Sustainable Hues: Exploring the Molecular Palette of Biowaste Dyes through LC-MS Metabolomics
Abstract
:1. Introduction
2. Results
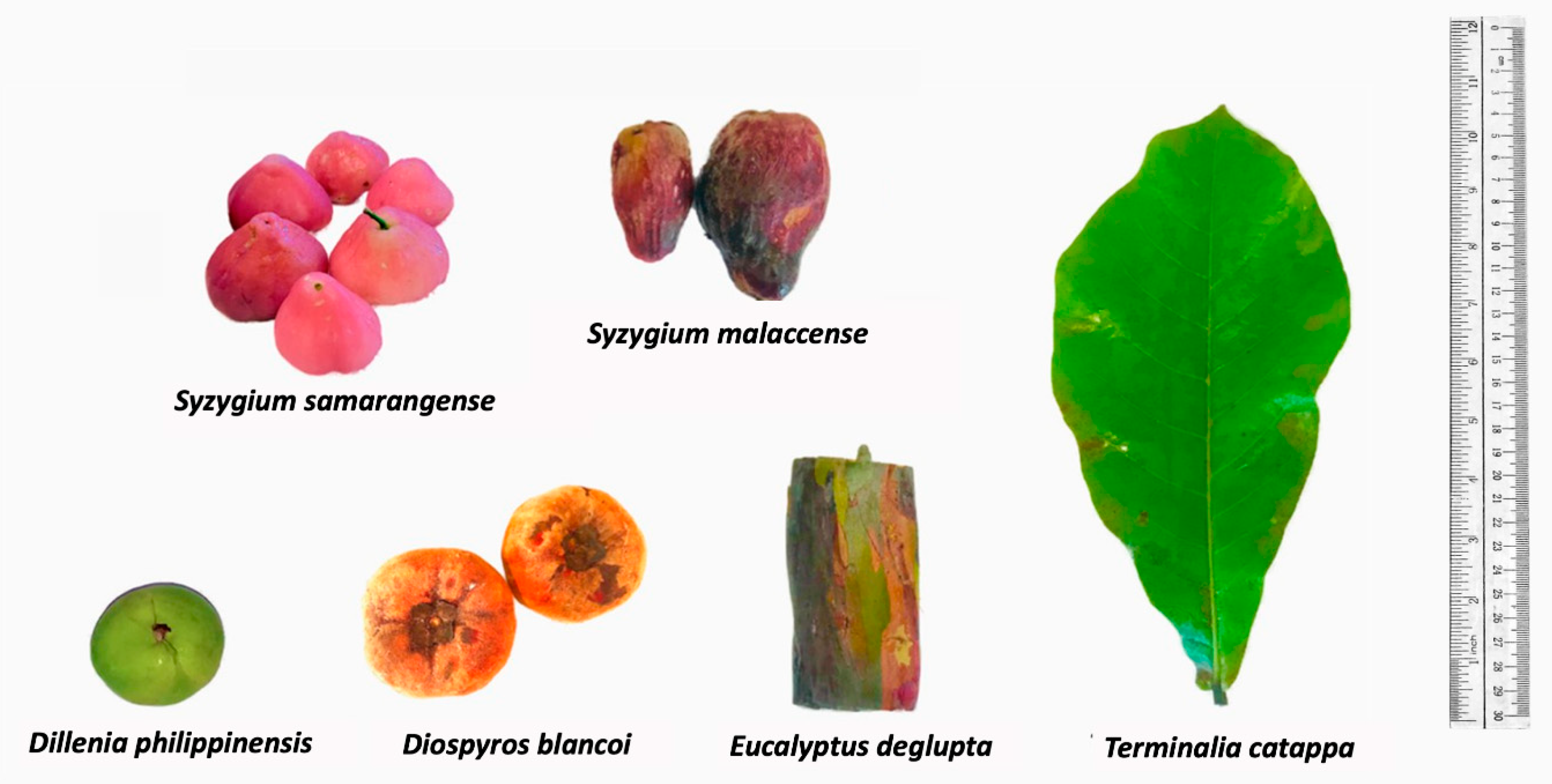
2.1. Extraction of Biowaste Natural Dyes and Dyeing on Textiles
2.2. UPLC®-ESI qTOF Profiling, Molecular Networking and Structural Analysis of Small Molecules from Extracted Natural Dyes
2.3. Chemometrics Analysis of Natural Dye Samples
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Extraction of Natural Dyes
4.3. LC-MS Analysis
4.4. Bioinformatics Analysis
4.5. UV-Vis Spectrophotometric Analysis
4.6. Textile Dyeing and Colorimetric Evaluation
4.7. Multivariate Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Adeel, S.; Amin, N.; Fazal-ur-Rehman; Ahmad, T.; Batool, F.; Hassan, A. Sustainable Isolation of Natural Dyes from Plant Wastes for Textiles. In Recycling from Waste in Fashion and Textiles; Pintu, P., Shakeel, A., Kunal, S., Sanjay, S., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 363–390. [Google Scholar]
- Khattab, T.A.; Abdelrahman, M.S.; Rehan, M. Textile Dyeing Industry: Environmental Impacts and Remediation. Environ. Sci. Pollut. Res. 2020, 27, 3803–3818. [Google Scholar] [CrossRef]
- Mussak, R.A.M.; Bechtold, T. Natural Colorants in Textile Dyeing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Lagashetti, A.C.; Dufossé, L.; Singh, S.K.; Singh, P.N. Fungal Pigments and Their Prospects in Different Industries. Microorganisms 2019, 7, 604. [Google Scholar] [CrossRef] [Green Version]
- Adeel, S.; Rehman, F.; Pervaiz, M.; Hussaan, M.; Amin, N.; Majeed, A.; Rehman, H. Microwave Assisted Green Isolation of Laccaic Acid from Lac Insect (Kerria Lacca) for Wool Dyeing. Prog. Color. Color. Coat. 2021, 14, 293–299. [Google Scholar] [CrossRef]
- Adeel, S.; Rafi, S.; Mustaan, M.A.; Salman, M.; Ghaffar, A. Animal Based Natural Dyes: A Short Review. In Handbook of Renewable Materials for Coloration and Finishing; Yusuf, M., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 41–74. [Google Scholar] [CrossRef]
- Mahdi, M.M.; Tuj-Zohra, F.; Ahmed, S. Dyeing of Shoe Upper Leather with Extracted Dye from Acacia Nilotica Plant Bark-An Eco-Friendly Initiative. Prog. Color. Color. Coat. 2020, 14, 241–258. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Veloso, C.M. Microencapsulation of natural dyes with biopolymers for application in food: A review. Food Hydrocoll. 2021, 112, 106374. [Google Scholar] [CrossRef]
- Patil, N.N.; Datar, A.G. Applications of natural dye from Ixora coccinea L. in the field of textiles and cosmetics. Color. Technol. 2016, 132, 98–103. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Yook-Heng, L.; Salam, F.; Mat Zaid, M.H.; Abu-Hanifah, S. A Colorimetric pH Sensor Based on Clitoria sp and Brassica sp for Monitoring of Food Spoilage Using Chromametry. Sensors 2019, 19, 4813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richhariya, G.; Kumar, A.; Tekasakul, P.; Gupta, B. Natural dyes for dye sensitized solar cell: A review. Renew. Sust. Energ. Rev. 2017, 69, 705–718. [Google Scholar] [CrossRef]
- Labrador, T.; Paz-Tauro, M.; Robis, E. Hibla Ng Lahing Filipino: The Artistry of Philippine Textiles; National Museum: Manila, Philippines, 2013; ISBN 9789715670210. [Google Scholar]
- Habal, L.; de Guzman, Z. Gampol: A Compendium of Philippine Dye Yielding Plants and Their Textile Application; Philipphine Textile Research Institute, Department of Science and Technology (DOST): Makati City, Philippines, 2003; Volume 1, ISBN 9718551344. [Google Scholar]
- Leaño, J.R. A Compendium of Philippine Dye-Yielding Plants and Their Extraction and Textile Application Technologies; Philipphine Textile Research Institute, Department of Science and Technology (DOST): Makati City, Philippines, 2008; Volume 2. [Google Scholar]
- Davidson, J. Use of Principal Components, Factor Analysis and Varimax Rotation to Describe Variability in Wood of Eucalyptus Deglupta Blume. Wood Sci. Technol. 1975, 9, 275–291. [Google Scholar] [CrossRef]
- Vankar, P.S. Natural Dyes for Textiles: Sources, Chemistry, and Applications, 1st ed.; Woodhead Publishing: Sawston, UK, 2017; ISBN 9780081018842. [Google Scholar] [CrossRef]
- El-Zawahry, M.M.; El-Shami, S.; El-Mallah, M.H. Optimizing a Wool Dyeing Process with Reactive Dye by Liposome Microencapsulation. Dyes Pigm. 2007, 74, 684–691. [Google Scholar] [CrossRef]
- Molino, R.J.E.J.; Junio, H.A. Profiling the Philippine Blue: Liquid Chromatography/Mass Spectrometry-Based Metabolomics Study on Philippine Indigofera. Rapid Commun. Mass Spectrom. 2021, 35, e9037. [Google Scholar] [CrossRef]
- Zhou, B.; Xiao, J.F.; Tuli, L.; Ressom, H.W. LC-MS-Based Metabolomics. Mol. Biosyst. 2012, 8, 470–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with GNPS. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [Green Version]
- Tautenhahn, R.; Patti, G.J.; Rinehart, D.; Siuzdak, G. XCMS Online: A Web-Based Platform to Process Untargeted Metabolomic Data. Anal. Chem. 2012, 84, 5035–5039. [Google Scholar] [CrossRef] [Green Version]
- Náthia-Neves, G.; Vardanega, R.; Meireles, M.A.A. Extraction of natural blue colorant from Genipa americana L. using green technologies: Techno-economic evaluation. Food Bioprod. Process. 2019, 114, 132–143. [Google Scholar] [CrossRef]
- Veldkamp, J.F. Nomenclature of Syzygium gracile (Myrtaceae). Blumea 2003, 48, 489–490. [Google Scholar] [CrossRef]
- Dacanay, F.; Ladra, M.; Junio, H.; Nellas, R. Molecular Affinity of Mabolo Extracts to an Octopamine Receptor of a Fruit Fly. Molecules 2017, 22, 1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loum, J.; Byamukama, R.; Wanyama, P.A.G. Efficient Extraction of Natural Dyes from Selected Plant Species. Chemistry Africa 2021, 4, 677–689. [Google Scholar] [CrossRef]
- Ramos AE, F.; Evanno, L.; Poupon, E.; Champy, P.; Beniddir, M.A. Natural Products Targeting Strategies Involving Molecular Networking: Different Manners, One Goal. Nat. Prod. Rep. 2019, 36, 960–980. [Google Scholar] [CrossRef] [PubMed]
- Nothias, L.F.; Nothias-Esposito, M.; da Silva, R.; Wang, M.; Protsyuk, I.; Zhang, Z.; Sarvepalli, A.; Leyssen, P.; Touboul, D.; Costa, J.; et al. Bioactivity-Based Molecular Networking for the Discovery of Drug Leads in Natural Product Bioassay-Guided Fractionation. J. Nat. Prod. 2018, 81, 758–767. [Google Scholar] [CrossRef] [Green Version]
- Montoro, P.; Tuberoso, C.I.G.; Perrone, A.; Piacente, S.; Cabras, P.; Pizza, C. Characterisation by Liquid Chromatography-Electrospray Tandem Mass Spectrometry of Anthocyanins in Extracts of Myrtus Communis L. Berries Used for the Preparation of Myrtle Liqueur. J. Chromatogr. A 2006, 1112, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Cuyckens, F.; Claeys, M. Mass Spectrometry in the Structural Analysis of Flavonoids. J. Mass. Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef]
- Kleinenkuhnen, N.; Büchel, F.; Gerlich, S.C.; Kopriva, S.; Metzger, S. A Novel Method for Identification and Quantification of Sulfated Flavonoids in Plants by Neutral Loss Scan Mass Spectrometry. Front. Plant Sci. 2019, 10, 885. [Google Scholar] [CrossRef]
- Chang, Z.; Zhang, Q.; Liang, W.; Zhou, K.; Jian, P.; She, G.; Zhang, L. A Comprehensive Review of the Structure Elucidation of Tannins from Terminalia Linn. Evid. Based Complementary Altern. Med. 2019, 2019, 8623909. [Google Scholar] [CrossRef] [Green Version]
- Zaccaron, S.; Ganzerla, R.; Bortoluzzi, M. Iron Complexes with Gallic Acid: A Computational Study on Coordination Compounds of Interest for the Preservation of Cultural Heritage. J. Coord. Chem. 2013, 66, 1709–1719. [Google Scholar] [CrossRef]
- Waridel, P.; Wolfender, J.L.; Ndjoko, K.; Hobby, K.R.; Major, H.J.; Hostettmann, K. Evaluation of Quadrupole Time-of-Flight Tandem Mass Spectrometry and Ion-Trap Multiple-Stage Mass Spectrometry for the Differentiation of C-Glycosidic Flavonoid Isomers. J. Chromatogr. A 2001, 926, 29–41. [Google Scholar] [CrossRef]
- Brazier-Hicks, M.; Evans, K.M.; Gershater, M.C.; Puschmann, H.; Steel, P.G.; Edwards, R. The C-Glycosylation of Flavonoids in Cereals. J. Biol. Chem. 2009, 284, 17926–17934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oelrichs, P.B.; Pearce, C.M.; Zhu, J.; Filippich, L.J. Isolation and Structure Determination of Terminalin a Toxic Condensed Tannin from Terminalia Oblongata. Nat. Toxins 1994, 2, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Deinzer, M.L. Tandem Mass Spectrometry for Sequencing Proanthocyanidins. Anal. Chem. 2007, 79, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, S.; Kumar, B. LC-MS Identification of Proanthocyanidins in Bark and Fruit of Six Terminalia Species. Nat. Prod. Commun. 2018, 13, 555–560. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, R.; Jayasinghe, L.; Kuhnert, N. Identification and Characterization of Proanthocyanidins of 16 Members of the Rhododendron Genus (Ericaceae) by Tandem LC-MS. J. Mass. Spectrom. 2012, 47, 502–515. [Google Scholar] [CrossRef]
- Matsubara, T.; Taniguchi, S.; Morimoto, S.; Yano, A.; Hara, A.; Wataoka, I.; Urakawa, H.; Yasunaga, H. Relationship between Dyeing Condition and Dyeability in Hair Colouring by Using Catechinone Prepared Enzymatically or Chemically from (+)-Catechin. J. Cosmet. Dermatol. Sci. Appl. 2015, 5, 94–106. [Google Scholar] [CrossRef] [Green Version]
- van den Berg, R.A.; Hoefsloot, H.C.J.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom 2006, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Ghouila, H.; Meksi, N.; Haddar, W.; Mhenni, M.F.; Jannet, H.B. Extraction, Identification and Dyeing Studies of Isosalipurposide, a Natural Chalcone Dye from Acacia Cyanophylla Flowers on Wool. Ind. Crop. Prod. 2012, 35, 31–36. [Google Scholar] [CrossRef]
- Formica, J.V.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Dyes and Pigments: Their Structure and Properties. In Dyes and Pigments; Springer International Publishing: Cham, Switzerland, 2016; pp. 13–29. [Google Scholar]
- Ge, X.; Timrov, I.; Binnie, S.; Biancardi, A.; Calzolari, A.; Baroni, S. Accurate and Inexpensive Prediction of the Color Optical Properties of Anthocyanins in Solution. J. Phys. Chem. A. 2015, 119, 3816–3822. [Google Scholar] [CrossRef]
- Bancirova, M. Changes of the Quercetin Absorption Spectra in Dependence on Solvent. Chemistry 2015, 1, 31–34. [Google Scholar]
- Park, H.R.; Daun, Y.; Park, J.K.; Bark, K.M. Spectroscopic Properties of Flavonoids in Various Aqueous-Organic Solvent Mixtures. Bull. Korean Chem. Soc. 2013, 34, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Cysewski, P.; Jeliński, T.; Przybyłek, M.; Shyichuk, A. Color Prediction from First Principle Quantum Chemistry Computations: A Case of Alizarin Dissolved in Methanol. New J. Chem. 2012, 36, 1836–1843. [Google Scholar] [CrossRef]
- Malcioǧlu, O.B.; Calzolari, A.; Gebauer, R.; Varsano, D.; Baroni, S. Dielectric and Thermal Effects on the Optical Properties of Natural Dyes: A Case Study on Solvated Cyanin. J. Am. Chem. Soc. 2011, 133, 15425–15433. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Anthocyanin Food Colorant and Its Application in PH-Responsive Color Change Indicator Films Change Indicator Films. Crit Rev. Food. 2020, 61, 2297–2325. [Google Scholar] [CrossRef]
- Burgos, G.; Amoros, W.; Muñoa, L.; Sosa, P.; Cayhualla, E.; Sanchez, C.; Díaz, C.; Bonierbale, M. Total Phenolic, Total Anthocyanin and Phenolic Acid Concentrations and Antioxidant Activity of Purple-Fleshed Potatoes as Affected by Boiling. J. Food Compos. Anal. 2013, 30, 6–12. [Google Scholar] [CrossRef]
- Briggs, T.R. The Physical Chemistry of Dyeing: Substantive Dyes. J. Phys. Chem. 1932, 28, 368–386. [Google Scholar] [CrossRef]
- Phan, K.; van den Broeck, E.; van Speybroeck, V.; de Clerck, K.; Raes, K.; de Meester, S. The Potential of Anthocyanins from Blueberries as a Natural Dye for Cotton: A Combined Experimental and Theoretical Study. Dye. Pigm. 2020, 176, 108180. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Giusti, M.M. Bathochromic and Hyperchromic Effects of Aluminum Salt Complexation by Anthocyanins from Edible Sources for Blue Color Development. J. Agric. Food Chem. 2014, 62, 6955–6965. [Google Scholar] [CrossRef] [PubMed]
- Mabry, T.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer: New York, NY, USA, 1970. [Google Scholar]
- Zheng, Y.Z.; Zhou, Y.; Liang, Q.; Chen, D.F.; Guo, R. Theoretical Studies on the Hydrogen-Bonding Interactions between Luteolin and Water: A DFT Approach. J. Mol. Model. 2016, 22, 257. [Google Scholar] [CrossRef]
- Amat, A.; Clementi, C.; Miliani, C.; Romani, A.; Sgamellotti, A.; Fantacci, S. Complexation of Apigenin and Luteolin in Weld Lake: A DFT/TDDFT Investigation. Phys. Chem. Chem. Phys. 2010, 12, 6672–6684. [Google Scholar] [CrossRef]
- Smith, G.J.; Thomsen, S.J.; Markham, K.R.; Andary, C.; Cardon, D. The Photostabilities of Naturally Occurring 5-Hydroxyflavones, Flavonols, Their Glycosides and Their Aluminium Complexes. J. Photochem. Photobiol. A 2000, 136, 87–91. [Google Scholar] [CrossRef]
- Pȩkal, A.; Biesaga, M.; Pyrzynska, K. Interaction of Quercetin with Copper Ions: Complexation, Oxidation and Reactivity towards Radicals. BioMetals 2011, 24, 41–49. [Google Scholar] [CrossRef]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florêncio, M.H.; Jennings, K.R. Interactions of Flavonoids with Iron and Copper Ions: A Mechanism for Their Antioxidant Activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef]
- Lekka, C.E.; Ren, J.; Meng, S.; Kaxiras, E. Structural, Electronic, and Optical Properties of Representative Cu-Flavonoid Complexes. J. Phys. Chem. 2009, 113, 6478–6483. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.T.; Mira, M.L.; Florêncio, M.H.; Jennings, K.R. Iron and Copper Chelation by Flavonoids: An Electrospray Mass Spectrometry Study. J. Inorg. Biochem. 2002, 92, 105–111. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron Chelation by the Powerful Antioxidant Flavonoid Quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef]
- Jeevitha, D.; Sadasivam, K.; Praveena, R.; Jayaprakasam, R. DFT Study of Glycosyl Group Reactivity in Quercetin Derivatives. J. Mol. Struct. 2016, 1120, 15–24. [Google Scholar] [CrossRef]
- Matsubara, T.; Wataoka, I.; Urakawa, H.; Yasunaga, H. Effect of Reaction PH and CuSO4 Addition on the Formation of Catechinone Due to Oxidation of (+)-Catechin. Int. J. Cosmet. Sci. 2013, 35, 362–367. [Google Scholar] [CrossRef]
- Mongkholrattanasit, R.; Klaichoi, C.; Rungruangkitkrai, N.; Punrattanasin, N.; Sriharuksa, K.; Nakpathom, M. Dyeing Studies with Eucalyptus, Quercetin, Rutin, and Tannin: A Research on Effect of Ferrous Sulfate Mordant. J. Text. 2013, 2013, 423842. [Google Scholar] [CrossRef]
- Elhabiri, M.; Carrër, C.; Marmolle, F.; Traboulsi, H. Complexation of Iron(III) by Catecholate-Type Polyphenols. Inorganica Chim. Acta 2007, 360, 353–359. [Google Scholar] [CrossRef]
- Jolliffe, I.T. Principal Component Analysis, 1st ed.; Springer: New York, NY, USA, 1986. [Google Scholar]
- Worley, B.; Powers, R. PCA as a practical indicator of OPLS-DA model reliability. Curr. Metab. 2016, 4, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Barani, H.; Montazer, M. A Review on Applications of Liposomes in Textile Processing. J. Liposome Res. 2008, 18, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Montazer, M.; Taghavi, F.A.; Toliyat, T.; Moghadam, M.B. Optimization of dyeing of wool with madder and liposomes by central composite design. J. Appl. Polym. Sci. 2007, 106, 1614–1621. [Google Scholar] [CrossRef]
- Martí, M.; Coderch, L.; de la Maza, A.; Parra, J.L. Liposomes of phosphatidylcholine: A biological natural surfactant as a dispersing agent. Color. Technol. 2007, 123, 237–241. [Google Scholar] [CrossRef]
- Villela, A.; van Vuuren, M.S.A.; Willemen, H.M.; Derksen, G.C.H.; van Beek, T.A. Photo-stability of a flavonoid dye in presence of aluminium ions. Dye. Pigm 2019, 162, 222–231. [Google Scholar] [CrossRef]
- Manian, A.P.; Paul, R.; Bechtold, T. Metal mordanting in dyeing with natural colourants. Color. Technol. 2016, 132, 107–113. [Google Scholar] [CrossRef]
- İşmal, Ö.E.; Yıldırım, L. Metal Mordants & Biomordants. In The Impact and Prospects of Green Chemistry for Textile Technology, 1st ed.; ul Islam, S., Butola, B.S., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 57–82. [Google Scholar]
- Oda, H. Improvement of light fastness of natural dyes. Part 2: Effect of functionl phenyl esters on the photofading of carthaminin polymeric substrate. Color. Technol. 2001, 117, 257–261. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.; Wang, J.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Sofwatre Environment for Integrated Models. Genome Res. 1971, 13, 426. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.; Li, S.; Xia, J. Metaboanalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, 388–396. [Google Scholar] [CrossRef]







| Compound | Mass (m/z) | p-Value 1 | Contribution to Variance (%) | Source 2 |
|---|---|---|---|---|
| Vitexin/Isovitexin | 433.1 | 8.81 × 10−8 | 5.12 | T. catappa, D. philippinensis |
| 2,4,6-trimethoxychalcone | 299.1 | 2.89 × 10−8 | 3.09 | S. samarangense |
| Quercetin | 303.1 | 6.05 × 10−5 | 4.25 | S. samarangense |
| Quercetin-O-arabinoside | 435.1 | 7.18 × 10−9 | 1.76 | S. samarangense |
| Tiliroside | 595.1 | 3.09 × 10−10 | 4.03 | D. philippinensis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molino, R.J.E.J.; Rellin, K.F.B.; Nellas, R.B.; Junio, H.A. Sustainable Hues: Exploring the Molecular Palette of Biowaste Dyes through LC-MS Metabolomics. Molecules 2021, 26, 6645. https://doi.org/10.3390/molecules26216645
Molino RJEJ, Rellin KFB, Nellas RB, Junio HA. Sustainable Hues: Exploring the Molecular Palette of Biowaste Dyes through LC-MS Metabolomics. Molecules. 2021; 26(21):6645. https://doi.org/10.3390/molecules26216645
Chicago/Turabian StyleMolino, Ralph John Emerson J., Klidel Fae B. Rellin, Ricky B. Nellas, and Hiyas A. Junio. 2021. "Sustainable Hues: Exploring the Molecular Palette of Biowaste Dyes through LC-MS Metabolomics" Molecules 26, no. 21: 6645. https://doi.org/10.3390/molecules26216645
APA StyleMolino, R. J. E. J., Rellin, K. F. B., Nellas, R. B., & Junio, H. A. (2021). Sustainable Hues: Exploring the Molecular Palette of Biowaste Dyes through LC-MS Metabolomics. Molecules, 26(21), 6645. https://doi.org/10.3390/molecules26216645






