5-diaryl pyridines by Cs2CO3-promoted annulation of aromatic terminal alkynes with benzamides as nitrogen sources in sulfolane, along with the formation of 1,3-diaryl propenes as by-product. Noteworthy features of this procedure include the one-pot manner with a wide range of readily available alkynes under transition-metal-free conditions to afford 3,5-diaryl pyridines with high chemoselectivity, and benzamides were firstly used as nitrogen sources in the construction of pyridine. In addition, the present pyridine ring formation resulted from a formal [2+2+1+1] cyclocondensation of three alkynes and benzamides, one of the alkynes provided one carbon and benzamides provided the nitrogen atom. This is a new transformation of alkyne and shows the new utility of benzamides in organic synthesis.
Supplementary Materials
The following are available online at www.mdpi.com/link. The characterization data of the known products, copies of 1H and 13C NMR charts of all products, X-ray structural details of 3aa, and part by-product’s NMR spectroscopic data and GC-MS.
Author Contributions
Investigation, writing—original draft preparation, H.M.; investigation, H.M. and M.A.I.; discussion, M.N.A.; conceptualization, supervision, writing—review and editing, R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (21673124, 21972072).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
H.M. and M.A.I. thank the China Scholarship Council (CSC) for offering generous support for their study at Tsinghua University as PhD candidates.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
The sample of compounds are not available from authors.
References
- Baumann, M.; Baxendale, I.R. An overview of the synthetic routes to the best-selling drugs containing 6-membered heterocycles. Beilstein J. Org. Chem. 2013, 9, 2265–2319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altaf, A.A.; Shahzad, A.; Gul, Z.; Rasool, N.; Badshah, A.; Lal, B.; Khan, E. A review on the medicinal importance of pyridine derivatives. J. Drug Des. Med. Chem. 2015, 1, 1–11. [Google Scholar]
- Prachayasittikul, S.; Pingaew, R.; Worachartcheewan, A.; Sinthupoom, N.; Prachayasittikul, V.; Ruchirawat, S.; Prachayasittikul, V. Roles of pyridine and pyrimidine derivatives as privileged scaffolds in anticancer agents. Mini-Rev. Med. Chem. 2017, 17, 869–901. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, H.A.; Moustafa, A.H.; El-Torky, A.E.; Abd El-Salam, E.A. A series of pyridines and pyridine-based sulfa-drugs as antimicrobial agents: Design, synthesis and antimicrobial activity. Russ. J. Gen. Chem. 2017, 87, 2401–2408. [Google Scholar] [CrossRef]
- Bagley, M.C.; Glover, C.; Merritt, E.A. The Bohlmann–Rahtz pyridine synthesis: From discovery to applications. Synlett 2007, 2459–2482. [Google Scholar] [CrossRef]
- Aida, W.; Ohtsuki, T.; Li, X.; Ishibashi, M. Isolation of new carbamate- or pyridine-containing natural products, fuzanins A, B, C, and D from Kitasatospora sp. IFM10917. Tetrahedron 2009, 65, 369–373. [Google Scholar] [CrossRef]
- Hudson, G.A.; Hooper, A.R.; DiCaprio, A.J.; Sarlah, D.; Mitchell, D.A. Structure prediction and synthesis of pyridine-based macrocyclic peptide natural products. Org. Lett. 2021, 23, 253–256. [Google Scholar] [CrossRef]
- Nakamura, I.; Yamamoto, Y. Transition-metal-catalyzed reactions in heterocyclic synthesis. Chem. Rev. 2004, 104, 2127–2198. [Google Scholar] [CrossRef]
- Henry, G.D. De novo synthesis of substituted pyridines. Tetrahedron 2004, 60, 6043–6061. [Google Scholar] [CrossRef]
- Heller, B.; Hapke, M. The fascinating construction of pyridine ring systems by transition metalcatalysed [2+2+2] cycloaddition reactions. Chem. Soc. Rev. 2007, 36, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Maryanoff, B.E.; Zhang, H.-C. Pyridine-containing macrocycles via cobalt-mediated [2+2+2] cycloadditions of α,ω-bis-alkynes. Arkivoc 2007, 7–35. [Google Scholar] [CrossRef] [Green Version]
- Varela, J.A.; Saá, C. Recent advances in the synthesis of pyridines by transition-metal-catalyzed [2+2+2] cycloaddition. Synlett 2008, 2571–2578. [Google Scholar]
- Hill, M.D. Recent strategies for the synthesis of pyridine derivatives. Chem. Eur. J. 2010, 16, 12052–12062. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.; Abrenica, M.V.A.; Wang, P. Cycloaddition of alkynes: Atom-economic protocols for constructing six-membered cycles. Curr. Org. Chem. 2011, 15, 712–729. [Google Scholar] [CrossRef]
- Gulevich, A.V.; Dudnik, A.S.; Chernyak, N.; Gevorgyan, V. Transition metal-mediated synthesis of monocyclic aromatic heterocycles. Chem. Rev. 2013, 113, 3084–3213. [Google Scholar] [CrossRef] [Green Version]
- Allais, C.; Grassot, J.-M.; Rodriguez, J.; Constantieux, T. Metal-free multicomponent syntheses of pyridines. Chem. Rev. 2014, 114, 10829–10868. [Google Scholar] [CrossRef] [PubMed]
- Vessally, E.; Hosseinian, A.; Edjlali, L.; Bekhradnia, A.; Esrafili, M.D. New page to access pyridine derivatives: Synthesis from N-propargylamines. RSC Adv. 2016, 6, 71662–71675. [Google Scholar] [CrossRef]
- Zheng, L.; Hua, R. C–H activation and alkyne annulation via automatic or intrinsic directing groups: Towards high step economy. Chem. Rec. 2018, 18, 556–569. [Google Scholar] [CrossRef]
- Zheng, L.; Hua, R. Recent advances in construction of polycyclic natural product scaffolds via one-pot reactions involving alkyne annulation. Front. Chem. 2020, 8, 580355. [Google Scholar] [CrossRef]
- Hua, R. Isoquinolone syntheses by annulation protocols. Catalysts 2021, 11, 620. [Google Scholar] [CrossRef]
- Zhou, Y.; Hua, R. Synthesis of 1-benzyl-, 1-alkoxyl-, and 1-aminoisoquinolines via rhodium(III)-catalyzed aryl C−H activation and alkyne annulation. J. Org. Chem. 2021, 86, 8862–8872. [Google Scholar] [CrossRef]
- Zheng, L.; Ju, J.; Bin, Y.; Hua, R. Synthesis of isoquinolines and heterocycle-fused pyridines via three-component cascade reaction of aryl ketones, hydroxylamine, and alkynes. J. Org. Chem. 2012, 77, 5794–5800. [Google Scholar] [CrossRef]
- Wang, P.; Hua, R.; Li, C.-J. One-pot synthesis of unsymmetrical 2,4,6-triaryl pyridines by the oxidative cyclocondensation of benzaldehydes, aromatic alkynes and ammonium bifluoride. Curr. Org. Synth. 2013, 10, 655–660. [Google Scholar] [CrossRef]
- Nizami, T.A.; Hua, R. Silver-catalyzed chemoselective annulation of propargyl amines with alkynes for access to pyridines and pyrroles. Tetrahedron 2017, 73, 6080–6084. [Google Scholar] [CrossRef]
- Su, J.; Chen, Q.; Lu, L.; Ma, Y.; Auyoung, G.H.L.; Hua, R. Base-promoted nucleophilic fluoroarenes substitution of C-F bonds. Tetrahedron 2018, 74, 303–307. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Lu, L.; Mehmood, H.; Khan, D.M.; Hua, R. Quinazolinone synthesis through base-promoted SNAr reaction of ortho-fluorobenzamides with amides followed by cyclization. ACS Omega 2019, 4, 8207–8213. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.A.; Mehmood, H.; Lv, J.; Hua, R. Base-promoted SNAr reactions of fluoro- and chloroarenes as a route to N-aryl indoles and carbazoles. Molecules 2019, 24, 1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Wang, Y.; Hua, R. Base-promoted chemodivergent formation of 1,4-benzoxazepin-5(4H)-ones and 1,3-benzoxazin-4(4H)-ones switched by solvents. Molecules 2019, 24, 3773. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, H.; Iqbal, M.A.; Lu, L.; Hua, R. Base-promoted annulation of amidoximes with alkynes: Simple access to 2,4-disubstituted imidazoles. Molecules 2020, 25, 3621. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Lu, L.; Mehmood, H.; Hua, R. Biaryl formation via base-promoted direct coupling reactions of arenes with aryl halides. ACS Omega 2021, 6, 15981–15987. [Google Scholar] [CrossRef]
- Li, Z.; Huang, X.; Chen, F.; Zhang, C.; Wang, X.; Jiao, N. Cu-catalyzed concise synthesis of pyridines and 2-(1H)-pyridones from acetaldehydes and simple nitrogen donors. Org. Lett. 2015, 17, 584–587. [Google Scholar] [CrossRef]
- Sathish, M.; Chetna, J.; Krishna, N.H.; Shankaraiah, N.; Alarifi, A.; Kamal, A. Iron-mediated one-pot synthesis of 3,5-diarylpyridines from β-nitrostyrenes. J. Org. Chem. 2016, 81, 2159–2165. [Google Scholar] [CrossRef]
- Ranjani, G.; Nagarajan, R. Insight into copper catalysis: In situ formed nano Cu2O in Suzuki-Miyaura cross-coupling of aryl/indolyl boronates. Org. Lett. 2017, 19, 3974–3977. [Google Scholar] [CrossRef]
- Tilstam, U. Sulfolane: A versatile dipolar aprotic solvent. Org. Process Res. Dev. 2012, 16, 1273–1278. [Google Scholar] [CrossRef]
- Kurc, B. Sulfolane with LiPF6, LiNTf2 and LiBOB—as a non-flammable electrolyte working in a lithium-ion batteries with a LiNiO2 cathode. Int. J. Electrochem. Sci. 2018, 13, 5938–5955. [Google Scholar] [CrossRef]
- Cox, R.A.; Druet, L.M.; Klausner, A.E.; Modro, T.A.; Wan, P.; Yates, K. Protonation acidity constants for some benzamides, acetamides, and lactams. Can. J. Chem. 1980, 59, 1568–1573. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).