In Vitro and In Silico Interaction Studies with Red Wine Polyphenols against Different Proteins from Human Serum †
Abstract
:1. Introduction
2. Results
2.1. Bioactive Properties of the Investigated Wines
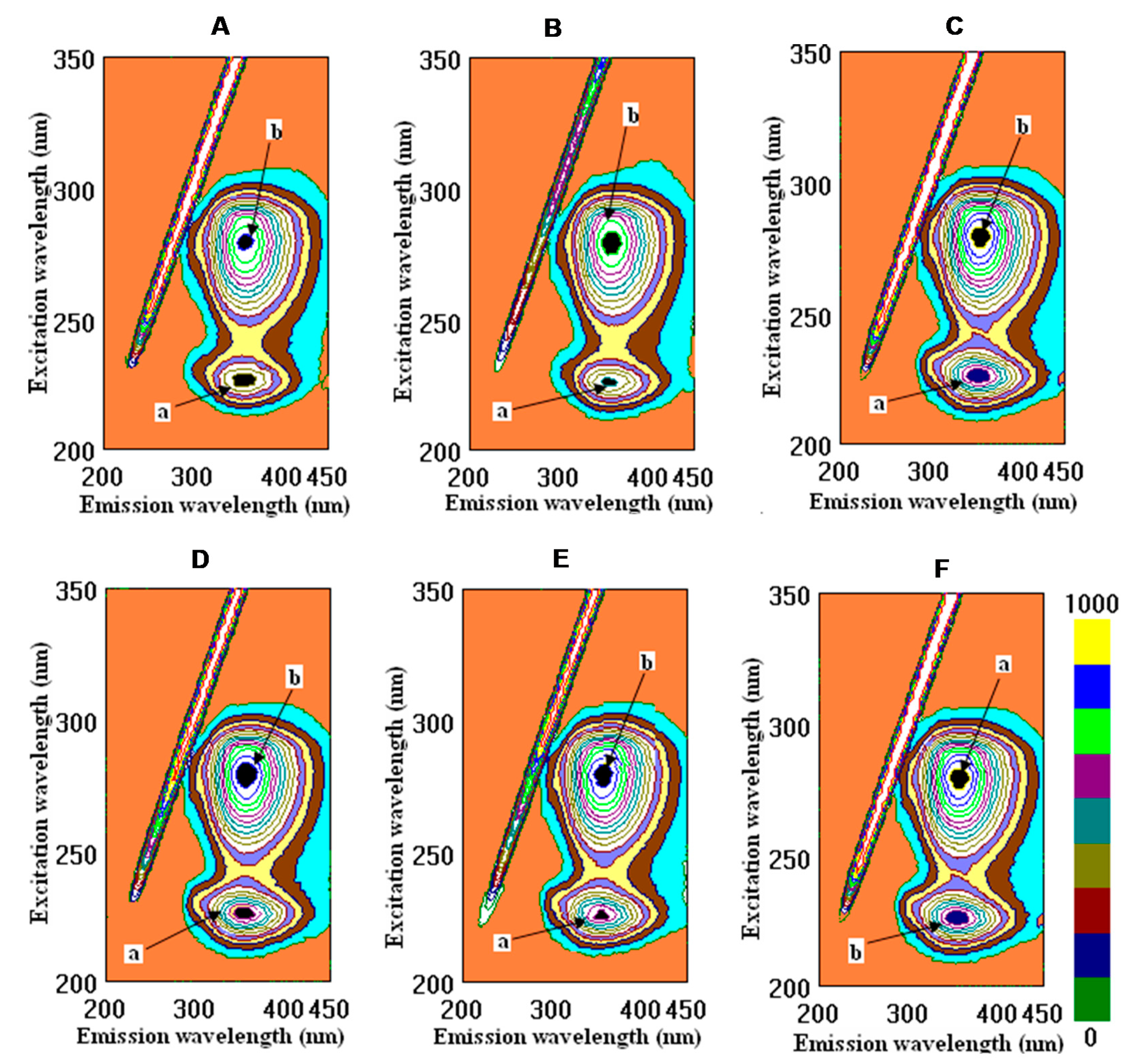
2.2. Binding Properties of Wines and Some Phenolic Compounds with the Main Human Proteins
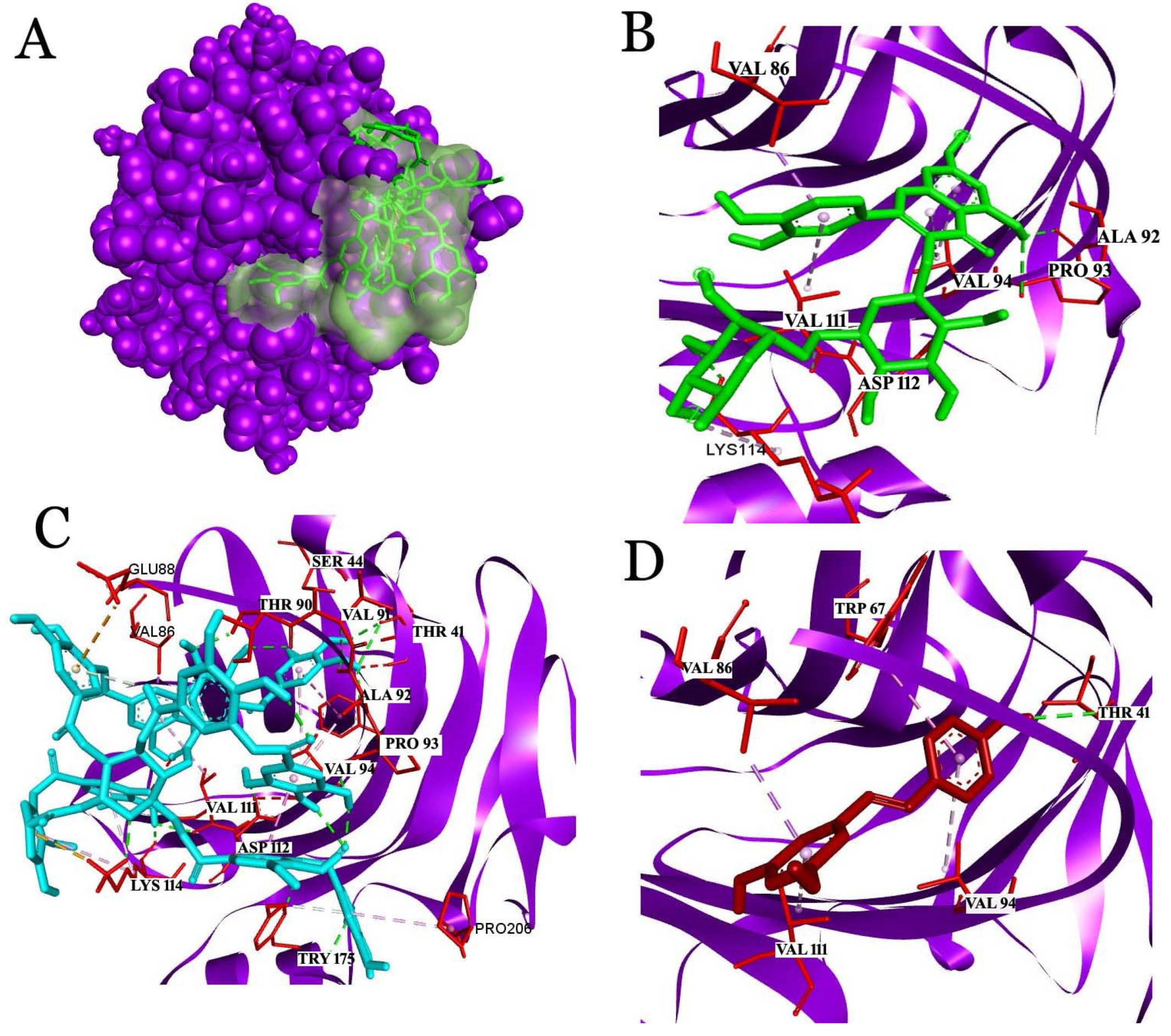
2.3. Docking Studies
2.3.1. Interaction Analysis with C-Reactive Protein (CRP)
2.3.2. Interaction Analysis with Fibrinogen
2.3.3. Interaction Analysis with Human Glutathione Peroxidase 3 (GPX3)
2.3.4. Analysis of the Interaction with Human Serum Albumin (HSA)
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Samples
4.3. Analyses of Bioactive Compounds
4.4. Fluorometric Studies
4.5. Molecular Docking of Ligands with the Serum Proteins
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Giglio, R.V.; Patti, A.M.; Cicero, A.F.; Lippi, G.; Rizzo, M.; Toth, P.P.; Banach, M. Polyphenols: Potential Use in the Prevention and Treatment of Cardiovascular Diseases. Curr. Pharm. Des. 2018, 24, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Santhakumar, A.B.; Bulmer, A.C.; Singh, I. A review of the mechanisms and effectiveness of dietary polyphenols in reducing oxidative stress and thrombotic risk. J. Hum. Nutr. Diet. 2013, 27, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Zachwieja, Z.; Katrich, E.; Pawelzik, E.; Haruenkit, R.; Trakhtenberg, S.; Martin-Belloso, O. Comparison of the contents of the main antioxidant compounds and the antioxidant activity of white grapefruit and his new hybrid. LWT 2004, 37, 337–343. [Google Scholar] [CrossRef]
- Gorinstein, S.; Zemser, M.; Weisz, M.; Halevy, S.; Martin-Belloso, O.; Trakhtenberg, S. The influence of alcohol-containing and alcohol-free beverages on lipid levels and lipid peroxides in serum of rats. J. Nutr. Biochem. 1998, 9, 682–686. [Google Scholar] [CrossRef]
- Garaguso, I.; Nardini, M. Polyphenols content, phenolics profile and antioxidant activity of organic red wines produced without sulfur dioxide/sulfites addition in comparison to conventional red wines. Food Chem. 2015, 179, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Nardini, M.; Virgili, F.; Scaccini, C. Role of dietary polyphenols in the platelet aggregation network—A review of the in vitro studies. Curr. Top. Nutraceutical Res. 2006, 4, 1–21. [Google Scholar]
- Tekos, F.; Makri, S.; Skaperda, Z.-V.; Patouna, A.; Terizi, K.; Kyriazis, I.; Kotseridis, Y.; Mikropoulou, E.; Papaefstathiou, G.; Halabalaki, M.; et al. Assessment of Antioxidant and Antimutagenic Properties of Red and White Wine Extracts In Vitro. Metabolites 2021, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Di Minno, G.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef] [Green Version]
- Shafreen, R.; Lakshmi, S.; Pandian, S.; Park, Y.; Kim, Y.; Paśko, P.; Deutsch, J.; Katrich, E.; Gorinstein, S. Unraveling the Antioxidant, Binding and Health-Protecting Properties of Phenolic Compounds of Beers with Main Human Serum Proteins: In Vitro and In Silico Approaches. Molecules 2020, 25, 4962. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Abas, F.; Park, Y.-S.; Park, Y.-K.; Ham, K.-S.; Kang, S.-G.; Lubinska-Szczygeł, M.; Ezra, A.; Gorinstein, S. Bioactivities of Phenolic Compounds from Kiwifruit and Persimmon. Molecules 2021, 26, 4405. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, I.; Spagnuolo, C.; Russo, G.; Russo, M.; Cervellera, C.; Moccia, S. The Pro-Oxidant Activity of Red Wine Polyphenols Induces an Adaptive Antioxidant Response in Human Erythrocytes. Antioxidants 2021, 10, 800. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, W.; Meng, Y.; Zhang, Y.; Jin, G.; Fang, Z. Wine phenolic profile altered by yeast: Mechanisms and influences. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3579–3619. [Google Scholar] [CrossRef]
- Giacosa, S.; Parpinello, G.P.; Segade, S.R.; Ricci, A.; Paissoni, M.A.; Curioni, A.; Marangon, M.; Mattivi, F.; Arapitsas, P.; Moio, L.; et al. Diversity of Italian red wines: A study by enological parameters, color, and phenolic indices. Food Res. Int. 2021, 143, 110277. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Rao, L.V.M.; Agrawal, A.; Pendurthi, U.R. Effect of wine phenolics on cytokine-induced C-reactive protein expression. J. Thromb. Haemost. 2007, 5, 1309–1317. [Google Scholar] [CrossRef] [Green Version]
- Bijak, M.; Nowak, P.; Borowiecka, M.; Ponczek, M.B.; Zbikowska, H.; Wachowicz, B. Protective effects of (−)-epicatechin against nitrative modifications of fibrinogen. Thromb. Res. 2012, 130, e123–e128. [Google Scholar] [CrossRef]
- Zhang, Q.-A.; Fu, X.; Martín, J.F.G. Effect of ultrasound on the interaction between (−)-epicatechin gallate and bovine serum albumin in a model wine. Ultrason. Sonochem. 2017, 37, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Bonaventura, A.; Montecucco, F.; Dallegri, F.; Carbone, F. Impact of Red Wine Consumption on Cardiovascular Health. Curr. Med. Chem. 2019, 26, 3542–3566. [Google Scholar] [CrossRef]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of Red Wine Consumption to Human Health Protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-K.; He, F.; Wang, Y.-X.; Liu, X.; Duan, C.-Q.; Wang, J. Influences of Berry Size on Fruit Composition and Wine Quality of Vitis vinifera L. cv. ‘Cabernet Sauvignon’ Grapes. S. Afr. J. Enol. Vitic. 2018, 39, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Bernal, Ó.A.; Coria-Oliveros, A.J.; Vazquez-Flores, A.A.; de la Rosa, L.A.; Núñez-Gastélum, A.; Rodrigo-García, J.; Ayala-Zavala, J.F.; Alvarez-Parrilla, E. Evolution of phenolic content, antioxidant capacity and phenolic profile during cold pre-fermentative maceration and subsequent fermentation of Cabernet Sauvignon red wine. S. Afr. J. Enol. Vitic. 2020, 41, 72–82. [Google Scholar] [CrossRef]
- Padilha, C.V.D.S.; Miskinis, G.A.; Souza, M.E.; Pereira, G.E.; Oliveira, D.; Bordignon-Luiz, M.; Lima, M.D.S. Rapid determination of flavonoids and phenolic acids in grape juices and wines by RP-HPLC/DAD: Method validation and characterization of commercial products of the new Brazilian varieties of grape. Food Chem. 2017, 228, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Montoro, M.; Olalla, M.; Gimenez-Martinez, R.; Navarro-Alarcon, M.; Rufián-Henares, J.A. Phenolic compounds and antioxidant activity of Spanish commercial grape juices. J. Food Compos. Anal. 2015, 38, 19–26. [Google Scholar] [CrossRef]
- Raczkowska, J.; Mielcarz, G.; Howard, A.; Raczkowski, M. UPLC and Spectrophotometric Analysis of Polyphenols in Wines Available in the Polish Market. Int. J. Food Prop. 2011, 14, 514–522. [Google Scholar] [CrossRef]
- Landrault, N.; Poucheret, P.; Ravel, P.; Gasc, F.; Cros, G.; Teissedre, P.-L. Antioxidant Capacities and Phenolics Levels of French Wines from Different Varieties and Vintages. J. Agric. Food Chem. 2001, 49, 3341–3348. [Google Scholar] [CrossRef]
- del Barrio-Galán, R.; Medel-Maraboli, M.; Peña-Neira, A. Effect of different aging techniques on the polysaccharide and phenoliccomposition and sensory characteristics of Syrah red wines fermented using different yeast strains. Food Chem. 2015, 179, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Polovka, M.; Martinez-Ayala, A.L.; González-Aguilar, G.A.; Ham, K.S.; Kang, S.G.; Park, Y.K.; Heo, B.G.; Namiesnik, J.; Gorinstein, S. Fluorescence studies by quenching and protein unfolding on the interaction of bioactive compounds in water extracts of kiwifruit cultivars with human serum albumin. J. Lumin. 2015, 160, 71–77. [Google Scholar] [CrossRef]
- Ren, M.; Du, G.; Tian, C.; Song, X.; Zhu, D.; Wang, X.; Zhang, J. Influence of Different Phenolic Fractions on Red Wine Astringency Based on Polyphenol/Protein Binding. S. Afr. J. Enol. Vitic. 2017, 38. [Google Scholar] [CrossRef]
- Mondal, P.; Bose, A. Spectroscopic overview of quercetin and its Cu(II) complex interaction with serum albumins. BioImpacts 2019, 9, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Hu, X.; Xu, M.; Wang, Y. Studies on interactions of pentagalloyl glucose, ellagic acid and gallic acid with bovine serum albumin: A spectroscopic analysis. Food Chem. 2020, 324, 126872. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-Y.; Zhao, P.; Wang, X.-Y.; Zhang, J.; Wang, X.H.; Tian, C.-R.; Ren, M.-M.; Chen, T.-G.; Yuan, H.-H. Evaluation of the potential astringency of the skins and seeds of different grape varieties based on polyphenol/protein binding. Food Sci. Technol. 2019, 39, 930–938. [Google Scholar] [CrossRef] [Green Version]
- Gorinstein, S.; Caspi, A.; Libman, I.; Trakhtenberg, S. Cardioprotective effect of alcohol consumption: Contemporary concepts. Nutr. Res. 2002, 22, 659–666. [Google Scholar] [CrossRef]
- Gorinstein, S.; Caspi, A.; Libman, I.; Trakhtenberg, S. Mechanism of cardioprotective effect and the choice of alcoholic beverage. Am. J. Cardiol. 2000, 85, 280–281. [Google Scholar] [CrossRef]
- Roumenina, L.T.; Ruseva, M.M.; Zlatarova, A.; Ghai, R.; Kolev, M.; Olova, N.; Gadjeva, M.; Agrawal, A.; Bottazzi, B.; Mantovani, A.; et al. Interaction of C1q with IgG1, C-reactive Protein and Pentraxin 3: Mutational Studies Using Recombinant Globular Head Modules of Human C1q A, B, and C Chains. Biochemistry 2006, 45, 4093–4104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altieri, D.; Plescia, J.; Plow, E. The structural motif glycine 190-valine 202 of the fibrinogen gamma chain interacts with CD11b/CD18 integrin (alpha M beta 2, Mac-1) and promotes leukocyte adhesion. J. Biol. Chem. 1993, 268, 1847–1853. [Google Scholar] [CrossRef]
- Ugarova, T.P.; Lishko, V.K.; Podolnikova, N.P.; Okumura, N.; Merkulov, S.M.; Yakubenko, V.P.; Yee, V.C.; Lord, A.S.T.; Haas, T.A. Sequence γ377−395(P2), but Not γ190−202(P1), Is the Binding Site for the αMI-Domain of Integrin αMβ2 in the γC-Domain of Fibrinogen. Biochemistry 2003, 42, 9365–9373. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.A.; Ryu, J.K.; Akassoglou, K. Fibrinogen in neurological diseases: Mechanisms, imaging and therapeutics. Nat. Rev. Neurosci. 2018, 19, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.A.; Bauer, J.; Flick, M.J.; Sikorski, S.L.; Nuriel, T.; Lassmann, H.; Degen, J.L.; Akassoglou, K. The fibrin-derived γ377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J. Exp. Med. 2007, 204, 571–582. [Google Scholar] [CrossRef]
- Song, J.; Yü, Y.; Xing, R.; Guo, X.; Liu, D.; Wei, J.; Song, H. Unglycosylated recombinant human glutathione peroxidase 3 mutant from Escherichia coli is active as a monomer. Sci. Rep. 2014, 4, 6698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, B.; Huang, W.; Åkesson, B.; Ladenstein, R. The crystal structure of seleno-glutathione peroxidase from human plasma at 2.9 Å resolution. J. Mol. Biol. 1997, 268, 869–885. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.F.; Nascimento, J.M.D.; Sobral, R.V.D.S.; de Amorim, E.L.C.; Silva, R.; Leite, A.C.L. Tannic acid as a precipitating agent of human plasma proteins. Eur. J. Pharm. Sci. 2019, 138, 105018. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhao, Y.; Wang, H.; Yuan, Y.; Yang, F.; Zhang, C.; Yamamoto, K. Noncovalent Interaction of Dietary Polyphenols with Common Human Plasma Proteins. J. Agric. Food Chem. 2011, 59, 10747–10754. [Google Scholar] [CrossRef]
- Coşan, D.T.; Saydam, F.; Özbayer, C.; Doğaner, F.; Soyocak, A.; Güneş, H.V.; Değirmenci, I.; Kurt, H.; Üstüner, M.C.; Bal, C. Impact of tannic acid on blood pressure, oxidative stress and urinary parameters in L-NNA-induced hypertensive rats. Cytotechnology 2013, 67, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Cui, L.; Han, X.; Zhang, Y.; Zhang, X.; Chu, X.; Zhang, F.; Zhang, Y.; Chu, L. Protective effects of tannic acid on acute doxorubicin-induced cardiotoxicity: Involvement of suppression in oxidative stress, inflammation, and apoptosis. Biomed. Pharmacother. 2017, 93, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Tikoo, K.; Sane, M.S.; Gupta, C. Tannic acid ameliorates doxorubicin-induced cardiotoxicity and potentiates its anti-cancer activity: Potential role of tannins in cancer chemotherapy. Toxicol. Appl. Pharmacol. 2011, 251, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.; Tabassum, H.; Parvez, S. Tannic Acid Provides Neuroprotective Effects against Traumatic Brain Injury through the PGC-1α/Nrf2/HO-1 Pathway. Mol. Neurobiol. 2020, 57, 2870–2885. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Anti-amyloidogenic activity of tannic acid and its activity to destabilize Alzheimer’s β-amyloid fibrils in vitro. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2004, 1690, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Zhong, L.; Yu, Z.; Qi, J. Anti-neuroinflammatory effects of tannic acid against lipopolysaccharide-induced BV2 microglial cells via inhibition of NF-κB activation. Drug Dev. Res. 2018, 80, 262–268. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Kim, J.; Jeong, H.-J.; Kim, H.-M. Potential anti-inflammatory effect of Madi-Ryuk and its active ingredient tannic acid on allergic rhinitis. Mol. Immunol. 2019, 114, 362–368. [Google Scholar] [CrossRef]
- Dong, G.; Liu, H.; Yu, X.; Zhang, X.; Lu, H.; Zhou, T.; Cao, J. Antimicrobial and anti-biofilm activity of tannic acid against Staphylococcus aureus. Nat. Prod. Res. 2017, 32, 2225–2228. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B. Tannic Acid with Antiviral and Antibacterial Activity as A Promising Component of Biomaterials—A Minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef]
- Fei, J.; Sun, Y.; Duan, Y.; Xia, J.; Yu, S.; Ouyang, P.; Wang, T.; Zhang, G. Low concentration of rutin treatment might alleviate the cardiotoxicity effect of pirarubicin on cardiomyocytes via activation of PI3K/AKT/mTOR signaling pathway. Biosci. Rep. 2019, 39, BSR20190546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, F.M.; Coelho, M.N.; José-Chagas, F.D.N.; Malvar, D.D.C.; Kanashiro, A.; Cunha, F.Q.; Vianna-Filho, M.D.M.; Pinto, A.D.C.; Vanderlinde, F.A.; Costa, S.S. Oral treatments with a flavonoid-enriched fraction from Cecropia hololeuca and with rutin reduce articular pain and inflammation in murine zymosan-induced arthritis. J. Ethnopharmacol. 2020, 260, 112841. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.S.; Quelhas, S.; Silva, A.M.; Souto, E.B. Nanoemulsions for delivery of flavonoids: Formulation and in vitro release of rutin as model drug. Pharm. Dev. Technol. 2014, 19, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A. Immunomodulatory effect of rutin, catechin, and hesperidin on macrophage function. Indian J. Biochem. Biophys. 2020, 57, 58–63. [Google Scholar]
- Na, J.Y.; Song, K.; Kim, S.; Kwon, J. Rutin protects rat articular chondrocytes against oxidative stress induced by hydrogen peroxide through SIRT1 activation. Biochem. Biophys. Res. Commun. 2016, 473, 1301–1308. [Google Scholar] [CrossRef]
- Luo, M.; Sui, Y.; Tian, R.; Lu, N. Formation of a bovine serum albumin diligand complex with rutin for the suppression of heme toxicity. Biophys. Chem. 2020, 258, 106327. [Google Scholar] [CrossRef]
- Naseer, B.; Iqbal, S.; Wahid, N.; Qazi, H.J.; Nadeem, M.; Nawaz, M. Evaluation of antioxidant and antimicrobial potential of rutin in combination with butylated hydroxytoluene in cheddar cheese. J. Food Process. Preserv. 2020, 45, e15046. [Google Scholar] [CrossRef]
- Lee, L.-C.; Hou, Y.-C.; Hsieh, Y.-Y.; Chen, Y.-H.; Shen, Y.-C.; Lee, I.-J.; Shih, M.-C.M.; Hou, W.-C.; Liu, H.-K. Dietary supplementation of rutin and rutin-rich buckwheat elevates endogenous glucagon-like peptide 1 levels to facilitate glycemic control in type 2 diabetic mice. J. Funct. Foods 2021, 85, 104653. [Google Scholar] [CrossRef]
- Rakshit, S.; Shukla, P.; Verma, A.; Nirala, S.K.; Bhadauria, M. Protective role of rutin against combined exposure to lipopolysaccharide and D-galactosamine-induced dysfunctions in liver, kidney, and brain: Hematological, biochemical, and histological evidences. J. Food Biochem. 2021, 45, e13605. [Google Scholar] [CrossRef]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef]
- Lin, S.C.; Ho, C.T.; Chuo, W.H.; Li, S.M.; Wang, T.T.; Lin, C.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017, 17, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedetti, F.; Sorrenti, V.; Buriani, A.; Fortinguerra, S.; Scapagnini, G.; Zella, D. Resveratrol, Rapamycin and Metformin as Modulators of Antiviral Pathways. Viruses 2020, 12, 1458. [Google Scholar] [CrossRef] [PubMed]
- Filardo, S.; Di Pietro, M.; Mastromarino, P.; Sessa, R. Therapeutic potential of resveratrol against emerging respiratory viral infections. Pharmacol. Ther. 2020, 214, 107613. [Google Scholar] [CrossRef] [PubMed]
- Ellen, B.M.; Kumar, N.D.; Bouma, E.M.; Troost, B.; van de Pol, D.P.I.; Van der Ende-Metselaar, H.H.; Ap-perloo, L.; van Gosliga, D.; van den Berge, M.; Nawijn, M.C. Resveratrol and Pterostilbene inhibit SARS-CoV-2 replication in air-liquid interface cultured human primary bronchial epithelial cells. Viruses 2021, 13, 1335. [Google Scholar] [CrossRef] [PubMed]
- Ramdani, L.H.; Bachari, K. Potential therapeutic effects of resveratrol against SARS-CoV-2. Acta Virol. 2020, 64, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Stromsnes, K.; Lagzdina, R.; Olaso-Gonzalez, G.; Gimeno-Mallench, L.; Gambini, J. Pharmacological Properties of Polyphenols: Bioavailability, Mechanisms of Action, and Biological Effects in In Vitro Studies, Animal Models, and Humans. Biomedicines 2021, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Guo, Y.; Xu, B.; Li, W.; Lin, Y.; Sun, X.; Ding, C.; Zhang, X. Effects of Tannic Acid, Green Tea and Red Wine on hERG Channels Expressed in HEK293 Cells. PLoS ONE 2015, 10, e0143797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.K.; Luo, J.; Lau, C.W.; Chen, Z.; Tian, X.Y.; Huang, Y. Pharmacological basis and new insights of resveratrol action in the cardiovascular system. Br. J. Pharmacol. 2019, 177, 1258–1277. [Google Scholar] [CrossRef] [PubMed]
- Kuntić, V.; Filipovic, I.; Vujić, Z. Effects of Rutin and Hesperidin and their Al(III) and Cu(II) Complexes on in Vitro Plasma Coagulation Assays. Molecules 2011, 16, 1378–1388. [Google Scholar] [CrossRef]
- dos Santos, I.; Bosman, G.; Aleixandre-Tudo, J.L.; du Toit, W. Direct quantification of red wine phenolics using fluorescence spectroscopy with chemometrics. Talanta 2022, 236, 122857. [Google Scholar] [CrossRef] [PubMed]
- Araujo, L.D.; Parr, W.V.; Grose, C.; Hedderley, D.; Masters, O.; Kilmartin, P.A.; Valentin, D. In-mouth attributes driving perceived quality of Pinot noir wines: Sensory and chemical characterization. Food Res. Int. 2021, 149, 110665. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Dai, L.; Sun, Y.; Li, C.; Ruan, S.; Li, J.; Xu, Y. Determination of the age of dry red wine by multivariate techniques using color parameters and pigments. Food Control. 2021, 129, 108253. [Google Scholar] [CrossRef]
- Bordiga, M.; Perestrelo, R.; Câmara, J.S.; Yang, Q.; Corke, H.; Travaglia, F.; Locatelli, M.; Arlorio, M.; Coïsson, J.D. Global volatile signature and polyphenols patterns in Vespolina wines according to vintage. Int. J. Food Sci. Technol. 2020, 56, 1551–1561. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Feucht, W.; Polster, J. Nuclei of Plants as a Sink for Flavanols. Z. Nat. C 2001, 56, 479–482. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, E.R.; Eisele, T.; Giusti, M.M.; Hofsommer, H.; Koswig, S.; Krueger, A.D.; Kupina, S.; Martin, S.K.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Mitrevska, K.; Grigorakis, S.; Loupassaki, S.; Calokerinos, A.C. Antioxidant Activity and Polyphenolic Content of North Macedonian Wines. Appl. Sci. 2020, 10, 2010. [Google Scholar] [CrossRef] [Green Version]
- Lamuela-Raventos, R.M.; Waterhouse, A.L. A direct HPLC separation of wine phenolics. Am. J. Enol. Vitic. 1994, 45, 1–5. [Google Scholar]
- Arsa, S.; Wipatanawin, A.; Suwapanich, R.; Makkerdchoo, O.; Chatsuwan, N.; Kaewthong, P.; Pinsirodom, P.; Taprap, R.; Haruenkit, R.; Poovarodom, S.; et al. Properties of Different Varieties of Durian. Appl. Sci. 2021, 11, 5653. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.; Hernández, M.T.; Martín-Álvarez, A.P.J.; Polo, M.C. Study of Low Molecular Weight Phenolic Compounds during the Aging of Sparkling Wines Manufactured with Red and White Grape Varieties. J. Agric. Food Chem. 2003, 51, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Indices | CSCarmel1 | CSCarmel2 | CSYarden1 | CSYarden2 |
|---|---|---|---|---|
| Polyph, mgGAE | 2190.83 ± 9.43 a | 2230.73 ± 8.72 a | 1560.33 ± 6.32 b | 1610.42 ± 6.21 b |
| Flavan, mgCE | 241.84 ± 3.62 b | 253.94 ± 2.92 ab | 272.51 ± 4.33 a | 283.63 ± 3.73 a |
| Flavon, mgCE | 408.63 ± 3.63 a | 418.63 ± 5.11 a | 292.42 ± 2.54 b | 302.62 ± 5.24 ab |
| Tannins, mgCE | 152.54 ± 1.82 a | 156.24 ± 1.42 a | 51.33 ± 0.92 b | 52.43 ± 0.73 b |
| Anthoc, mgCGE | 137.53 ± 2.24 a | 140.23 ± 2.93 a | 97.91 ± 1.83 b | 101.22 ± 2.93 b |
| ABTS, mMTE | 19.84 ± 0.34 a | 20.45 ± 1.12 a | 13.94 ± 1.11 b | 14.85 ± 1.23 b |
| FRAP, mMTE | 5.84 ± 0.54 a | 6.18 ± 0.61 a | 4.12 ± 0.34 b | 4.46 ± 0.25 ab |
| CUPRAC, mMTE | 27.11 ± 1.14 a | 28.33 ± 1.65 a | 19.64 ± 1.65 b | 20.47 ± 1.76 b |
| DPPH, mMTE | 9.65 ± 0.87 a | 10.52 ± 1.12 a | 7.14 ± 0.65 b | 7.36 ± 0.73 b |
| Rutin, mg | 8.63 ± 0.54 a | 9.25 ± 0.87 a | 6.81 ± 0.56 b | 6.53 ± 0.55 b |
| Resveratro, mg | 2.15 ± 0.18 ab | 2.98 ± 0.12 a | 1.71 ± 0.17 b | 1.91 ± 0.17 ab |
| Quercetin, mg | 7.32 ± 0.41 ab | 8.24 ± 0.61 a | 5.74 ± 0.43 c | 6.49 ± 0.43 b |
| Caffeic acid, mg | 10.15 ± 0.97 a | 11.24 ± 1.12 a | 8.64 ± 0.76 b | 9.45 ± 0.75 ab |
| Catechin, mg | 40.21 ± 0.37 a | 42.17 ± 0.46 a | 31.18 ± 0.23 b | 34.15 ± 0.22 ab |
| Epicatechin, mg | 26.14 ± 2.33 a | 28.65 ± 2.43 a | 21.94 ± 2.09 b | 23.18 ± 1.89 ab |
| Indices | CSCarmel 1 | CSCarmel 2 | CSYarden 1 | CSYarden 2 | Tannic Acid | Quercetin | Caffeic Acid | Ethanol | Fib/HSA |
|---|---|---|---|---|---|---|---|---|---|
| λem/ex, nm, peak a Fib | 230/341 | 230/341 | 230/340 | 230/341 | 229/343 | 228/340 | 229/340 | 228/341 | 229/342 |
| FI, A.U., peak a Fib | 468.8 ± 7.2 c | 478.4 ± 8.2 c | 484.6 ± 5.6 c | 499.6 ± 7.3 c | 657.4 ± 5.9 b | 861.0 ± 8.5 a | 836.1 ± 8.9 a | 865.5 ± 7.9 a | 883.6 ± 7.9 a |
| BP, %, peak a Fib | 46.9 ± 3.8a | 45.9 ± 6.9 a | 45.2 ± 3.4 a | 43.5 ± 7.1 a | 26.6 ± 3.9 b | 2.6 ± 0.7 d | 5.4 ± 0.7 c | 2.1 ± 0.3 d | - |
| λem/ex, nm, peak b Fib | 280/345 | 281/345 | 278/344 | 278/345 | 280/347 | 280/343 | 278/341 | 281/342 | 282/341 |
| FI, A.U., peak b Fib | 524.6 ± 9.3 c | 535.3 ± 6.9 c | 679.3 ± 5.3 bc | 702.3 ± 8.3 b | 595.9 ± 5.4 c | 706.9 ± 8.2 b | 768.7 ± 7.1 ab | 797.1 ± 7.8 ab | 811.7 ± 8.4 a |
| BP, %, peak b Fib | 35.4 ± 4.4 a | 34.1 ± 2.8 a | 16.3 ± 1.4 bc | 13.7 ± 1.2 bc | 26.6 ± 5.1 b | 12.9 ± 1.1 c | 5.3 ± 0.7 d | 1.8 ± 0.1 e | - |
| λem/ex, nm, peak a HSA | 227/356 | 228/357 | 226/354 | 226/355 | 226/358 | 229/356 | 225/358 | 228/355 | 228/353 |
| FI, A.U., peak a HSA | 406.2 ± 7.3 c | 414.5 ± 7.2 c | 411.5 ± 4.3 c | 424.9 ± 3.1c | 579.6 ± 4.7 b | 582.8 ± 5.1 b | 626.9 ± 7.4 a | 633.0 ± 9.1 a | 643.0 ± 6.3 a |
| BP, %, peak a HSA | 36.9+3.8 a | 35.5 ± 1.8 a | 36.0 ± 3.2 a | 34.0 ± 3.2 a | 9.9 ± 0.9 b | 9.4 ± 0.7 b | 2.5 ± 0.4 c | 1.6 ± 0.4 c | - |
| λem/ex, nm, peak b HSA | 278/359 | 278/359 | 279/360 | 280/360 | 280/359 | 280/356 | 279/360 | 280/356 | 280/357 |
| FI, A.U., peak b HSA | 843.0 ± 6.4 ab | 860.2 ± 7.4 ab | 771.0 ± 7.4 b | 794.8 ± 5.2 b | 818.9 ± 9.9 ab | 867.4 ± 8.8 ab | 865.5 ± 7.9 ab | 910.9 ± 8.3 a | 920.1 ± 10.3 a |
| BP, %, peak b HSA | 8.4 ± 0.7 bc | 6.5 ± 0.8 c | 16.2 ± 1.5 a | 13.6 ± 1.9 ab | 11.0 ± 1.0 b | 5.7 ± 0.4 c | 5.9 ± 0.6 c | 1.0 ± 0.9 d | - |
| Compound Name | PubChem ID | Binding Affinity (kcal/mol) | |||
|---|---|---|---|---|---|
| CRP | Fibrinogen | GPX3 | HSA | ||
| Epicatechin | 72276 | −7.8 | −5.1 | −6.6 | −8.9 |
| Epigallocatechin | 72277 | −8.3 | −6.3 | −6.4 | −8.6 |
| Resveratrol | 445154 | −7.4 | −6.1 | −6.8 | −9.1 |
| Rutin | 5280805 | −8.7 | −7.9 | −7.4 | −9.9 |
| Quercetin | 5280343 | −8.7 | −5.3 | −6.8 | −9.2 |
| Gallic acid | 370 | −6.3 | −5.7 | −6.2 | −6.2 |
| Tannic acid | 16129778 | −7.7 | −6.4 | −7.3 | −10.4 |
| Myricetin | 5281672 | −8.4 | −6.3 | −6.8 | −9 |
| Caffeic acid | 689043 | −6.4 | −5 | −5.7 | −7.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafreen, R.M.B.; Lakshmi, S.A.; Pandian, S.K.; Kim, Y.-M.; Deutsch, J.; Katrich, E.; Gorinstein, S. In Vitro and In Silico Interaction Studies with Red Wine Polyphenols against Different Proteins from Human Serum. Molecules 2021, 26, 6686. https://doi.org/10.3390/molecules26216686
Shafreen RMB, Lakshmi SA, Pandian SK, Kim Y-M, Deutsch J, Katrich E, Gorinstein S. In Vitro and In Silico Interaction Studies with Red Wine Polyphenols against Different Proteins from Human Serum. Molecules. 2021; 26(21):6686. https://doi.org/10.3390/molecules26216686
Chicago/Turabian StyleShafreen, Raja Mohamed Beema, Selvaraj Alagu Lakshmi, Shunmugiah Karutha Pandian, Young-Mo Kim, Joseph Deutsch, Elena Katrich, and Shela Gorinstein. 2021. "In Vitro and In Silico Interaction Studies with Red Wine Polyphenols against Different Proteins from Human Serum" Molecules 26, no. 21: 6686. https://doi.org/10.3390/molecules26216686
APA StyleShafreen, R. M. B., Lakshmi, S. A., Pandian, S. K., Kim, Y.-M., Deutsch, J., Katrich, E., & Gorinstein, S. (2021). In Vitro and In Silico Interaction Studies with Red Wine Polyphenols against Different Proteins from Human Serum. Molecules, 26(21), 6686. https://doi.org/10.3390/molecules26216686