Antimicrobial Effects of Basil, Summer Savory and Tarragon Lyophilized Extracts in Cold Storage Sausages
Abstract
:1. Introduction
2. Results
2.1. Plant Extracts Characterization
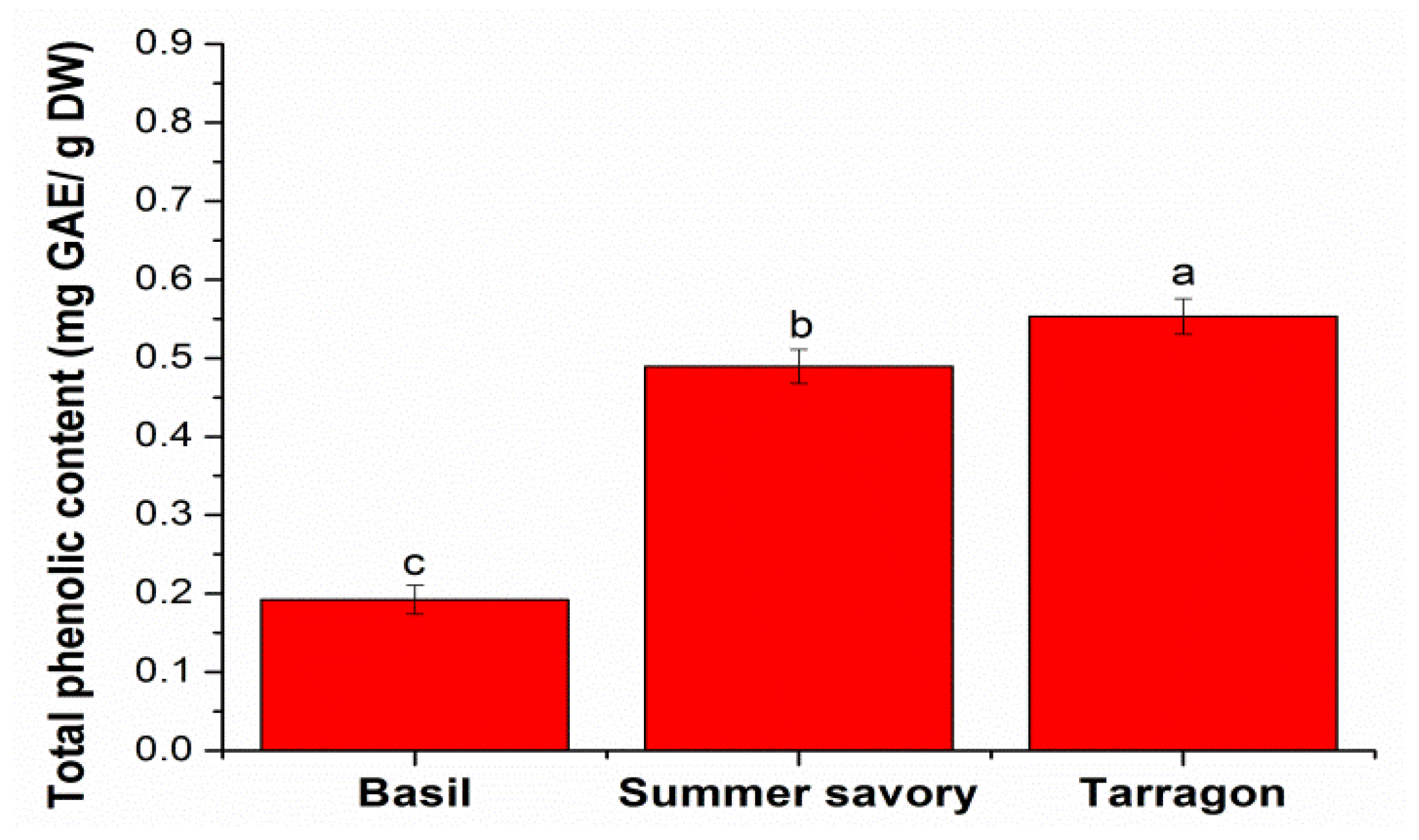
2.1.1. Total Phenolic Content
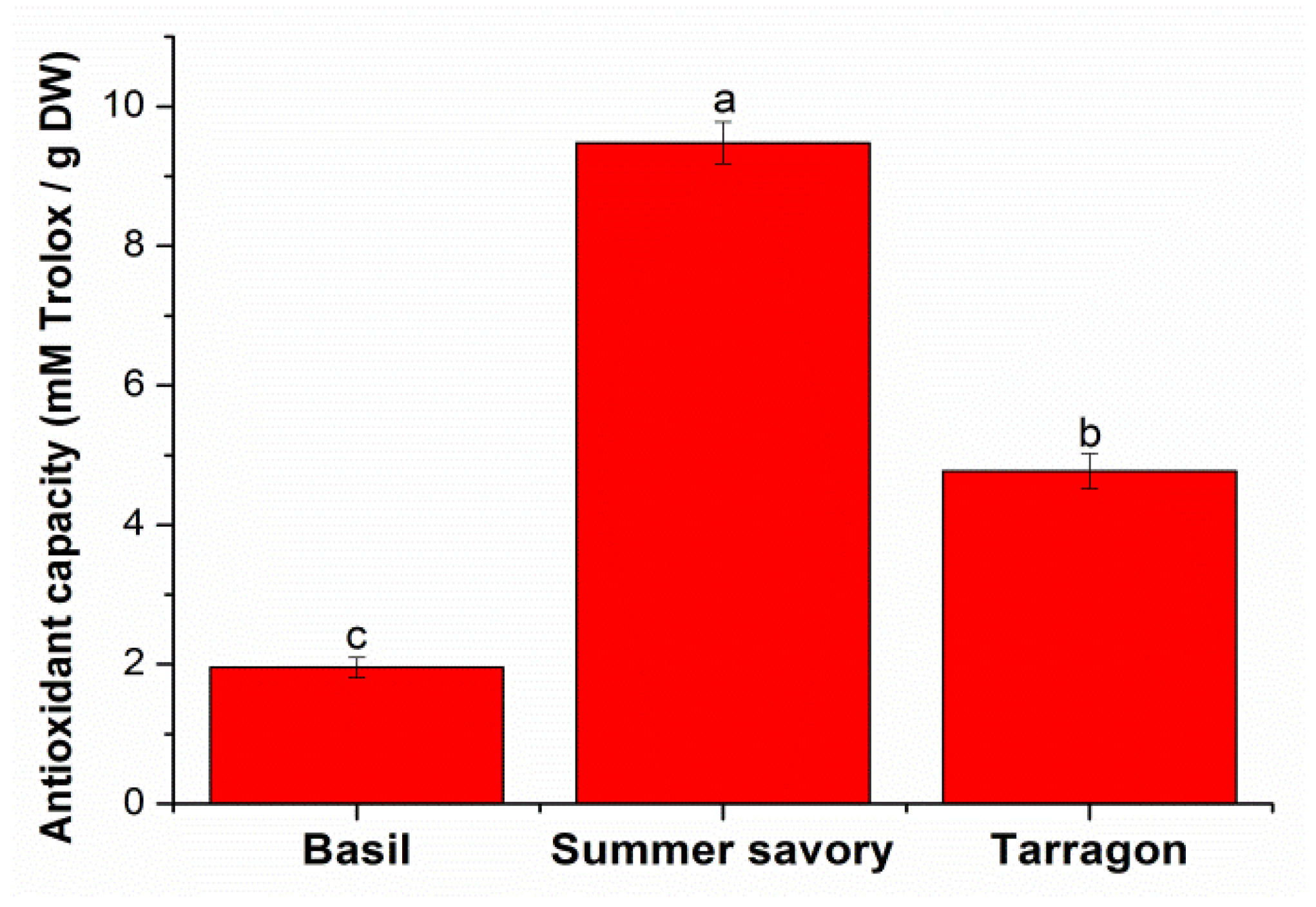
2.1.2. Antioxidant Capacity
2.1.3. Antimicrobial Capacity
2.2. Plant Extracts Characterization
3. Materials and Methods
3.1. Hydroalcoholic Extract Preparation
3.2. Characterization of the Obtained Extracts
3.2.1. Total Phenolic Content
3.2.2. Antioxidant Capacity
3.2.3. Antimicrobial Activity
3.3. Sausages Preparation
3.4. Sausages Quality Analysis
3.4.1. Sensory Analysis of Sausages
3.4.2. Moisture Content
3.4.3. pH Determination
3.4.4. Water Activity Determination
3.5. Microbiological Analysis of the Sausages
3.5.1. TNG Determination
3.5.2. Coliforms Bacteria Determination
3.5.3. Staphylococcus Genus Determination
3.5.4. Determination of Genus Salmonella
3.6. Microbiological Analysis of the Infected (Contaminated) Sausages with Reference Strains
3.7. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hasler, C.M. Functional Foods: Benefits, Concerns and Challenges—A Position Paper from the American Council on Science and Health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Bhat, Z.; Bhat, H. Functional Meat Products: A Review. Int. J. Meat Sci. 2010, 1, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Poltorak, A.; Marcinkowska-Lesiak, M.; Lendzion, K.; Onopiuk, A.; Moczkowska, M.; Wojtasik-Kalinowska, I.; Wierzbicka, A. The effect of bioactive components of plant origin on the physicochemical and sensory characteristics of functional sausages. Food Sci. Technol. 2019, 39, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Smaoui, S.; Ben Hsouna, A.; Lahmar, A.; Ennouri, K.; Mtibaa-Chakchouk, A.; Sellem, I.; Najah, S.; Bouaziz, M.; Mellouli, L. Bio-preservative effect of the essential oil of the endemic Mentha piperita used alone and in combination with BacTN635 in stored minced beef meat. Meat Sci. 2016, 117, 196–204. [Google Scholar] [CrossRef]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.R.; Babuskin, S.; Babu, P.A.S.; Sasikala, M.; Sabina, K.; Archana, G.; Sivarajan, M.; Sukumar, M. Antimicrobial and antioxidant effects of spice extracts on the shelf life extension of raw chicken meat. Int. J. Food Microbiol. 2014, 171, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.; de Kok, T.M.; Verbeke, W. Consumer attitude and purchase intention towards processed meat products with natural compounds and a reduced level of nitrite. Meat Sci. 2016, 121, 119–126. [Google Scholar] [CrossRef]
- Mariem, C.; Sameh, M.; Nadhem, S.; Soumaya, Z.; Najiba, Z.; Raoudha, E.G. Antioxidant and antimicrobial properties of the extracts from Nitraria retusa fruits and their applications to meat product preservation. Ind. Crop. Prod. 2014, 55, 295–303. [Google Scholar] [CrossRef]
- Turtoi, M. Replacement of Nitrites (Nitrites) in Meat Products. In: MeatMilk Journal [online]. Bucharest. 2015. Available online: https://www.meat-milk.ro/inlocuirea-azotitilor-nitritilor-in-produsele-din-carne/ (accessed on 15 September 2021). (In Romanian).
- Lombraña, J.I. Fundamentals and Tendencies in Freeze-Drying of Foods. In Advances in Food Dehydration, 1st ed.; Ratti, C., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 209–235. [Google Scholar] [CrossRef]
- Fellows, P. Freeze drying and freeze concentration. In Food Processing Technology: Principles and Practice, 4th ed.; Woodhead Publishing/Elsevier Science: Kent, UK, 2017; pp. 929–940. [Google Scholar]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Barley, J.; SP Scientific. Basic Principles of Freeze Drying. 2020. Available online: https://www.spscientific.com/freeze-drying-lyophilization-basics/ (accessed on 2 June 2021).
- Raț, A.M.; Muste, S.; Pop, C.; Rotar, A.M.; Nistor, A.L.; Chiș, M.S. Characterization of different plant extract types obtained from two varieties of basil (Ocimum Basilicum L.). Hop Med. Plants 2020, 1–2, 246–253. [Google Scholar]
- Cioroi, M.; Dumitriu, D. Studies on total polyphenols content and antioxidant activity of aqueous extracts from selected Lamiaceae species. Ann. Univ. Dunarea Jos Galati Fascicle VI. Food Technol. 2009, 34, 42–46. [Google Scholar]
- Leahu, A.; Damian, C.; Oroian, M.; Miclescu, V.; Ropciuc, S. Variation in content of antioxidant and free radical scavenging activity of basil (Ocimum basilicum), dill (Anethum graveolens) and parsley (Petroselinum sativum). Food Environ. Saf. 2013, XII, 347–353. [Google Scholar]
- Aburigal, Y.A.A.; Mirghani, M.E.S.; Elmogtaba, E.Y.; Sirible, A.A.M.; Hamza, N.B.; Hussein, I.H. Total phenolic content and antioxidant capacity of basil (Ocimum basilicum L.) leaves from different locations. Int. Food Res. J. 2017, 24, S378–S381. [Google Scholar]
- Chan, E.W.C.; Kong, L.Q.; Yee, K.Y.; Chua, W.Y.; Loo, T.Y. Antioxidant and antibacterial properties of some fresh and dried Labiatae herbs. Free. Radic. Antioxid. 2012, 2, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Słowianek, M.; Leszczyńska, J. Antioxidant properties of selected culinary spices. Herba Pol. 2016, 62, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Petkova, N.; Ivanov, I.; Raeva, M.; Topuzova, M.; Todorova, M.; Denev, P. Fructans and antioxidants in leaves of culinary herbs from Asteraceae and Amaryllidaceae families. Food Res. 2019, 3, 407–415. [Google Scholar] [CrossRef]
- Khezrilu, B.J.; Heidari, R. The evaluation of antioxidant activities and phenolic compounds in leaves and inflorescence of Artemisia dracunculus L. by HPLC. J. Med. Plants 2014, 13, 41–50. [Google Scholar]
- Predescu, C.; Papuc, C.; Ștefan, G.; Petcu, C. Phenolics content, antioxidant and antimicrobial activities of some extracts obtained from Romanian summer savory and Lebanon wild thyme. Sci. Work. Ser. C. Vet. Med. 2020, LXVI, 17–22. [Google Scholar]
- Mašković, P.; Veličković, V.; Mitić, M.; Đurović, S.; Zeković, Z.; Radojković, M.; Cvetanović, A.; Švarc-Gajić, J.; Vujić, J. Summer savory extracts prepared by novel extraction methods resulted inenhanced biological activity. Ind. Crop. Prod. 2017, 109, 875–881. [Google Scholar] [CrossRef]
- Coppo, E.; Marchese, A. Antibacterial activity of polyphenols. Curr. Pharm. Biotechnol. 2014, 15, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Ștefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. The Chemical and Biological Profiles of Leaves from Commercial Blueberry Varieties. Plants 2020, 9, 1193. [Google Scholar] [CrossRef]
- Ștefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. Chemical Composition and Biological Activities of the Nord-West Romanian Wild Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.) Leaves. Antioxidants 2020, 9, 495. [Google Scholar] [CrossRef]
- Sharma, H.; Mendiratta, S.K.; Agarwal, R.K.; Kumar, S.; Soni, A. Evaluation of antioxidant and anti-microbial activity of various essential oils in fresh chicken sausages. J. Food Sci. Technol. 2017, 54, 279–292. [Google Scholar] [CrossRef] [PubMed]
- de Oliveiraa, T.L.C.; de Araújo Soaresa, R.; Ramosa, E.M.; das Graças Cardosob, M.; Alvesc, E.; Piccoli, R.H. Antimicrobial activity of Satureja montana L. essential oil against Clostridium perfringens type A inoculated in mortadella-type sausages formulated with different level soft sodium nitrite. Int. J. Food Microbiol. 2011, 144, 546–555. [Google Scholar] [CrossRef] [Green Version]
- Gaio, I.; Saggiorato, A.G.; Treichel, H.; Cichoski, A.J.; Astolfi, V.; Cardoso, R.I.; Toniazzo, G.; Valduga, E.; Paroul, N.; Cansian, R.L. Antibacterial activity of basil essential oil (Ocimum basilicum L.) in Italian-type sausage. J. Consum. Prot. Food Saf. 2015, 10, 323–329. [Google Scholar] [CrossRef]
- Tomović, V.; Šojić, B.; Savanović, J.; Kocić-Tanackov, S.; Pavlić, B.; Jokanović, M.; Đorđević, V.; Parunović, N.; Martinović, A.; Vujadinović, D. New Formulation towards Healthier Meat Products: Juniperus communis L. Essential Oil as Alternative for Sodium Nitrite in Dry Fermented Sausages. Foods 2020, 9, 1066. [Google Scholar] [CrossRef]
- Ghendov-Moșanu, A. Biologically Active Compounds of Horticultural Origin for Functional Foods; Sturza, R., Ed.; Tehnica-UTM: Chisinau, Moldova, 2018; p. 236. (In Romanian) [Google Scholar]
- Fierascu, I.; Dinu-Pirvu, C.E.; Fierascu, R.C.; Velescu, B.S.; Anuta, V.; Ortan, A.; Jinga, V. Phytochemical Profile and Biological Activities of Satureja hortensis L.: A Review of the Last Decade. Molecules 2018, 23, 2458. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.F.; Attia, F.A.; Liu, Z.; Li, C.; Wei, J.; Kang, W. Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants. Food Sci. Hum. Wellness 2019, 8, 299–305. [Google Scholar] [CrossRef]
- Rappoport, Z. (Ed.) The Chemistry of Phenols, Parts 1 and 2; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003; p. 1658. [Google Scholar]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. Phenolic Compounds-Biological Activity. Marcos Soto-Hernandez, Mariana Palma-Tenango and Maria del Rosario Garcia-Mateos; IntechOpen: London, UK, 2017; Available online: https://www.intechopen.com/chapters/53128 (accessed on 2 June 2021).
- GD No. 624 from 19-09-2020 Regarding the Approval of the Quality Requirements for Meat Preparations and Products. Available online: https://www.legis.md/cautare/getResults?doc_id=123163& (accessed on 2 June 2021).
- Sandulachi, E. Water Activity in Food Products; Tehnica-UTM: Chisinau, Moldova, 2020; p. 160. (In Romanian) [Google Scholar]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus Aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, V.; Stefova, M.; Chinnici, F. Determination of the polyphenol contents in Macedonian grapes and wines by standardized spectrophotometric methods. J. Serb. Chem. Soc. 2010, 75, 45–59. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Weinstein, M.P. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2018; ISBN 978-1-56238-836-2. [Google Scholar]
- Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. Antimicrobial Susceptibility Testing Protocols; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-1-4200-1449-5. [Google Scholar]
- ISO 6658:2017. Sensory Analysis—Methodology—General Guidance. Available online: https://www.iso.org/standard/65519.html (accessed on 2 June 2021).
- Bradley, R.L. Moisture and Total Solids Analysis. In Food Analysis; Springer: Boston, MA, USA, 2010. [Google Scholar]
- ISO 4833-1:2013. Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony count at 30 °C by the Pour Plate Technique. Available online: https://www.iso.org/standard/53728.html (accessed on 2 June 2021).
- ISO. ISO 24444:2010 Cosmetics—Sun Protection Test Methods—In Vivo Determination of the Sun Protection Factor (SPF). Available online: https://www.iso.org/obp/ui/#iso:std:iso:24444:ed-1:v1:en (accessed on 15 November 2018).
- Sandulachi, E.; Rubțov, S.; Popescu, L. Microbiological Control of Food; Tehnica-UTM: Chisinau, Moldova, 2017. (In Romanian) [Google Scholar]
- ISO 6579-1:2017. Microbiology of the food chain—Horizontal method for the detection, enumeration and serotyping of Salmonella—Part 1: Detection of Salmonella spp. Available online: https://www.iso.org/standard/56712.html (accessed on 2 June 2021).
- Galețchi, P.; Buiuc, D.; Plugaru, Ș. Practical Guide to Medical Microbiology; Technical Bucharest: Chisinau, Moldova, 1997; pp. 86–99. (In Romanian) [Google Scholar]
- Preparation of McFarland Turbidity Standards. Available online: https://microbeonline.com/preparation-mcfarland-turbidity-standards/ (accessed on 2 June 2021).
| Bacterial Strain | Standard Antibiotic | MIC, mg/mL | MBC, mg/mL | ||||
|---|---|---|---|---|---|---|---|
| Basil | Summer Savory | Tarragon | Basil | Summer Savory | Tarragon | ||
| Listeria monocytogenes | 0.03 | 1.25 | 0.31 | 1.25 | 2.50 | 0.62 | 2.50 |
| Pseudomonas aeruginosa | 0.12 | 5.00 | 5.00 | 2.50 | 10.00 | 10.00 | 5.00 |
| Salmonella typhimurium | 0.12 | 5.00 | 5.00 | 5.00 | 10.00 | 10.00 | 10.00 |
| Escherichia coli | 0.24 | 5.00 | 2.50 | 5.00 | 10.00 | 5.00 | 10.00 |
| Quality Indicators | Storage Time | Control | SBE 0.1% | SBE 0.2% | SBE 0.3% | SSE 0.05% | SSE 0.1% | SSE 0.2% | STE 0.1% | STE 0.2% | STE 0.3% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture content, % | 1st day | 64.37 ± 0.09 f | 67.38 ± 0.06 e | 69.07 ± 0.06 d | 69.16 ± 0.15 c,d | 69.33 ± 0.09 b,c,d | 69.44 ± 0.06 b,c | 71.48 ± 0.09 a | 69.48 ± 0.12 b,c | 69.56 ± 0.03 b | 69.64 ± 0.06 b |
| 3rd day | 58.1 ± 0.15 g | 59.3 ± 0.03 f | 59.99 ± 0.03 e | 61.06 ± 0.09 c,d | 59.32 ± 0.15 f | 60.28 ± 0.06 e | 60.78 ± 0.03 d | 62.51 ± 0.09 a | 62.16 ± 0.06 b | 61.09 ± 0.03 c | |
| 6th day | 54.89 ± 0.15 d | 55.52 ± 0.06 c | 55.57 ± 0.06 c | 55.94 ± 0.12 b | 55.04 ± 0.03 d | 55.11 ± 0.09 d | 55.83 ± 0.03 b,c | 55.74 ± 0.12 b,c | 56.41 ± 0.03 a | 55.55 ± 0.06 c | |
| Active acidity pH | 1st day | 6.38 ± 0.06 a | 6.15 ± 0.12 a | 6.13 ± 0.06 a | 6.12 ± 0.06 a | 6.16 ± 0.09 a | 6.12 ± 0.03 a | 6.10 ± 0.09 a | 6.29 ± 0.12 a | 6.14 ± 0.03 a | 6.12 ± 0.06 a |
| 3rd day | 6.40 ± 0.06 a | 6.17 ± 0.09 a | 6.26 ± 0.06 a | 6.22 ± 0.09 a | 6.33 ± 0.03 a | 6.24 ± 0.12 a | 6.28 ± 0.12 a | 6.41 ± 0.12 a | 6.33 ± 0.09 a | 6.28 ± 0.03 a | |
| 6th day | 6.32 ± 0.09 a | 6.24 ± 0.06 a | 6.34 ± 0.12 a | 6.32 ± 0.06 a | 6.30 ± 0.09 a | 6.24 ± 0.12 a | 6.24 ± 0.12 a | 6.28 ± 0.12 a | 6.35 ± 0.06 a | 6.26 ± 0.12 a | |
| Water activity aw, c.u. | 1st day | 0.875 ± 0.003 a | 0.873 ± 0.003 a | 0.873 ± 0.003 a | 0.873 ± 0.003 a | 0.876 ± 0.003 a | 0.875 ± 0.002 a | 0.874 ± 0.000 a | 0.874 ± 0.000 a | 0.871 ± 0.003 a | 0.868 ± 0.003 a |
| 3rd day | 0.869 ± 0.000 a | 0.871 ± 0.003 a | 0.871 ± 0.000 a | 0.872 ± 0.000 a | 0.871 ± 0.000 a | 0.871 ± 0.003 a | 0.869 ± 0.003 a | 0.874 ± 0.000 a | 0.871 ± 0.003 a | 0.868 ± 0.003 a | |
| 6th day | 0.866 ± 0.000 a | 0.869 ± 0.000 a | 0.869 ± 0.003 a | 0.870 ± 0.003 a | 0.869 ± 0.003 a | 0.869 ± 0.000 a | 0.869 ± 0.002 a | 0.874 ± 0.003 a | 0.871 ± 0.003 a | 0.868 ± 0.003 a | |
| Average score of sensory profile | 3rd day | 5.00 ± 0.00 a | 4.95 ± 0.02 a,b | 4.68 ± 0.03 c | 4.32 ± 0.04 e | 4.99 ± 0.01 a,b | 4.66 ± 0.03 c | 4.35 ± 0.03 e | 4.89 ± 0.01 b | 4.51 ± 0.03 d | 3.99 ± 0.04 f |
| Exterior appearance | 5.00 ± 0.00 a | 5.00 ± 0.00 a | 4.95 ± 0.01 a,b | 4.90 ± 0.03 b,c | 5.00 ± 0.00 a | 4.98 ± 0.01 a | 4.94 ± 0.02 a,b,c | 5.00 ± 0.00 a | 4.93 ± 0.02 a,b,c | 4.87 ± 0.05 c | |
| Color and appearance in section | 5.00 ± 0.00 a | 5.00 ± 0.00 a | 4.75 ± 0.02 b | 4.45 ± 0.01 d | 5.00 ± 0.00 a | 4.75 ± 0.02 b | 4.60 ± 0.05 c | 5.00 ± 0.00 a | 4.50 ± 0.02 c,d | 3.75 ± 0.06 e | |
| Odor | 5.00 ± 0.00 a | 5.00 ± 0.00 a | 4.87 ± 0.04 b | 3.83 ± 0.06 f | 5.00 ± 0.00 a | 4.37 ± 0.02 d | 4.00 ± 0.01 e | 4.75 ± 0.02 c | 3.83 ± 0.04 f | 3.63 ± 0.02 g | |
| Taste | 5.00 ± 0.00 a | 4.75 ± 0.11 b | 3.83 ± 0.05 d | 3.46 ± 0.07 e | 4.95 ± 0.02 a | 4.27 ± 0.08 c | 3.35 ± 0.10 e,f | 4.70 ± 0.10 b | 4.33 ± 0.05 c | 3.25 ± 0.05 f | |
| Cosistency | 5.00 ± 0.00 a | 4.99 ± 0.01 a | 4.98 ± 0.02 a,b | 4.98 ± 0.01 a,b | 4.99 ± 0.01 a,b | 4.95 ± 0.02 a,b | 4.87 ± 0.07 b | 4.99 ± 0.01 a | 4.98 ± 0.02 a,b | 4.45 ± 0.05 c |
| Sample/Storage Time | TNMAFA, CFU/g | Coliforms Bacteriain 1 g | Staphylococcus aureus in 1 g | Salmonella spp. in 25 g |
|---|---|---|---|---|
| After 24 h | ||||
| Control | 103 | - | - | - |
| SBE 0.1% | 103 | - | - | - |
| SBE 0.2% | 103 | - | - | - |
| SBE 0.3% | 103 | - | - | - |
| SSE 0.05% | 103 | - | - | - |
| SSE 0.1% | 102 | - | - | - |
| SSE 0.2% | 103 | - | - | - |
| SET 0.1% | 103 | - | - | - |
| SET 0.2% | 103 | - | - | - |
| SET 0.3% | 102 | - | - | - |
| After 96 h (4 days) | ||||
| Control | 104 | - | - | - |
| SBE 0.1% | 103 | - | - | - |
| SBE 0.2% | 103 | - | - | - |
| SBE 0.3% | 102 | - | - | - |
| SSE 0.05% | 103 | - | - | - |
| SSE 0.1% | 103 | - | - | - |
| SSE 0.2% | 103 | - | - | - |
| SET 0.1% | 102 | - | - | - |
| SET 0.2% | 103 | - | - | - |
| SET 0.3% | 104 | - | - | - |
| After 168 h (7 days) | ||||
| Control | 105 | - | - | - |
| SBE 0.1% | 104 | - | - | - |
| SBE 0.2% | 103 | - | - | - |
| SBE 0.3% | 104 | - | - | - |
| SSE 0.05% | 104 | - | - | - |
| SSE 0.1% | 104 | - | - | - |
| SSE 0.2% | 104 | - | - | - |
| SET 0.1% | 104 | - | - | - |
| SET 0.2% | 104 | - | - | - |
| SET 0.3% | 105 | - | - | - |
| Sample | Staphylococcus aureus mln/g | Escherichia coli mln/g | ||
|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |
| Control | 320 | 650 | 528 | 788 |
| SBE 0.1% | 312 | 26 | 67 | 232 |
| SBE 0.2% | 286 | 160 | 52 | 184 |
| SBE 0.3% | 242 | 74 | 46 | 242 |
| SSE 0.05% | 263 | 316 | 216 | 244 |
| SSE 0.1% | 146 | 136 | 104 | 103 |
| SSE 0.2% | 102 | 216 | 88 | 196 |
| SET 0.1% | 316 | 236 | 146 | 702 |
| SET 0.2% | 206 | 256 | 128 | 248 |
| SET 0.3% | 112 | 221 | 106 | 256 |
| Sensory Characteristic | Product Description |
|---|---|
| Exterior appearance | Small sticks with a clean, dry surface, without stains, adhesions, affluences of composition and ruptures of the membrane. The ends of the membranes of small bars are twisted or tied with string or thread. |
| Color and appearance in section | The color of the composition from light pink (control) to the color characteristic of the type of plant extract, finely chopped with pieces of food ingredients other than meat, according to the recipe, mixed evenly and without gaps. The presence of fine porosity in the form of gaps and the insignificant presence of coarse connective tissue are allowed. |
| Odor | Characteristic of the type of product with an odor of respective plant extract, without a foreign odor. |
| Taste | Characteristic of the type of product with a taste of respective plant extract, without foreign taste. |
| Consistency | Fine, juicy (hot) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macari, A.; Sturza, R.; Lung, I.; Soran, M.-L.; Opriş, O.; Balan, G.; Ghendov-Mosanu, A.; Vodnar, D.C.; Cojocari, D. Antimicrobial Effects of Basil, Summer Savory and Tarragon Lyophilized Extracts in Cold Storage Sausages. Molecules 2021, 26, 6678. https://doi.org/10.3390/molecules26216678
Macari A, Sturza R, Lung I, Soran M-L, Opriş O, Balan G, Ghendov-Mosanu A, Vodnar DC, Cojocari D. Antimicrobial Effects of Basil, Summer Savory and Tarragon Lyophilized Extracts in Cold Storage Sausages. Molecules. 2021; 26(21):6678. https://doi.org/10.3390/molecules26216678
Chicago/Turabian StyleMacari, Artur, Rodica Sturza, Ildiko Lung, Maria-Loredana Soran, Ocsana Opriş, Greta Balan, Aliona Ghendov-Mosanu, Dan Cristian Vodnar, and Daniela Cojocari. 2021. "Antimicrobial Effects of Basil, Summer Savory and Tarragon Lyophilized Extracts in Cold Storage Sausages" Molecules 26, no. 21: 6678. https://doi.org/10.3390/molecules26216678







