Carbon Nanotubes, Graphene, and Carbon Dots as Electrochemical Biosensing Composites
Abstract
:1. Introduction
2. Experimental and Discussion
2.1. Carbon Nanotube Composites
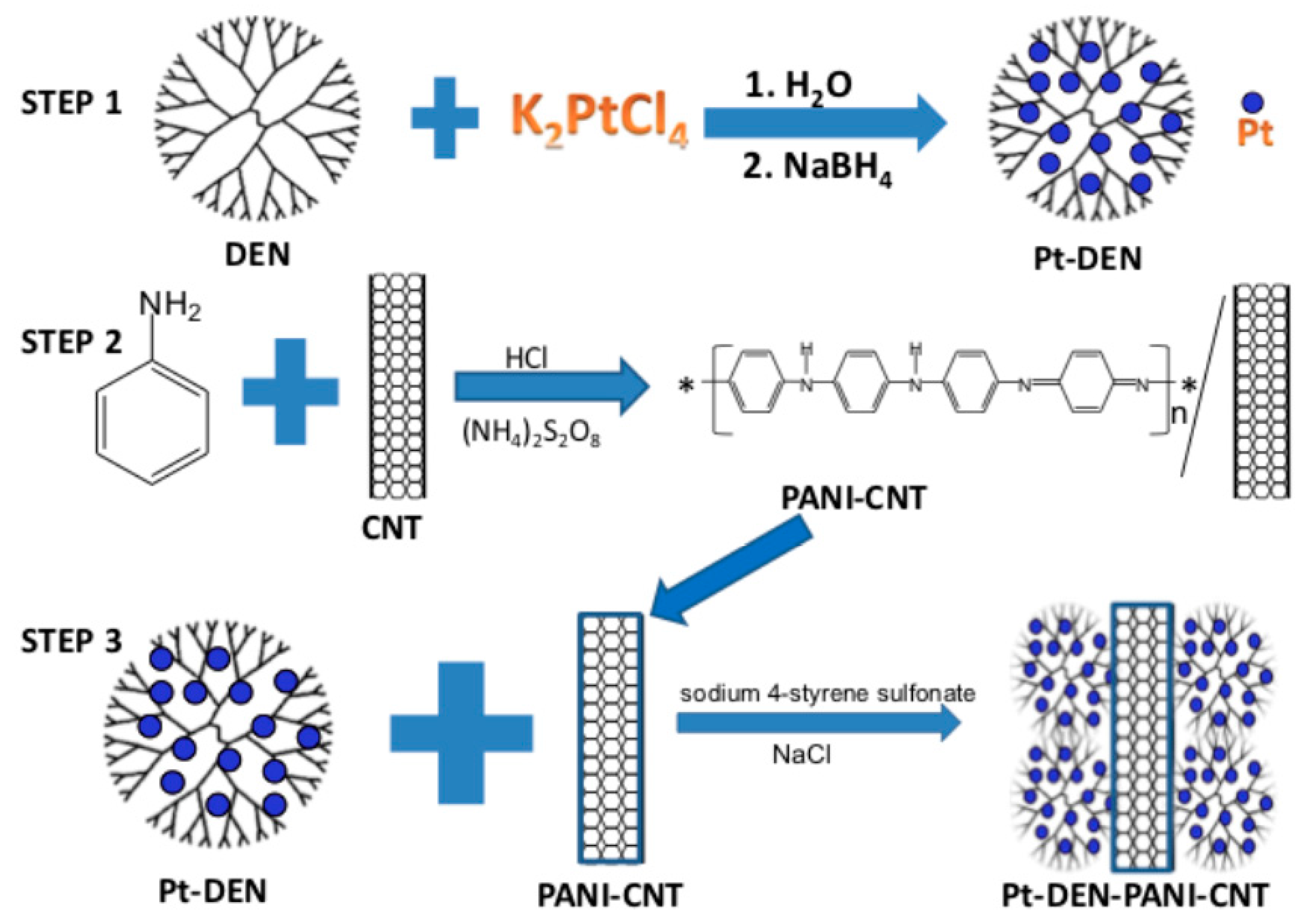
2.1.1. Functionalizing CNTs
2.1.2. Strategies to Modify the Working Electrode Surface
2.1.3. Biosensing Applications of CNT Composites
Noble Metal-Based Composites
Transition Metal Nanoparticle CNT Composites
2.2. Graphene and Reduced Graphene Oxide
2.2.1. Covalent Attachment of Functionalities to Graphene Oxide
2.2.2. Graphene and Reduced Graphene Oxide Noble Metal Composites
2.2.3. Graphene Oxide and Reduced Graphene Oxide Transition Metal Composites
| Sensor | Technique | Analyte | LOD | Reference |
|---|---|---|---|---|
| ssProbed modified SPCE/rGO/Au NP | DPV | PAH mutation | 21.3 fM | [75] |
| CuO/Graphene | CA | Glucose | 5.04 µM | [76] |
| Nd2O5 NPs/GO | CV | Raloxifene | 18.43 nM | [77] |
| CNT/GO/Fe3O4 | DPV | Diclofenac (DCF) | 33 pM | [79] |
| CuSe/rGO | LSV | Eugenol | 0.41 µg/kg | [80] |
| Pd-Mn/rGO | CA | Glucose | 1.25 µM | [81] |
| RGO/Fe3O4 | SWV | Melatonin | 8.4 nM | [82] |
| RGO/ZrO2/Co3O4 | CV, DPV | Gallic acid | 1.56 nM | [83] |
| RGO/Au NPs/SPE | CV, DPV | Ascorbic acid | 1.04 µM | [84] |
| RGO/GCE | CV, DPV | Resveratrol | 0.2 µM | [85] |
| TiO2/rGO | CV, SWV | Oleuropein, Hydroxytyrosol | 0.57 nM | [86] |
| NF/ERGO/GCE | DPV | Tocopherol | 0.06 µM | [87] |
| RGO/GCE | CV, DPV | Curcumin | 0.9 pM | [88] |
| GO-CMF/PdSPs | CV | Dopamine | 23 nM | [89] |
| RGO/SnO2-Co3O4 | CV, SWV | Melatonin | 4.1 nM | [90] |
| RGO/CuO | CV, CA | Flutamide | 0.001 µM | [91] |
| SPCE/AuNP/GO | LSV, EIS | Alkaline phosphatase | 9.1 U/L | [92] |
| Gd2O3 NPs@rGO | CV, CA | H2O2 | 1.57 nM | [93] |
2.3. Carbon Dots
2.3.1. Graphene Oxide and Reduced Graphene Oxide Transition Metal Composites
2.3.2. Functionalization of Carbon Dots
2.3.3. Biosensing Applications of Carbon Dots
Carbon Dot Transition Metal Oxide Composites
Carbon Dots Modified with Noble Metals
3. Summary and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Robinson, J.T.; Tabakman, S.M.; Yang, K.; Dai, H. Carbon materials for drug delivery and cancer therapy. Mater. Today 2011, 14, 316–323. [Google Scholar] [CrossRef]
- Mendes, R.G.; Bachmatiuk, A.; Büchner, B.; Cuniberti, G.; Ruemmeli, M.H. Carbon nanostructures as multi-functional drug delivery platforms. J. Mater. Chem. B 2013, 1, 401–428. [Google Scholar] [CrossRef]
- Francis, A.P.; Thiyagarajan, D. Toxicity of carbon nanotubes: A review. Toxicol. Ind. Health 2018, 34, 200–218. [Google Scholar] [CrossRef] [PubMed]
- Mottier, A.; Mouchet, F.; Pinelli, E.; Gauthier, L.; Flahaut, E. Environmental impact of engineered carbon nanoparticles: From releases to effects on the aquatic biota. Curr. Opin. Biotechnol. 2017, 46, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avant, B.; Bouchard, D.; Chang, X.; Hsieh, H.-S.; Acrey, B.; Han, Y.; Spear, J.; Zeep, R.; Knightes, C.A. Environmental fate of multiwalled carbon nanotubes and graphene oxide across diferent aquatic ecosystems. Nanoimpact 2019, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kurapati, R.; Mukherjee, S.P.; Martin, C.; Bepete, G.; Vázquez, E.; Pénicaud, A.; Fadeel, B.; Bianco, A. Degradation of single-layer and few-layer graphene by neutropil myeloperoxidase. Angew. Chem. Int. Ed. Engl. 2018, 57, 11722–11727. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, I.; Sar, D.; Mukherjee, P.; Schwartz-Duval, A.S.; Huang, Z.; Jaramillo, C.; Civantos, A.; Tripathi, I.; Allain, J.P.; Bhargava, R.; et al. Enzyme-catalyzed biodegradation of carbon dots follows sequential oxidation in a time dependent manner. Nanoscale 2019, 11, 8226–8236. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Chen, X.; Ren, T.; Zhang, P.; Yang, D. Carbon nanotube based biosensors. Sens. Actuators B Chem. 2015, 207, 690–715. [Google Scholar] [CrossRef]
- Radushkevich, V.; Lukayanovich, V.M. The structure of carbon forming in thermal decomposition of carbon monoxide on an iron catalyst. Russ. J. Phys. Chem. 1952, 25, 88–95. [Google Scholar]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iijima, S. Carbon nanotubes: Past, present and future. Phys. B Condens. Matter 2002, 323, 1–5. [Google Scholar] [CrossRef]
- Deb, A.K.; Chusuei, C.C. Aqueous Solution Surface Chemistry of Carbon Nanotubes. In Physical and Chemical Properties of Carbon Nanotubes; Suzuki, S., Ed.; InTech: Rijeka, Croatia, 2013; pp. 263–283. [Google Scholar]
- Sahoo, N.G.; Cheng, H.K.F.; Li, L.; Chan, S.H.; Judeh, Z.; Zhao, J. Specific functionalization of carbon nanotubes for advanced polymer nanocomposites. Adv. Funct. Mater. 2009, 19, 3962–3971. [Google Scholar] [CrossRef]
- Moore, E.; Wang, P.Y.; Vogt, A.P.; Gibson, C.T.; Haridas, V.; Voelcker, N.H. Clicking dendritic peptides onto single walled carbon nanotubes. RSC Adv. 2012, 2, 1289–1291. [Google Scholar] [CrossRef]
- Liao, W. Effects of multiwalled carbon nanotubes functionalization on the morphology of mechanical and thermal properties of carbon fiber/vinyl ester composites. ACS Appl. Mater. 2013, 5, 3975–3982. [Google Scholar] [CrossRef] [PubMed]
- Yoonessi, M.; Lebron-Colon, M.; Scheiman, D.; Meador, M.A. Carbon nanotube epoxy nanocomposites: The effects of interfacial modifcations on the dynamic mechanical properties of the nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 16621–16630. [Google Scholar] [CrossRef] [PubMed]
- Mammeri, F.; Teyssandier, J.; Darche-Dugares, C.; Debacker, S.; Le Bourhis, E.; Chehimi, M.M. Carbon nanotube-poly(methyl methacrylate) hybrid films: Preparation using diazonium salt chemistry and mechanical properties. J. Colloid Interface Sci. 2014, 433, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, Q.; Li, Q.; Chen, G.X. Controlled dielectric properties of polymer composites from coating multiwalled carbon nanotubes with octa-acrylate silesquioxane through Diels-Alder cycloaddition and atom transfer radical polymerization. Ind. Eng. Chem. Res. 2014, 53, 6699–6707. [Google Scholar] [CrossRef]
- Najafi-Shoa, S.; Roghani-Mamaqani, H.; Salami-Kalajahi, M.; Azimi, R. Incorporation of epoxy resin and carbon nanotube into silica/siloxane network for improving thermal properties. J. Mater. Sci. 2016, 51, 9057–9073. [Google Scholar] [CrossRef]
- Abdollahi, A.; Roghani-Mamaqani, H.; Salami-Kalajah, M.; Mousavi, A.; Razavi, B.; Shahi, S. Preparation of organic-inorganic hybrid nanocomposites from chemically modified epoxy and novolac resins and silica-attached carbon nanotubes by sol-gel process: Investigation of thermal degradation and stability. Prog. Org. Coat. 2018, 117, 154–165. [Google Scholar] [CrossRef]
- Stobinski, L. Multiwall carbon nanotubes purification and oxidation by nitric acid studied by the FTIR and electron spectroscopy methods. J. Alloys Compd. 2010, 501, 77–84. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.; Zhang, J.; Gao, Y. Comparative investigation of the resistance to electrochemical oxidation of carbon black and carbon nanotubes in aqueous sulfuric acid solution. Electrochim. Acta 2006, 51, 5853–5857. [Google Scholar] [CrossRef]
- Jiang, L.C.; Zhang, W.D. Electrodeposition of TiO2 nanoparticles on multiwalled carbon nanotube arrays for hydrogen peroxide sensing. Electroanalysis 2009, 21, 988–993. [Google Scholar] [CrossRef]
- Hu, X.; Zou, C.; Zou, X. The formation of supramolecular carbon fiber via amidation reaction on the surface of amino single walled carbon nanotubes for selective adsorption organic pollutants. J. Colloid Interf. Sci. 2019, 54, 112–122. [Google Scholar] [CrossRef] [PubMed]
- D’Arlas, B.F.; Goyanes, S.; Rubiolo, G.H.; Mondragon, I.; Corcuera, M.A.; Eceiza, A. Surface modification of multiwalled carbon nanotubes via esterification using a biodegradable polyol. J. Nanosci. Nanotechnol. 2009, 9, 6064–6071. [Google Scholar] [CrossRef]
- Iannazzo, D.; Piperno, A.; Ferlazzo, A.; Pistone, A.; Milone, C.; Lanza, M.; Cimino, F.; Speciale, A.; Trombetta, D.; Saija, A.; et al. Functionalization of multiwalled carbon nanotubes with coumarin derivatives and their biological evaluation. Org. Biomol. Chem. 2012, 10, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, F.; Huang, S.; Li, M.; Guo, T.; Mo, C.; Pang, X.; Chen, L.; Li, X. In-situ facile preparation of highly efficient Copper/Nickel bimetallic nanocatalyst on chemically grafted carbon nanotubes for non-enzymatic sensing of glucose. J. Colloid Interface Sci. 2019, 557, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Alemany, L.B.; Margrave, J.L.; Khabashesku, V.N. Sidewall carboxylic acid functionalization of single-walled carbon nanotubes. J. Am. Chem. Soc. 2003, 125, 15174–15182. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.J.; Cheng, J.; Jin, J.; Cheng, S.H.; Wong, W.T. Development and evaluation of pH-responsive single walled carbon nanotube-doxorubicin complexes in cancer cells. Int. J. Nanomed. 2011, 6, 2889–2898. [Google Scholar]
- Ensafi, A.A.; Zandi-Atashbar, N.; Rezaei, B.; Ghiaci, M.; Chermahini, M.E.; Moshiri, P. Non-enzymatic glucose electrochemical sensor based on silver nanoparticle dcorated organic functionalized multiwall carbon nanotubes. RSC Adv. 2016, 6, 60926–60932. [Google Scholar] [CrossRef]
- Xing, Y.; Li, L.; Chusuei, C.C.; Hull, R.V. Sonochemical oxidation of multiwalled carbon nanotubes. Langmuir 2005, 21, 4185–4190. [Google Scholar] [CrossRef] [PubMed]
- Chusuei, C.C.; Wayu, M. Characterizing Functionalized Carbon Nanotubes for Improved Fabrication in Aqueous Solution Environments. In Electronic Properties of Carbon Nanotubes/Book 5; Marulanda, J.M., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 55–66. [Google Scholar]
- Hull, R.V.; Li, L.; Xing, Y.; Chusuei, C.C. Pt nanoparticle binding on functionalized multiwalled carbon nanotubes. Chem. Mater. 2006, 18, 1780–1788. [Google Scholar] [CrossRef]
- Das, S.C.; Pandey, R.R.; Devkota, T.; Chusuei, C.C. Raman spectroscopy as an assay to disentangle zinc oxide carbon nanotube composites for optimized uric acid detection. Chemosensors 2018, 6, 65. [Google Scholar] [CrossRef] [Green Version]
- Kader, M.S.; Chusuei, C.C. A cobalt (II) oxide carbon nanotube composite to assay dopamine. Chemosensors 2020, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Aoki, K.J.; Chen, J.; Nishiumi, T. Examination of the Gouy-Chapman theory for double layer capacitance in deionized latex suspensions. RSC Adv. 2014, 4, 63171–63181. [Google Scholar] [CrossRef]
- Park, J.; Regalbuto, J.R. A simple and accurate determination of oxide PZC and the strong buffering effect of oxide surfaces at incipient wetness. Colloid Interface Sci. 1995, 175, 239–252. [Google Scholar] [CrossRef]
- McPhail, M.R.; Sells, J.A.; He, Z.; Chusuei, C.C. Charging Nanowalls: Adjusting the Carbon Nanotube Isoelectric Point via Surface Chemical Functionalization. J. Phys. Chem. C 2009, 113, 14102–14109. [Google Scholar] [CrossRef]
- Pandey, R.R.; Guo, Y.; Gao, Y.; Chusuei, C.C. A Prussian Blue ZnO carbon nanotube composite for chronoamperometrically assaying H2O2 in BT20 and 4T1 breast cancer cells. Anal. Chem. 2019, 91, 10573–10581. [Google Scholar] [CrossRef]
- Gatabi, M.P.; Moghaddam, H.M.; Ghorbani, M. Point of zero charge of maghemite decorated multiwalled carbon nanotubes fabricated by chemical precipitation method. J. Mol. Liq. 2016, 216, 117–125. [Google Scholar] [CrossRef]
- Manasa, G.; Bhakta, A.K.; Mekhalif, Z.; Mascarenhas, R.J. Voltammetric study and rapid quantification of rescorcinol in hair dye and biological samples using ultrasensitive maghemite/MWCNT modified cargon paste electrode. Electroanalysis 2019, 31, 1363–1372. [Google Scholar] [CrossRef]
- Deb, A.K.; Das, S.C.; Saha, A.; Wayu, M.B.; Marksberry, M.H.; Baltz, R.J.; Chusuei, C.C. Ascorbic acid, acetaminophen, and hydrogen peroxide detection using a dendrimer-encapsulated Pt nanoparticle carbon nanotube composite. J. Appl. Electrochem. 2016, 46, 289–298. [Google Scholar] [CrossRef]
- Wang, X.; Wu, M.; Tang, W.; Zhu, Y.; Wang, L.; Wang, Q.; He, P. Simulataneous electrochemical determination of ascorbic acid, dopamine, and uric acid using a Palladium nanoparticles/graphene/chitosan modified electrode. J. Electroanal. Chem. 2013, 695, 10–15. [Google Scholar] [CrossRef]
- Heitner-Wirguin, C. Recent advances in perfluorinated ionomer membranes: Structures, properties, and applications. J. Mem. Sci. 1996, 120, 1–33. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef] [PubMed]
- Poletti, F.; Favarette, L.; Kovtun, A.; Treossi, E.; Corticelli, F.; Gazzano, M.; Palermo, V.; Zanardi, C.; Melucci, M. Electrochemical sensing of glucose by chitosan modified graphene oxide. J. Phys. Mater. 2020, 3, 14011. [Google Scholar] [CrossRef]
- Kuppens, I.; Van Maanen, M.; Rosing, H.; Schellens, J.; Beijen, J. Quantitative analysis of docetaxel in human plasma using liquid chromatography coupled with tandem mass spectrometry. Biomed. Chromatogr. 2005, 19, 355–361. [Google Scholar] [CrossRef]
- Najari, S.; Bagheri, H.; Monsef-Khoshhesab, Z.; Hajian, A.; Afkhami, A. Electrochemical sensor based on gold nanoparticle-multiwall carbon nanotube nanocomposite for the sensitive determination of docetaxel as an anticancer drug. Ionics 2018, 24, 3209–3219. [Google Scholar] [CrossRef]
- Sheng, Q.; Liu, D.; Zheng, J. A nonenzymatic electrochemical nitrite sensor based on Pt nanoparticles loaded Ni(OH)2/multiwalled carbon nanotube composites. J. Electroanal. Chem. 2017, 796, 9–16. [Google Scholar] [CrossRef]
- Freitas, M.; Nouws, H.P.A.; Delerue-Matos, C. Electrochemical sensing platforms for HER2-ECD breast cancer biomarker detection. Electroanalysis 2019, 31, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.; Zeng, P.; Ma, C.; Peng, H.; Yang, J.; Huang, J.; Zhang, M.; Cheng, F. Electrochemical sensor for sensitive detection of luteolin based on multi-walled carbon nanotubes/poly(3,4-ethylenedioxythiophene)-gold nanocomposites. New J. Chem. 2020, 44, 1953–1961. [Google Scholar] [CrossRef]
- Yi, J.; Qiao, J.; Wang, Y.; Gao, K.; Zhao, R.; Meng, X. Electrochemical sensor platform for 8-hydroxy-2′-deoxyguanosine detection based on carboxyl-functionalized carbon-allotropic nanomaterials wrapped gold nanoparticles modified electrode. Int. J. Electrochem. Sci. 2019, 14, 9098–9111. [Google Scholar] [CrossRef]
- Keerthi, M.; Boopathy, G.; Chen, S.M.; Chen, T.W.; Lou, B.S. A core-shell molybdenum nanoparticles entrapped f-MWCNTs hybrid nanostructured material based non-enzymatic biosensor for electrochemical detection of dopamine neurotransmitter in biological samples. Sci. Rep. 2019, 9, 13075. [Google Scholar] [CrossRef]
- Patel, B.R.; Imran, S.; Ye, W.; Weng, H.; Noroozifar, M. Simultaneous voltammetric detection of six molecules using a nanocomposite of titanium dioxide nanorods with multi-walled carbon nanotubes. Electrochim. Acta 2020, 362, 137094. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Liu, X.; Cheng, J.; Liu, W.; Liu, T.; Sun, M.; Zhao, L.; Ding, F.; Lu, Z.; et al. CuCO2O4/N-doped CNTs with molecularly imprinted polymer for electrochemical sensor: Preparation, characterization, and detection of metronidazole. Biosens. Bioelectron. 2019, 142, 111483. [Google Scholar] [CrossRef] [PubMed]
- Nontawong, N.; Amatatongchai, M.; Jarujamrus, P.; Tamuang, S.; Chairam, S. Non-enzymatic glucose sensors for sensitive amperometric detection based on simple method of nickel nanoparticles decorated on magnetite carbon nanotubes modified glassy carbon electrodes. Int. J. Electrochem. Sci. 2017, 12, 1362–1376. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, C.; Jiang, Y.; Shen, J.; Geng, P.; Zhang, W.; Huang, Q. An electrochemical glycan biosensor based on a thionine-bridged multiwalled carbon nanotube/gold nanoparticle composite-modified electrode. RSC Adv. 2016, 6, 112981–112987. [Google Scholar] [CrossRef]
- Du, P.; Wu, P.; Cai, C. A glucose biosensor based on electrocatalytic oxidation of NADPH at single-walled carbon nanotubes functionalized with poly(Nile blue A). Electroanal. Chem. 2008, 624, 21–26. [Google Scholar] [CrossRef]
- Du, P.; Liu, S.; Wu, P.; Cai, C. Single-walled carbon nanotubes functionalized with poly(nile blue A) and their application to dehydrogenase-based biosensors. Electrochim. Acta 2007, 53, 1811–1823. [Google Scholar] [CrossRef]
- Wang, Z.; Etienne, M.; Pöller, S.; Schuhmann, W.; Kohring, G.-W.; Mamane, V.; Walcarius, A. Dehydrogenase-based reagentless biosensors: Electrochemically assisted deposition of sol-gel thin films on functionalized carbon nanotubes. Electroanalysis 2012, 24, 376–385. [Google Scholar] [CrossRef]
- Zeng, J.; Wei, W.; Wu, L.; Liu, X.; Liu, K.; Li, Y. Fabrication of poly(toluidine blue O)/carbon nanotube composite nanowires and its stable low-potential detection of NADH. J. Electroanal. Chem. 2006, 595, 152–160. [Google Scholar] [CrossRef]
- Peña, R.C.; Bertotti, M.; Brett, C.M.A. Methylene blue/multiwall carbon nanotube modified electrode for the amperometric determination of hydrogen peroxide. Electroanalysis 2011, 23, 2290–2296. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Baghayeri, M. Fabrication of layer-by-layer deposited films containing carbon nanotubes and poly(malachite green) as a sensor for simultaneous determination of ascorbic acid, epinenphrine, and uric acid. Turk. J. Chem. 2013, 37, 36–50. [Google Scholar]
- Gligor, D.; Walcarius, A. Glassy carbon electrode modified with a film of poly(toluidine blue O) and carbon nanotubes for nitrite detection. J. Solid State Electrochem. 2014, 18, 1571–1580. [Google Scholar] [CrossRef]
- Ghica, M.E.; Winsterstellar, Y.; Brett, C.M.A. Poly(brilliant green)/carbon nanotube-modified carbon film electrodes and application as sensors. J. Solid State Electrochem. 2013, 17, 1571–1580. [Google Scholar] [CrossRef]
- Yogeswaran, U.; Chen, S.-M. Multi-walled carbon nanotubes with poly(methene blue) composite film for the enhancement and separation of electroanalytical responses of catecholamine and ascorbic acid. Sensor Actuat. B. 2008, 130, 730–749. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Beitollahi, H.; Ganjipour, B.; Naeimi, H.; Nejati, M. Electrochemical and catalytic investigations of dopamine and uric acid by modified carbon nanotube paste electrode. Bioelectrochemistry 2009, 75, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, I.; Edgeworth, J.P.; Unwin, P.R.; Macpherson, J.V. Ultrathin carbon nanotube mat electrodes for enhanced amperometric detection. Adv. Mat. 2009, 21, 3105–3110. [Google Scholar] [CrossRef]
- Beitollahi, H.; Karimi-Maleh, H.; Khabazzadeh, H. Nanomolar and selective determination of epinephrine in the presence of nonepinephrine using carbon paste electrode modified with carbon nanotubes and novel 2-(4-oxo-3-phenyl-3, 4-dihydro-quinaZolinyl-)N’-phenyl-hydrazinecarbothioamide. Anal. Chem. 2008, 80, 9848–9851. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Dong, X.Y.; Li, J. An inlaying ultra-thin carbon paste electrode modified with functional single-wall carbon nanotubes for simultaneous determination of three purine derivatives. Sens. Actuators B-Chem. 2008, 131, 411–416. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dobonos, S.V.; Grigorievo, I.V.; Frisov, A.A. Electric field effect in atomically thin carbon film. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Pumero, M.; Ambrosi, A.; Bonanni, A.; Chng, E.L.K.; Poh, H.L. Graphene for electrochemical sensing and biosensing. Trends Analyt. Chem. 2010, 29, 954–965. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives, and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Vishwanathan, P.; Ramaraj, R. Chapter 2-Functionalized graphene nanocomposite for electrochemical sensors. In Graphene-Based Electrochemical Sensors for Biomolecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 43–65. [Google Scholar] [CrossRef]
- Seifali, S.M.; Nasirizadeh, N.; Azimazadeh, M. Nano-sensor based on reduced graphene oxide and gold nanoparticles, for detection of phenylketonuria-associated DNA mutation. IET Nanobiotechnol. 2018, 12, 417–422. [Google Scholar] [CrossRef]
- Fouroughi, F.; Rhasepar, M.; Hadianfard, M.J.; Kim, H. Microwave-assisted synthesis of graphene modified CuO nanoparticles for voltammetric enzyme-free sensing of glucose at biological pH values. Microchim. Acta 2018, 185, 57. [Google Scholar] [CrossRef]
- Chen, T.-W.; Merlin, J.P.; Chen, S.-M.; Anandraj, S.; Elshikh, M.S.; Tseng, T.-W.; Wang, K.; Qi, D.; Jiang, J. Sonochemical synthesis and fabrication of neodyminum sesquioxide entrapped with graphene oxide based hierarchical nanocomposite for highly sensitive electrochemical sensor of anti-cancer (Raloxifene) drug. Ultrason. Sonochem. 2020, 64, 104717. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Yu, J.; He, J.; Yang, H.; Ye, Y.; Song, Y. A green and simple strategy to prepare graphene foam-like three-dimensional porous carbon/Ni nanoparticles for glucose sensing. Sens. Actuat. B 2017, 239, 172–179. [Google Scholar] [CrossRef]
- Azadbakht, A.; Derikvandi, Z. Aptamer-based sensor for diclofenac quantification using carbon nanotubes and graphene oxide decorated with magnetic nanomaterials. J. Iran. Chem. Soc. 2018, 15, 595–606. [Google Scholar] [CrossRef]
- Murtada, K.; Moreno, V.; Ríos, A.; Zougagh, M. Decoration of graphene oxide with copper selenide in supercritical carbon dioxide medium as a novel approach for electrochemical sensing of eugenol in various samples. J. Supercrit. Fluids 2019, 153, 104597. [Google Scholar] [CrossRef]
- Waqas, M.; Lan, J.; Zhang, X.; Fan, Y.; Zhang, P.; Liu, C.; Jiang, Z.; Wang, X.; Zeng, J.; Chen, W. Fabrication of non-enzymatic electrochemical glucose sensor based on Pd-Mn alloy nanoparticles supported on reduced graphene oxide. Electroanalysis 2020, 32, 1–12. [Google Scholar] [CrossRef]
- Bagheri, H.; Afkhami, A.; Hashemi, P.; Ghanei, M. Simultaneous and sensitive determination of melatonin and dopamine with Fe3O4 nanoparticle-decorated reduced graphene oxide modified electrode. RSC Adv. 2015, 5, 21659. [Google Scholar] [CrossRef]
- Puangjan, A.; Chaiyasith, S. An efficient ZrO2CO3O4 reduced graphene oxide nanocomposite electrochemical sensor for simultaneous determination of gallic acid, caffeic acid and protocatechuic acid natural antioxidants. Electrochim. Acta 2016, 211, 273–288. [Google Scholar] [CrossRef]
- Ji, D.; Liu, Z.; Liu, L.; Low, S.S.; Lu, Y.; Yu, X.; Zhu, L.; Li, C.; Liu, Q. Smartphone-based integrated voltammetry system for simultaneous detection of ascorbic acid, dopamine, and uric acid with graphene and gold nanoparticles modified screen-printed electrodes. Biosens. Bioelectron. 2018, 119, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, Y.; Kang, Y.; Huang, H.; Li, C.; Xu, M.; Ye, B. Electrochemical evaluation of trans-resveratrol levels in red wine based on the interaction between resveratrol and graphene. Anal. Methods Chem. 2017, 2017, 5749025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazar, S.; Kurtulbaş, E.; Ortaboy, S.; Atun, G.; Şahin, S. Screening of the antioxidant properties of olive (Olea europaea) leaf extract by titanium based reduced graphene oxide electrode. Korean J. Chem. Eng. 2019, 36, 1184–1192. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A.; Aydar, S.; Çakar, Ş. Determination of Tocopherol using reduced graphene oxide-Nafion hybrid-modified electrode in pharmaceutical capsules and vegetable oil samples. Food Anal. Methods 2016, 9, 1745–1753. [Google Scholar] [CrossRef]
- Dey, N.; Devasena, T.; Sivalingam, T. A comparative evaluation of graphene oxide based materials for electrochemical non-enzymatic sensing of curcumin. Mater. Res. Express 2018, 5, 25406. [Google Scholar] [CrossRef]
- Palanisamy, S.; Velusamy, V.; Ramaraj, S.; Chen, S.-W.; Yang, T.C.K.; Balu, S.; Banks, C.E. Facile synthesis of cellulose microfibers supported palladium nanospindles on graphene oxide for selective detection of dopamine in pharmaceutical and biological samples. Mater. Sci. Eng. C 2019, 98, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Zeinali, H.; Bagheri, H.; Monsef-Khoshhesab, Z.; Khoshsafar, H.; Hajian, A. Nanomolar simultaneous determination of tryptophan and melatonin by a new ionic liquid carbon paste electrode modified with SnO2-CO3O4@rGO nanocompsite. Mater. Sci. Eng. C 2017, 71, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Sakthinatan, S.; Kokulnathan, T.; Chen, S.-M.; Karthik, R.; Tamizhdurai, P.; Chiu, T.-W.; Shanthi, K. Simple sonochemical synthesis of cupric oxide sphere decorated reduced graphene oxide composite for the electrochemical detection of flutamide drug in biological samples. J. Electrochem. Soc. 2019, 166, B68–B75. [Google Scholar] [CrossRef]
- Mahato, K.; Purohit, B.; Kumar, A.; Chandra, P. Clinically comparable impedimetric immunosensor for serum alkaline phosphatase detection based on electrochemically engineered Au-nano-dendroids and graphene oxide nanocomposite. Biosens. Bioelectron. 2020, 148, 111815. [Google Scholar] [CrossRef] [PubMed]
- Manavalan, S.; Rajaji, U.; Chen, S.-M.; Chen, T.-W.; Ramalingam, R.J.; Maiyalagan, T.; Sathiyan, A.; Hao, Q.; Lei, W. Microwave-assisted synthesis of gadolinium (II) oxide decorated reduced graphene oxide nanocomposite for detection of hydrogen peroxide in biological and clinical samples. J. Electroanal. Chem. 2019, 837, 167–174. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Kour, R.; Arya, S.; Young, S.-J.; Gupta, V.; Bandhoria, P.; Khosla, A. Recent advances in carbon nanomaterials as electrochemical sensors. Electrochem. Soc. 2020, 167, 37555. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, H.; Shang, P.; Zeng, X.; Chi, Y. Immobilizing water-soluble graphene quantum dots with gold nanoparticles for low potential electrochemiluminescence immunosensor. Nanoscale 2015, 7, 16366–16371. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.W.; Soomro, R.A.; Kalwar, N.H.; Zahoor, M.; Avci, A.; Pehlivan, E.; Hallam, K.R.; Willander, M. Carbon quantum dot coated Fe3O4 hybrid composites for sensitive electrochemical detection of uric acid. Microchem. J. 2019, 146, 517–524. [Google Scholar] [CrossRef]
- Shiri, S.; Pajouheshpour, N.; Khossafar, H.; Amidi, S.; Bagheri, H. An electrochemical sensor for the simulataneous determination of rifampicin and isoniazid using a C-dots@CuFe2O4 nanocomposite modified carbon paste electrode. New J. Chem. 2017, 41, 15564–15573. [Google Scholar] [CrossRef]
- Roushani, M.; Ghanbari, K.; Hoseini, S.J. Designing an electrochemical aptasensor based on immobilization of the aptamer onto nanocomposite for detection of the streptomycin antibiotic. Microchem. J. 2018, 141, 96–103. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Karimzadeh, A.; Shadjou, N. Magnetic graphene quantum dots as a functional nanomaterial towards voltammetric detection of L-tryptophan at physiological pH. J. Nanostruct. 2018, 8, 21–30. [Google Scholar]
- Muthusankar, G.; Devi, R.K.; Gopu, G. Nitrogen-doped carbon quantum dots embedded CO3O4 with multiwalled carbon nanotubes: An efficient probe for the simultaneous determination of anti-cancer and antibiotic drugs. Biosens. Bioelectron. 2020, 150, 111947. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, J.; Yang, R.; Qu, L.; Harrington, P.D.B. A sensitive and selective electrochemical sensor based on graphene quantum dot/golds nanoparticle nanocomposite modified electrode for the determination of quercertin in biological samples. Electroanalysis 2016, 28, 1322–1330. [Google Scholar] [CrossRef]
- Xi, J.; Xie, C.; Zhang, Y.; Wang, L.; Xiao, J.; Duan, X.; Ren, J. Pd nanoparticles decorated N-doped graphene quantum dots@N-doped carbon hollow nanospheres with high electrochemical sensing performance in cancer detection. ACS Appl. Mater. Interfaces 2016, 8, 22563–22573. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. A novel detection approach for serotonin by graphene quantum dots/two dimensional (2D) hexagonal boron nitride nanosheets with molecularly imprinted polymer. Appl. Surf. Sci. 2018, 458, 648–655. [Google Scholar] [CrossRef]
- Canevari, T.C.; Cincotto, F.H.; Gomes, D.; Landers, R.; Toma, H.E. Magnetite nanoparticles bonded carbon quantum dots magnetically confined onto screen printed carbon electrodes and their performance as electrochemical sensor for NADH. Electroanalysis 2017, 29, 1968–1975. [Google Scholar] [CrossRef]
- Shadjou, N.; Hasanzadeh, M.; Talebi, F.; Marjani, A.P. Integration of Beta-cyclodextrin in graphene quantum dot nano-structure and its application towards detection of vitamin C at physiological pH: A new electrochemical approach. Mater. Sci. Eng. C. 2016, 67, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Baluta, S.; Lesiak, A.; Cabaj, J. Graphene quantum dots-based electrochemical biosensors for catecholamine neurotransmitters detection. Electroanalysis 2018, 30, 1781–1790. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Doong, R. Functionalized N-doped graphene quantum dots for electrochemical determination of cholesterol through host-guest inclusion. Microchim. Acta. 2018, 185, 526. [Google Scholar] [CrossRef] [PubMed]
- Maaoui, H.; Teodoresu, F.; Wang, Q.; Pan, C.H.; Addad, A.; Chtourou, R.; Szanerits, S.; Boukherroub, R. Non-enzymatic glucose sensing using carbon quantum dots decorated with copper oxide nanoparticles. Sensors 2016, 16, 1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, J.; Zhao, M.; Qu, H.; Li, H.; Chen, S. Fabrication of CQDs/MoS2/Mo foil for the improved electrochemical detection. Anal. Chim. Acta 2019, 1079, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Beitollah, H.; Dourandish, Z.; Ganjali, M.R.; Shakeri, S. Voltammetric determination of dopamine in the presence of tyrosine using graphite screen-printed electrode modified with graphene quantum dots. Ionics 2018, 24, 4023–4031. [Google Scholar] [CrossRef]
- Chen, J.; He, P.; Bai, H.; He, S.; Zhang, T.; Zhang, X.; Dong, F. Poly (Beta-cyclodextrin)/carbon quantum dots modified glassy carbon electrode: Preparation, characterization, and simultaneous determination of dopamine, uric acid and tryptophan. Sens. Actuators B 2017, 252, 9–16. [Google Scholar] [CrossRef]
- Sadhukhan, M.; Bhowmik, T.; Kundu, M.K.; Barman, S. Facile synthesis of carbon quantum dots and thin graphene sheets for non-enzymatic sensing of hydrogen peroxide. RSC Adv. 2014, 4, 4998–5005. [Google Scholar] [CrossRef]
- Jahanbakhshi, M.; Habibi, B. A novel and facile synthesis of carbon quantum dots via salep hydrothermal treatment as the silver nanoparticles support: Application to electroanalytical determination of H2O2 in fetal bovine serum. Biosens. Bioelectron. 2016, 81, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Weng, W.; Niu, X.; Li, X.; Men, Y.; Sun, W.; Li, G.; Dong, L. Boron-doped graphene quantum dots modified electrode for electrochemistry and electrocatalysis of hemoglobin. J. Electroanal. Chem. 2018, 823, 137–145. [Google Scholar] [CrossRef]
- Habibi, E.; Heidari, H. Renewable surface carbon composite electrode bulk modified with GQD-RuC13 nano-composite for high sensitive detection of L-tyrosine. J. Electroanal. Chem. 2016, 28, 2559–2564. [Google Scholar] [CrossRef]
- Wang, L.; Tricard, S.; Yue, P.; Zhao, J.; Fang, J.; Shen, W. Polypyrrole and graphene carbon dots@Prussian Blue hybrid film on graphite felt electrodes: Application for amperometric determination of L-cysteine. Biosens. Bioelectron. 2016, 77, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Muthansankar, G.; Rajkumar, C.; Chen, S.M.; Karkuzhali, R.; Gopu, G.; Sangili, A.; Sengottuvelan, N.; Sankar, R. Sonochemical driven simple preparation of nitrogen-doped carbon quantum dots/SnO2 nanocomposite: A novel electrocatalyst for sensitive voltammetric determination of riboflavin. Sens. Actuators B Chem. 2019, 281, 602–612. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and synthesis of carbon dots: From carbon dots to carbonized polymer dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zahran, E.M.; Quiroga, B.A.; Perez, J.; Mintz, K.J.; Peng, Z.; Liyanage, P.Y.; Pandey, R.R.; Chusuei, C.C.; Leblanc, R.M. Size-dependent photocatalytic activity of carbon does with surface-state determined photoluminescence. Appl. Catal. B 2019, 248, 157–166. [Google Scholar] [CrossRef]
- Cilingir, E.K.; Seven, E.S.; Zhou, Y.; Walters, B.M.; Mintz, K.J.; Pandey, R.R.; Wikramanayake, A.H.; Chusuei, C.C.; Vanni, S.; Graham, R.M.; et al. Metformin derived carbon dots: Highly biocompatible fluorescent nanomaterials as mitochondrial targeting and blood-brain barrier penetrating biomarkers. J. Colloid Interface Sci. 2021, 592, 485–497. [Google Scholar] [CrossRef]
- Peng, Z.; Zhou, Y.; Ji, C.; Pardo, J.; Mintz, K.J.; Pandey, R.R.; Chusuei, C.C.; Graham, R.M.; Yan, G.; Leblanc, R.M. Facile synthesis of ‘boron-doped’ carbon dots and their application in visible-light-driven photocatalytic degradation of organic dyes. Nanomaterials 2020, 10, 1560. [Google Scholar] [CrossRef]
- Mintz, K.J.; Bartoli, M.; Rovere, M.; Zhou, Y.; Hettiarachchi, S.D.; Paudyal, S.; Chen, J.; Domena, J.B.; Liyanage, P.Y.; Sampson, R.; et al. A deep investigation into the structure of carbon dots. Carbon 2021, 173, 433–447. [Google Scholar] [CrossRef]
- Zhou, Y.; Mintz, K.J.; Cheng, L.; Chen, J.; Ferreira, B.C.L.B.; Hettiarachchi, S.D.; Liyanage, P.Y.; Seven, E.S.; Miloserdov, N.; Pandey, R.R.; et al. Direct conjugation of distinct carbon dots as Lego-like building blocks for the assembly of versatile drug nanocarriers. J. Colloid Interface Sci. 2020, 576, 412–425. [Google Scholar] [CrossRef]
- Seven, E.S.; Sharma, S.K.; Meziane, D.; Zhou, Y.; Mintz, K.J.; Pandey, R.R.; Chusuei, C.C.; Leblanc, R.M. Close-packed Langmuir monolayers of saccharide-based carbon dots at the air-subphase interface. Langmuir 2019, 35, 6708–6718. [Google Scholar] [CrossRef]
- Mintz, J.K.; Mercado, G.; Zhou, Y.; Ji, Y.; Hettiarachchi, D.S.; Liyanage, Y.P.; Pandey, R.R.; Chusuei, C.C.; Dallman, J.; Leblanc, M.R. Tryptophan carbon dots and their ability to cross the blood-brain barrier. Colloids Surf. B 2019, 176, 488–493. [Google Scholar] [CrossRef]
- Liyanage, P.Y.; Geleroff, D.L.; Peng, Z.; Mintz, K.J.; Hettiarachch, S.D.; Pandey, R.R.; Chusuei, C.C.; Blackwelder, P.L.; Leblanc, R.M. Carbon Nitride Dots: A Selective Bioimaging Nanomaterial. Bioconjug. Chem. 2019, 12, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liyanage, P.Y.; Geleroff, D.L.; Peng, Z.; Mintz, K.J.; Hettiarrachchi, S.D.; Pandey, R.R.; Chusuei, C.C.; Leblanc, R.M. Photoluminescent carbon dots: A mixture of heterogeneous fractions. ChemPhysChem 2018, 19, 2589–2597. [Google Scholar] [CrossRef]
- Ji, Y.; Zhou, Y.; Waidely, E.; Desserre, A.; Marksberry, M.H.; Chusuei, C.C.; Dar, A.A.; Chat, O.A.; Li, S.; Leblanc, R.M. Rheology of a carbon dot gel. Inorg. Chim. Acta 2017, 468, 119–124. [Google Scholar] [CrossRef]
- Zhou, Y.; Desserre, A.; Sharma, S.K.; Li, S.; Marksberry, M.H.; Chusuei, C.C.; Blackwelder, P.L.; Leblanc, R.M. Gel-like carbon dots: Characterization and their potential applications. ChemPhysChem 2017, 18, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Fiuza, T.; Gomide, G.; Campos, A.F.C.; Messina, F.; Depyrot, J. On the colloidal stability of nitrogen-rich carbon nanodots aqueous dispersions. C J. Carbon Res. 2019, 5, 74. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Jiang, Y.; Sun, X.; Bai, Z.; Zhang, Y.; Zhou, X. Surface modification and chemical functionalization of carbon dots: A review. Microchim. Acta 2018, 185, 424. [Google Scholar] [CrossRef]
- Yan, F.; Kong, D.; Luo, Y.; Ye, Q.; He, J.; Guo, X.; Chen, L. Carbon dots serve as an effective probe for the quantitative determination and for intracellular imaging of mercury (II). Microchim. Acta 2016, 183, 1611–1618. [Google Scholar] [CrossRef]
- Yin, J.Y.; Liu, H.J.; Jiang, S.; Chen, Y.; Yao, Y. Hyperbranched polymer functionalized carbon dots with multi stimuli-responsive property. ACS Macro Lett. 2013, 2, 1033–1037. [Google Scholar] [CrossRef]
- Shi, W.; Li, X.; Ma, H. A tunable ratiometric pH sensor based on carbon nanodots for the quantitative measurement of the intracellular pH of whole cells. Angew. Chem. Int. Ed. Eng. 2012, 51, 6432–6435. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, D.; Fang, Y.; Wang, H.; Liu, Y.; Xu, Z. Detection of ascorbic acid using green synthesized carbon quantum dots. J. Sens. 2019, 2019, 9869682. [Google Scholar] [CrossRef]
- Shao, X.; Gu, H.; Wang, Z.; Chai, X.; Tian, Y.; Shi, G. Highly selective electrochemical strategy for monitoring of cerebral Cu2+ based on a carbon dot-TPEA hybridized surface. Anal. Chem. 2013, 85, 418–425. [Google Scholar] [CrossRef]









| Sensor | Technique | Analyte | LOD | Reference |
|---|---|---|---|---|
| Pt/Ni(OH)2/MWCNT/GCE | CV | Nitrite | 0.13 mM | [49] |
| SPCE-MWCNT/AuNPs | LSV | HER2-ECD | 0.16 ng∙mL–1 | [50] |
| MWCNTs/PEDT-Au | EIS, CV, SWV | Luteolin | 0.2 nM | [51] |
| GO-COOH/MWCNT-COOH/PEI/Au | CV | Urinary 8-OHdG | 7.06 nM | [52] |
| Mo NP/f-MWCNTs | CV | Dopamine | 1.26 nM | [53] |
| CuCo2O4/N-MWCNT/GCE | CV | Metronidazole | 0.48 nM | [55] |
| Fe3O4-MWCNT-Ni NPs | CV, CA | Glucose | 6.7 µM | [56] |
| MWCNTs/Th/Au NP | DPV | Mannose | 0.015 µM | [57] |
| GlcDH/PNb-SWNT/GCE | CA | Glucose | 5.0 µM | [58] |
| AlcDH/PNb-SWNT/GCE | CA | Ethanol | 50 µM | [59] |
| DSDH/PMG/MWCNT/GCE | CA | Sorbitol | 100µM | [60] |
| PTBO/MWCNT/GCE | CA | NADH | 0.5 µM | [61] |
| MWCNT/PMB/GCE | CA | H2O2 | 20.7 µM | [62] |
| PmalG/MWCNT/GCE | DPV | AA | 0.23 µM | [63] |
| SWCNT/PTBO/GCE | CA | Nitrite | 0.37 µM | [64] |
| PBG/MWCNT/CFE | DPV | DA | 1.60 µM | [65] |
| PMB/MWCNT/GCE | CV | EP | 69.6 µM | [66] |
| PmalG/MWCNT/GCE | DPV | UA | 0.12 µM | [63] |
| EBNBH modified CNT-CPE | DPV | UA | 15 µM | [67] |
| SWCNT mat grown on Si | CV | DA | 1 µM | [68] |
| CNT paste with 2-PHC | SWV | Epinephrine | 9 nM | [69] |
| SWCNT-inlaying ultrathin CPE | DPV | Xanthine | 0.1 µM | [70] |
| Sensor | Technique | Analyte | LOD | References |
|---|---|---|---|---|
| HM-GQD-AuNPs | CV, ECL | Carcinoembryonic antigen | 0.01 ng∙mL–1 | [96] |
| CDs/Fe3O4 | DCAMP | Uric acid | 0.006 µM | [97] |
| CDs/CuFe2O4/CPE | SWV | RIF, NIZ | 0.022, 0.041 µM | [98] |
| AgNPs/CD-N-S/Au NPs | DPV | Streptomycin | 0.036 pg∙mL–1 | [99] |
| Fe3O4MNP-GQDs | DPV | L-tryptophan | 0.08 µM | [100] |
| N-CDs/Co3O4/MWCNTs | DPV | Flu, NF | 0.0169, 0.044 µM | [101] |
| AuNP/GQDs | DPV | Quercetin | 2.0 nM | [102] |
| NGQDs/NC/Pd | CA | H2O2 | 20 nM | [103] |
| GQDs/2D-hBN/GCE | DPV | Serotonin | 0.2 pM | [104] |
| Mag/NP/CQDs/SPE | DPV | NADH | 20 nM | [105] |
| Β-CD-GQD/GCE | SWV | AA | 0.49 µM | [106] |
| CQDs/Lac/GCE | CV | Epinephrine | 83 nM | [107] |
| Β-CD@N-GQDs/Fc/GCE | DPV | Cholesterol | 0.08 µM | [108] |
| CQDs/Cu2O/GCE | CA | Glu | 6 µM | [109] |
| CQDs/MoS2/Mo foil | CV | DA | 0.0090 µM | [110] |
| CQDs/SPE | DPV | DA | 0.05 µM | [111] |
| Β-CD/CQDs/GCE | DPV | UA | 0.01 µM | [112] |
| CQDs/GCE | CA | H2O2 | 300 nM | [113] |
| Ag NPs/CDs/GCE | CA, CV | H2O2 | 80 nM | [114] |
| NF/Hb/β-GQDs/CILE | DPV | H2O2 | 0.04 mM | [115] |
| GD-RuCl3/GCE | DPV | L-tyrosine | 0.23 µM | [116] |
| Ppy/CDs@PB/GF | CV, CA | L-Cysteine | 0.15 µM | [117] |
| N-CQD/SnO2/SPCE | DPV | Riboflavin | 8 nM | [118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, R.R.; Chusuei, C.C. Carbon Nanotubes, Graphene, and Carbon Dots as Electrochemical Biosensing Composites. Molecules 2021, 26, 6674. https://doi.org/10.3390/molecules26216674
Pandey RR, Chusuei CC. Carbon Nanotubes, Graphene, and Carbon Dots as Electrochemical Biosensing Composites. Molecules. 2021; 26(21):6674. https://doi.org/10.3390/molecules26216674
Chicago/Turabian StylePandey, Raja Ram, and Charles C. Chusuei. 2021. "Carbon Nanotubes, Graphene, and Carbon Dots as Electrochemical Biosensing Composites" Molecules 26, no. 21: 6674. https://doi.org/10.3390/molecules26216674
APA StylePandey, R. R., & Chusuei, C. C. (2021). Carbon Nanotubes, Graphene, and Carbon Dots as Electrochemical Biosensing Composites. Molecules, 26(21), 6674. https://doi.org/10.3390/molecules26216674







