Modern Methods for the Sustainable Synthesis of Metalloporphyrins
Abstract
:1. Introduction
2. Results
2.1. Mechanochemistry for the Synthesis of Metalloporphyrins
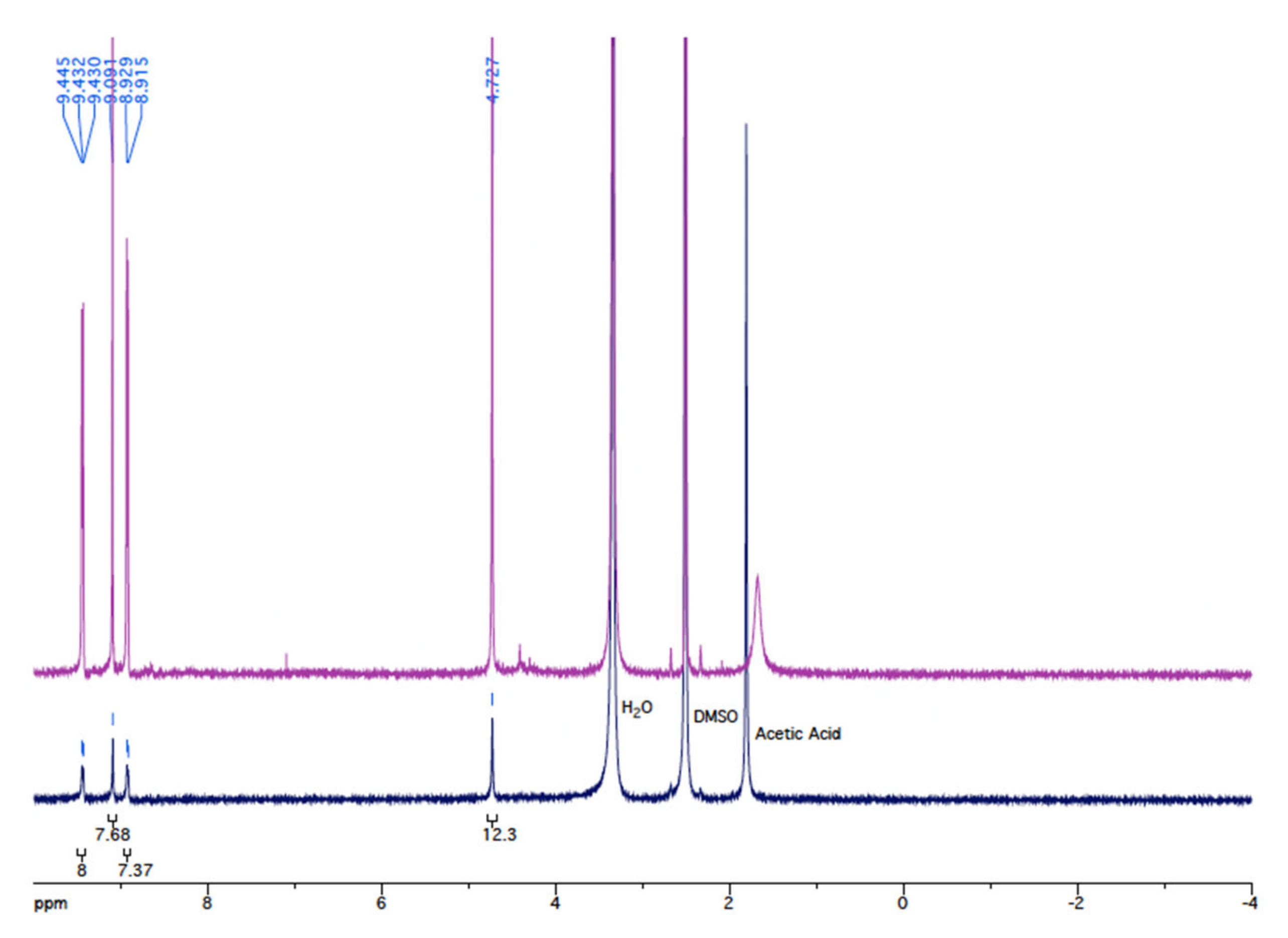
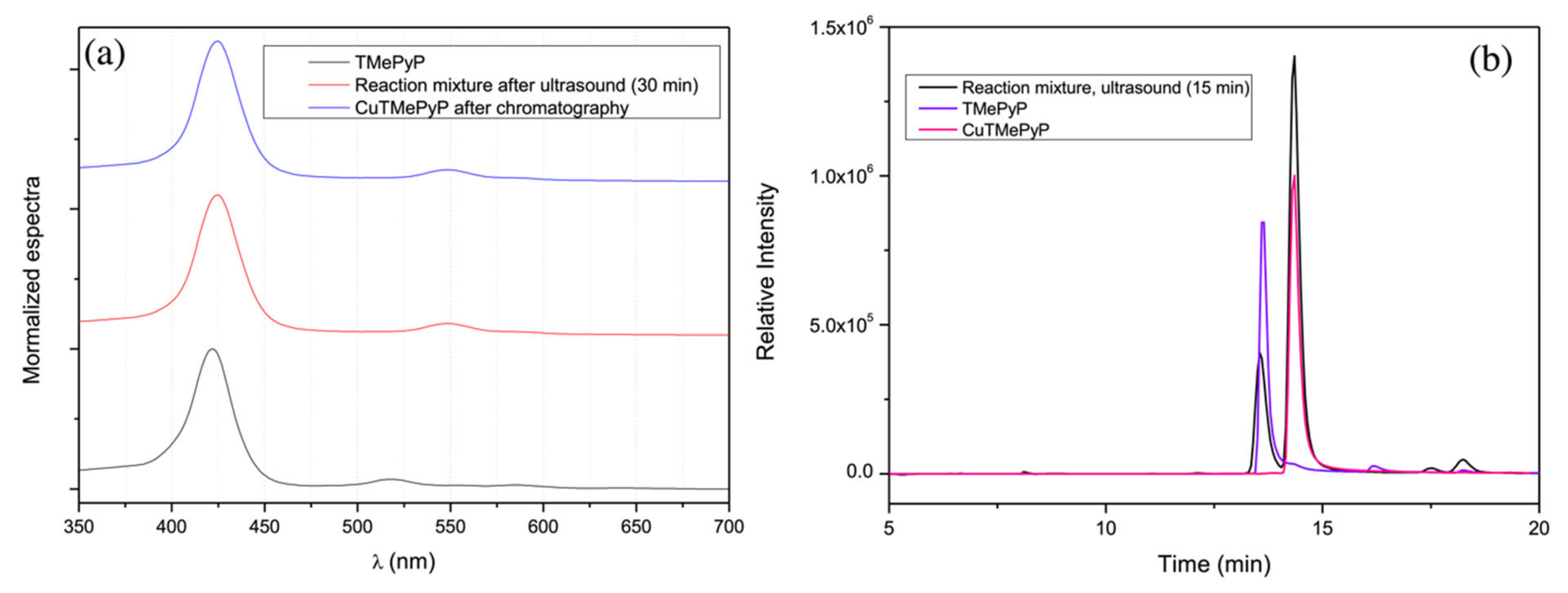
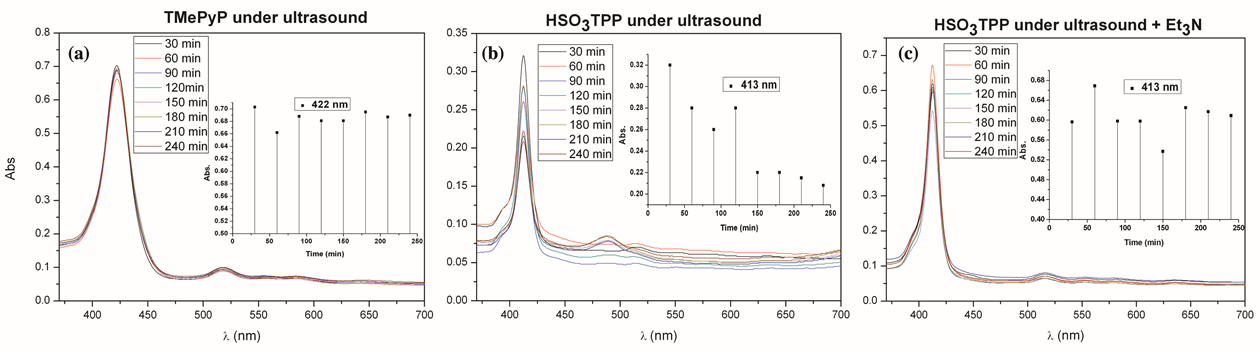
2.2. Ultrasound for the Synthesis of Metalloporphyrins
2.3. Sustainability Assesment
3. Discussion
4. Materials and Methods
4.1. Reactants and Equipment
4.2. HPLC Analysis Method
4.3. General Mechanochemical Synthesis Metalloporphyrins
4.4. General Synthesis of Metalloporphyrins under Ultrasound
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Smith, K.M. Porphyrins and Metalloporphyrins; Elsevier: Amsterdam, The Netherlands; New York, NY, USA; Oxford, UK, 1975. [Google Scholar]
- Costas, M. Selective C–H oxidation catalyzed by metalloporphyrins. Coord. Chem. Rev. 2011, 255, 2912–2931. [Google Scholar] [CrossRef]
- Shao, S.; Rajendiran, V.; Lovell, J.F. Metalloporphyrin nanoparticles: Coordinating diverse theranostic functions. Coord. Chem. Rev. 2019, 379, 99–120. [Google Scholar] [CrossRef]
- Simonneaux, G.; Maux, P.L.; Ferrand, Y.; Rault-Berthelot, J. Asymmetric heterogeneous catalysis by metalloporphyrins. Coord. Chem. Rev. 2006, 250, 2212–2221. [Google Scholar] [CrossRef]
- Doctorovich, F.; Bikiel, D.; Pellegrino, J.; Suárez, S.A.; Larsen, A.; Martí, M.A. Nitroxyl (azanone) trapping by metalloporphyrins. Coord. Chem. Rev. 2011, 255, 2764–2784. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Pucelik, B.; Regiel-Futyra, A.; Brindell, M.; Mazuryk, O.; Kyzioł, A.; Stochel, G.; Macyk, W.; Arnaut, L.G. Engineering of relevant photodynamic processes through structural modifications of metallotetrapyrrolic photosensitizers. Coord. Chem. Rev. 2016, 325, 67–101. [Google Scholar] [CrossRef]
- Pratviel, G. Porphyrins in complex with DNA: Modes of interaction and oxidation reactions. Coord. Chem. Rev. 2016, 308, 460–477. [Google Scholar] [CrossRef]
- Jurow, M.; Schuckman, A.E.; Batteas, J.D.; Drain, C.M. Porphyrins as molecular electronic components of functional devices. Coord. Chem. Rev. 2010, 254, 2297–2310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valderrey, V.; Aragay, G.; Ballester, P. Porphyrin tweezer receptors: Binding studies, conformational properties and applications. Coord. Chem. Rev. 2014, 258–259, 137–156. [Google Scholar] [CrossRef]
- Calvete, M.J.F.; Pineiro, M.; Dias, L.D.; Pereira, M.M. Hydrogen Peroxide and Metalloporphyrins in Oxidation Catalysis: Old Dogs with Some New Tricks. ChemCatChem 2018, 10, 3615–3635. [Google Scholar] [CrossRef]
- Zanatta, L.D.; Barbosa, I.A.; de Sousa Filho, P.C.; Zanardi, F.B.; Bolzon, L.B.; A Serra, O.; Iamamoto, Y. Metalloporphyrins in Drug and Pesticide Catalysis as Powerful Tools to Elucidate Biotransformation Mechanisms. Mini-Rev. Org. Chem. 2021, 18, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Calvete, M.J.F.; Pinto, S.M.A.; Pereira, M.M.; Geraldes, C.F.G.C. Metal coordinated pyrrole-based macrocycles as contrast agents for magnetic resonance imaging technologies: Synthesis and applications. Coord. Chem. Rev. 2017, 333, 82–107. [Google Scholar] [CrossRef]
- Ni, Y. Metalloporphyrins and Functional Analogues as MRI Contrast Agents. Curr. Med. Imaging Rev. 2008, 4, 96–112. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Ramzan, M.; Qureshi, A.; Khan, M.; Tariq, M. Emerging Applications of Porphyrins and Metalloporphyrins in Biomedicine and Diagnostic Magnetic Resonance Imaging. Biosensors 2018, 8, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koifman, O.I.; Ageeva, T.A. Metalloporphyrins in macromolecular chemistry. Russ. Chem. Bull. 2015, 64, 2001–2011. [Google Scholar] [CrossRef]
- Kielmann, M.; Prior, C.; Senge, M.O. Porphyrins in troubled times: A spotlight on porphyrins and their metal complexes for explosives testing and CBRN defense. New J. Chem. 2018, 42, 7529–7550. [Google Scholar] [CrossRef]
- Nascimento, B.F.O.; Pineiro, M.; Rocha Gonsalves, A.M.d.A.; Silva, M.R.; Beja, A.M.; Paixão, J.A. Microwave-assisted synthesis of porphyrins and metalloporphyrins: A rapid and efficient synthetic method. J. Porphyr. Phthalocyan. 2007, 11, 77–84. [Google Scholar] [CrossRef]
- Nascimento, B.F.O.; Rocha Gonsalves, A.M.d.A.; Pineiro, M. MnO2 instead of quinones as selective oxidant of tetrapyrrolic macrocycles. Inorg. Chem. Commun. 2010, 13, 395–398. [Google Scholar] [CrossRef]
- Gomes, C.; Peixoto, M.; Pineiro, M. Porphyrin synthesis using mechanochemistry: Sustainability assessment. J. Porphyr. Phthalocya 2019, 23, 889–897. [Google Scholar] [CrossRef]
- Gomes, C.; Vinagreiro, C.S.; Damas, L.; Aquino, G.; Quaresma, J.; Chaves, C.; Pimenta, J.; Campos, J.; Pereira, M.; Pineiro, M. Advanced Mechanochemistry Device for Sustainable Synthetic Processes. ACS Omega 2020, 5, 10868–10877. [Google Scholar] [CrossRef] [PubMed]
- Isoni, V.; Wong, L.L.; Khoo, H.H.; Halim, I.; Sharratt, P. Q-SA√ESS: A methodology to help solvent selection for pharmaceutical manufacture at the early process development stage. Green Chem. 2016, 18, 6564–6572. [Google Scholar] [CrossRef]
- Trost, B.M. The atom economy—A search for synthetic efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E Factor 25 Years on: The Rise of Green Chemsitry and Sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Van Aken, K.; Strekowski, L.; Patiny, L. EcoScale, a Semi-Quantitative Tool to Select an Organic Preparation Based on Economical and Ecological Parameters. Beil. J. Org. Chem. 2006, 2, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ralphs, K.; Zhang, C.; James, S.L. Solventless mechanochemical metallation of porphyrins. Green Chem. 2017, 19, 102–105. [Google Scholar] [CrossRef]
- Atoyebi, A.O.; Brückner, C. Observations on the Mechanochemical Insertion of Zinc(II), Copper(II), Magnesium(II), and Select Other Metal(II) Ions into Porphyrins. Inorg. Chem. 2019, 58, 9631–9642. [Google Scholar] [CrossRef]
- Nomura, H.; Koda, S.; Yasuda, K.; Kojima, Y. Ultrasonic irradiation effect on porphyrin and its application for quantification of ultrasonic intensity. Ultrasonics 1996, 34, 555–557. [Google Scholar] [CrossRef]
- Nomura, H.; Koda, S.; Yasuda, K.; Kojima, Y. Quantification of ultrasonic intensity based on the decomposition reaction of porphyrin. Ultrason. Sonochem. 1996, 3, S153–S156. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; But-Terworth-Heinemann: Oxford, MA, USA, 1997. [Google Scholar]
- Kumar, A.; Maji, S.; Dubey, P.; Abhilash, G.J.; Pandey, S.; Sarkar, S. One-pot general synthesis of metalloporphyrins. Tetrahedron Lett. 2007, 48, 7287–7290. [Google Scholar] [CrossRef]
- Sousa, J.F.M.; Pina, J.; Gomes, C.; Dias, L.D.; Pereira, M.M.; Murtinho, D.; Dias, P.; Azevedo, J.; Mendes, A.; Seixas de Melo, J.S.; et al. Transport and photophysical studies on porphyrin-containing sulfonated poly(etheretherketone) composite membranes. Mater. Today 2021, 29, 102781. [Google Scholar] [CrossRef]
- Jain, N.; Kumar, A.; Chauhan, S.M.S. Synthesis of Transition Metal Porphyrins from Free-Base 5,10,15,20-Tetraarylporphyrins Under Microwave Irradiation in Ionic Liquids. Synth. Commun. 2005, 35, 1223–1230. [Google Scholar] [CrossRef]
- Sankar, M.; Bhyrappa, P.; Varghese, B.; Praneeth, K.K.; Vaijayanthimala, G. Meso-tetrakis(3′,5′-di-substituted-phenyl)porphyrins: Structural, electrochemical redox and axial ligation properties. J. Porphyr. Phthalocyanines 2012, 9, 413–422. [Google Scholar] [CrossRef]
- Chen, E.X.; Qiu, M.; Zhang, Y.F.; Zhu, Y.S.; Liu, L.Y.; Sun, Y.Y.; Bu, X.; Zhang, J.; Lin, Q. Acid and Base Resistant Zirconium Polyphenolate-Metalloporphyrin Scaffolds for Efficient CO2 Photoreduction. Adv. Mater. 2018, 30, 1704388. [Google Scholar] [CrossRef]
- Joseph, R.; Kumar, K.G. Electrochemical sensing of acyclovir at a gold electrode modified with 2-mercaptobenzothiazole-[5,10,15,20-tetrakis-(3-methoxy-4-hydroxyphenyl)porphyrin ato]copper(II). Anal. Sci. 2011, 27, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Wyrębek, P.; Ostrowski, S. Synthesis of some β-nitro-meso-tetraphenylporphyrin derivatives. J. Porphyr. Phthalocyanines 2012, 11, 822–828. [Google Scholar] [CrossRef]
- Pasternack, R.F.; Francesconi, L.; Raff, D.; Spiro, E. Aggregation of Nickel(II), Copper(II), and Zinc(I1) Derivatives ofWater-Soluble Porphyrins. Inorg. Chem. 1973, 12, 2606–2611. [Google Scholar] [CrossRef]
- Casas, C.; Lacey, C.J.; Meunier, B. Preparation of hybrid “DNA cleaver-oligonucleotide” molecules based on a metallotris(methylpyridiniumyl)porphyrin motif. Bioconjug. Chem. 1993, 4, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Pasternack, R.F.; Spiro, E.G.; Teach, M. Solution properties of nickel(II) tetra(4-N-methylpyridyl) porphine and the influence of acetone, pyridine and imidazole. J. Inorg. Nucl. Chem. 1974, 36, 599–606. [Google Scholar] [CrossRef]
- Manna, B.K.; Sen, D.; Bera, S.C.; Rohatgi-Mukherjee, K.K. Spectral and flash kinetic studies of water-soluble zinc porphyrins in poly-N-vinyl-2-pyrrolidone matrix. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1992, 48, 1657–1669. [Google Scholar] [CrossRef]





| Entry | Metal Salt | Equiv | NaOH (Equiv) | Milling Freq (Hz) | t(min) | Yield 1 (%) |
|---|---|---|---|---|---|---|
| 1 | Zn(OAc)2·2H2O | 5 | 25 | 30 | 97 | |
| 2 | Cu(OAc)2·H2O | 5 | 25 | 90 | 84 | |
| 3 | Co(OAc)2·4H2O | 5 | 25 | 150 | 46 | |
| 4 | Co(OAc)2·4H2O | 5 | 30 | 250 | 61 | |
| 5 | Mn(OAc)2·4H2O | 10 | 30 | 300 | 12 | |
| 6 | Mn(OAc)2·4H2O | 10 | 5 | 30 | 300 | 30 |
| 7 | PdCl2 | 10 | 5 | 30 | 30 | 70 |
| 8 | PtCl2 | 10 | 5 | 30 | 150 | 8 |
| 9 | PtCl2 | 10 | 10 | 30 | 300 | 50 2 |
| Entry | Porphyrin | Cu(OAc)2·H2O Equiv | t(min) | Yield 1 (%) |
|---|---|---|---|---|
| 1 | 3,4-OMeTPP | 5 | 90 | 84 |
| 2 | TPP | 5 | 90 | 75 |
| 3 | 3,5-OMeTPP | 5 | 30 | 89 |
| 4 | 3,4,5-OMeTPP | 5 | 30 | 88 |
| 5 | 3-OH-4-OMeTPP | 5 | 30 | 90 |
| 6 | 3,5-ClTPP | 5 | 45 | 97 |
| 7 | 3-OHTPP | 5 | 30 | 74 |
| 7 | 3-NO2TPP | 5 | 30 | 70 |
| 8 | TMePyP | 5 | 30 | 35 2 |
| 9 | TMePyP | 10 | 60 | 45 2 |
| 10 | TMePyP | 10 | 90 | 46 2 |
| Entry | Metal Salt | Equiv | t(min) | Yield 1 (%) |
|---|---|---|---|---|
| 1 | Zn(OAc)2·2H2O | 1 | 30 | 90 |
| 2 | Cu(OAc)2·H2O | 1 | 30 | 53 (79) 2 |
| 3 | Mn(OAc)2·4H2O | 1 | 180 | 79 |
| 4 | Ni(OAc)2·4H2O | 5 | 330 | 18 |
| 5 | Co(OAc)2·4H2O | 2 | 120 | 32 |
| Entry | Porphyrin | Metal Salt | Equiv | t(min) | Yield 1 (%) |
|---|---|---|---|---|---|
| 1 | 4-SO3HTPP | Zn(OAc)2·2H2O | 1 | 60 | 65 |
| 2 | 4-CO2HTPP | Zn(OAc)2·2H2O | 1 | 30 | 46 |
| 3 | 4-CO2HTPP | Cu(OAc)2·H2O | 1 | 60 | 85 |
| 4 | 4-CO2HTPP | Cu(OAc)2·H2O | 2 | 60 | 99 |
| 5 | 4-PO3H2TPP | Zn(OAc)2·2H2O | 1 | 90 | 32 |
| 6 | 3-OHTPP | Zn(OAc)2·2H2O | 1 | 30 | 89 2 |
| Porphyrin | Method | Cu(OAc)2·H2O Equiv | Solvent | T (min) | Yield 1 (%) | Atom Economy | E-Factor | Ecoscale | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | TMePyP | Mechanochemistry | 10 | 60 | 45 | 57% | 4.7 | 50.5 | |
| 2 | TMePyP | Ultrasound | 1 | 1 mL H2O | 30 | 53 | 90% | 37.0 | 54.5 |
| 3 | 3-OHTPP | Mechanochemistry | 5 | 30 | 74 | 44% | 2.1 | 72 | |
| 4 | 3-OHTPP | Ultrasound | 1 | 1 mL NaOH (2 M) | 30 | 74 | 84% | 27.3 | 67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, C.; Peixoto, M.; Pineiro, M. Modern Methods for the Sustainable Synthesis of Metalloporphyrins. Molecules 2021, 26, 6652. https://doi.org/10.3390/molecules26216652
Gomes C, Peixoto M, Pineiro M. Modern Methods for the Sustainable Synthesis of Metalloporphyrins. Molecules. 2021; 26(21):6652. https://doi.org/10.3390/molecules26216652
Chicago/Turabian StyleGomes, Carla, Mariana Peixoto, and Marta Pineiro. 2021. "Modern Methods for the Sustainable Synthesis of Metalloporphyrins" Molecules 26, no. 21: 6652. https://doi.org/10.3390/molecules26216652
APA StyleGomes, C., Peixoto, M., & Pineiro, M. (2021). Modern Methods for the Sustainable Synthesis of Metalloporphyrins. Molecules, 26(21), 6652. https://doi.org/10.3390/molecules26216652