Untargeted Metabolomics Combined with Bioassay Reveals the Change in Critical Bioactive Compounds during the Processing of Qingzhuan Tea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Chemical Reagents
2.2. Sample Preparation
2.3. Sensory Evaluation
2.4. Determination the Contents of Main Compounds by HPLC
2.5. Untargeted Metabolomics Analysis
2.6. Inhibitory Effects of QZT Samples on α-Amylase and α-Glucosidase
2.7. In Vitro Antioxidant Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Sensory Evaluation
3.2. Contents of Main Compounds in QZT Samples
3.3. Contents of Catechins during Processing
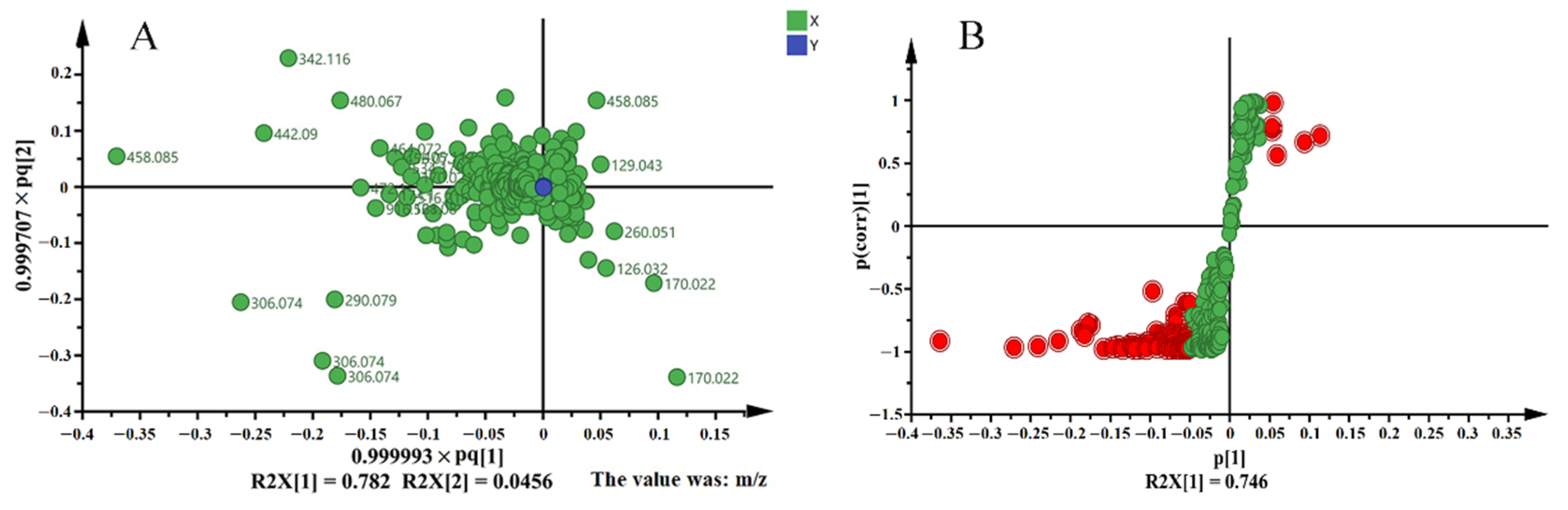
3.4. Multivariate Analysis of LC-MS Based Metabolomics Data
3.5. Inhibitory Effects on α-Amylase and α-Glucosidase
3.6. Antioxidant Activities
3.7. Correlation Analysis between Chemical Compositions and Biological Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Samples Availability
References
- Cheng, Q.; Cai, S.; Ni, D.; Wang, R.; Zhou, F.; Ji, B.; Chen, Y. In vitro antioxidant and pancreatic α-amylase inhibitory activity of isolated fractions from water extract of Qingzhuan tea. J. Food Sci. Technol. 2013, 52, 928–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Huang, M.; Qin, C.; Lv, B.; Mao, Q.; Liu, Z. Structural characterization and evaluation of the antioxidant activities of polysaccharides extracted from Qingzhuan brick tea. Int. J. Biol. Macromol. 2017, 101, 768–775. [Google Scholar] [CrossRef]
- Zhang, L.; Ho, C.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and Biological Activities of Processed Camellia sinensis Teas: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Yang, Q.-Q.; Chen, Z.; Zhang, J.-R.; Chen, Q.; Wang, Y.; Wei, X. Distinct Changes of Metabolic Profile and Sensory Quality during Qingzhuan Tea Processing Revealed by LC-MS-Based Metabolomics. J. Agric. Food Chem. 2020, 68, 4955–4965. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.-J.; Wan, X.-C.; Bao, G.-H. Brick dark tea: A review of the manufacture, chemical constituents and bioconversion of the major chemical components during fermentation. Phytochem. Rev. 2015, 14, 499–523. [Google Scholar] [CrossRef]
- Feng, L.; Zhou, J.; Zhang, L.; Liu, P.; Zheng, P.; Gao, S.; Song, C.; Yu, Y.; Gong, Z.; Wan, X. Gut microbiota-mediated improvement of metabolic disorders by Qingzhuan tea in high fat diet-fed mice. J. Funct. Foods 2021, 78, 104366. [Google Scholar] [CrossRef]
- Li, P.; Dai, W.; Lu, M.; Xie, D.; Tan, J.; Yang, C.; Zhu, Y.; Lv, H.; Peng, Q.; Zhang, Y.; et al. Metabolomic analysis reveals the composition differences in 13 Chinese tea cultivars of different manufacturing suitabilities. J. Sci. Food Agric. 2017, 98, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Deng, W.-W.; Wan, X.-C. Advantage of LC-MS metabolomics to identify marker compounds in two types of Chinese dark tea after different post-fermentation processes. Food Sci. Biotechnol. 2014, 23, 355–360. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.-Z.; Zhou, Y.-B.; Ling, T.-J.; Wan, X.-C. Chinese dark teas: Postfermentation, chemistry and biological activities. Food Res. Int. 2013, 53, 600–607. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Y.; Long, P.; Ho, C.-T.; Wang, Y.; Kan, Z.; Cao, L.; Zhang, L.; Wan, X. LC-MS-Based Metabolomics Reveals the Chemical Changes of Polyphenols during High-Temperature Roasting of Large-Leaf Yellow Tea. J. Agric. Food Chem. 2019, 67, 5405–5412. [Google Scholar] [CrossRef]
- Long, P.; Wen, M.; Granato, D.; Zhou, J.; Wu, Y.; Hou, Y.; Zhang, L. Untargeted and targeted metabolomics reveal the chemical characteristic of pu-erh tea (Camellia assamica) during pile-fermentation. Food Chem. 2019, 311, 125895. [Google Scholar] [CrossRef] [PubMed]
- Shalini, V.; Hussain, M.E. Obesity and diabetes: An update. Diabetes Metab. Syndr. 2017, 11, 73–79. [Google Scholar]
- Cai, X.; Liu, Z.; Dong, X.; Wang, Y.; Zhu, L.; Li, M.; Xu, Y. Hypoglycemic and lipid lowering effects of theaflavins in high-fat diet-induced obese mice. Food Funct. 2021, 12, 9922–9931. [Google Scholar] [CrossRef] [PubMed]
- Falla, N.; Demasi, S.; Caser, M.; Scariot, V. Phytochemical Profile and Antioxidant Properties of Italian Green Tea, a New High Quality Niche Product. Horticulturae 2021, 7, 91. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.; Sun, L.; Lai, X.; Li, Q.; Zhang, W.; Xiang, L.; Sun, S.; Cao, F. Six types of tea reduce high-fat-diet-induced fat accumulation in mice by increasing lipid metabolism and suppressing inflammation. Food Funct. 2019, 10, 2061–2074. [Google Scholar] [CrossRef] [PubMed]
- Roda, G.; Marinello, C.; Grassi, A.; Picozzi, C.; Aldini, G.; Carini, M.; Regazzoni, L. Ripe and Raw Pu-Erh Tea: LC-MS Profiling, Antioxidant Capacity and Enzyme Inhibition Activities of Aqueous and Hydro-Alcoholic Extracts. Molecules 2019, 24, 473. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Liu, P.; Zheng, P.; Zhang, L.; Zhou, J.; Gong, Z.; Yu, Y.; Gao, S.; Zheng, L.; Wang, X.; et al. Chemical profile changes during pile fermentation of Qingzhuan tea affect inhibition of α-amylase and lipase. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.-J.; Wei, X.-L.; Liu, H.-Y.; Li, H.; Xia, Y.; Wu, D.-T.; Zhang, P.-Z.; Gandhi, G.R.; Li, H.-B.; Gan, R.-Y. State-of-the-art review of dark tea: From chemistry to health benefits. Trends Food Sci. Technol. 2021, 109, 126–138. [Google Scholar] [CrossRef]
- Zhu, M.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.-M.; Xu, W.; Li, J.; Lin, H.-Y.; Zhang, Z.; Xiao, J.; et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2019, 312, 126043. [Google Scholar] [CrossRef]
- Guo, X.; Long, P.; Meng, Q.; Ho, C.-T.; Zhang, L. An emerging strategy for evaluating the grades of Keemun black tea by combinatory liquid chromatography-Orbitrap mass spectrometry-based untargeted metabolomics and inhibition effects on α-glucosidase and α-amylase. Food Chem. 2018, 246, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. Simultaneous automated measurement of total ‘antioxidant’ (reducing) capacity and ascorbic acid concentration. Redox Rep. 1997, 3, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Zhang, L.; Santos, J.S.; Cruz, T.M.; Marques, M.B.; Carmo, M.A.V.D.; Azevedo, L.; Wang, Y.; Granato, D. Multivariate effects of Chinese keemun black tea grades (Camellia sinensis var. sinensis) on the phenolic composition, antioxidant, antihemolytic and cytotoxic/cytoprotection activities. Food Res. Int. 2019, 125, 108516. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, Y.; Zhang, J.; Xu, L.; Zhou, H.; Wei, K.; Peng, L.; Zhang, J.; Liu, Z.; Wei, X. Integration of non-targeted metabolomics and E-tongue evaluation reveals the chemical variation and taste characteristics of five typical dark teas. LWT 2021, 150, 111875. [Google Scholar] [CrossRef]
- Li, Q.; Jin, Y.; Jiang, R.; Xu, Y.; Zhang, Y.; Luo, Y.; Huang, J.; Wang, K.; Liu, Z. Dynamic changes in the metabolite profile and taste characteristics of Fu brick tea during the manufacturing process. Food Chem. 2020, 344, 128576. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.-P.; Zhang, Y.-J.; Lin, Z.; Liang, Y.-R. Processing and chemical constituents of Pu-erh tea: A review. Food Res. Int. 2013, 53, 608–618. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, C.; Ma, C.; Ma, B.; Wang, J.; Zhou, B.; Xia, T. Comparative analysis of chemical constituents and antioxidant activity in tea-leaves microbial fermentation of seven tea-derived fungi from ripened Pu-erh tea. LWT 2021, 142, 111006. [Google Scholar] [CrossRef]
- Lv, H.-P.; Zhang, Y.; Shi, J.; Lin, Z. Phytochemical profiles and antioxidant activities of Chinese dark teas obtained by different processing technologies. Food Res. Int. 2017, 100, 486–493. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, S.; Dai, J.; Wang, L.; Xu, Y.; Peng, X.; Xie, X.; Peng, C. Molecular mechanisms and applications of tea polyphenols: A narrative review. J. Food Biochem. 2021, 45, e13910. [Google Scholar] [CrossRef]
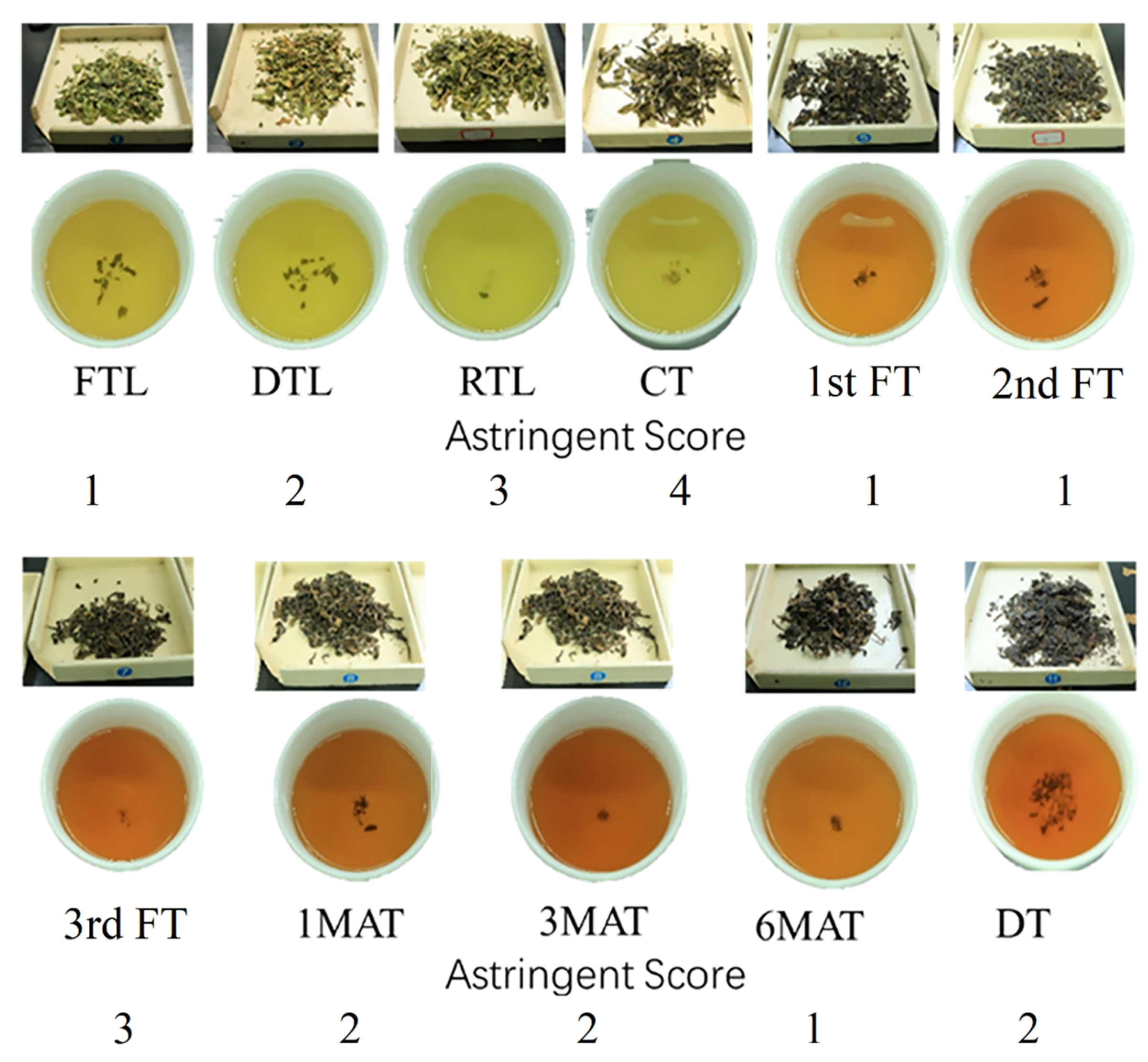



| Samples | Aqueous Extracts | Total Polyphenols | Total Amino Acids | Total Flavonoids | Total Soluble Sugars | Gallic Acid | Caffeine | Theobromine |
|---|---|---|---|---|---|---|---|---|
| FTL | 37.71 ± 2.21 a | 18.18 ± 1.06 a | 1.37 ± 0.08 a | 2.24 ± 0.03 a | 5.92 ± 0.62 a | 0.08 ± 0.00 c | 2.80 ± 0.16 a,b | 0.08 ± 0.00 d |
| DTL | 35.57 ± 0.51 a,b | 16.33 ± 1.11 a,b | 1.13 ± 0.04 a,b | 2.07 ± 0.02 b | 5.49 ± 1.18 a | 0.09 ± 0.00 c | 2.57 ± 0.09 c,d | 0.07 ± 0.00 e |
| RTL | 36.31 ± 0.63 a,b | 15.99 ± 0.85 b | 0.95 ± 0.04 b | 2.09 ± 0.02 b | 5.69 ± 0.49 a | 0.07 ± 0.01 c | 2.50 ± 0.11 c,d | 0.07 ± 0.00 f |
| CT | 35.47 ± 0.52 a,b | 15.61 ± 0.49 b | 0.83 ± 0.25 b,c | 1.95 ± 0.09 b | 4.93 ± 0.05 a,b | 0.15 ± 0.01 bc | 2.92 ± 0.15 a | 0.09 ± 0.00 b,c |
| 1st FT | 34.69 ± 0.15 b | 12.87 ± 0.34 c | 0.76 ± 0.12 b,c | 1.78 ± 0.11 c | 6.05 ± 0.39 a,b | 0.07 ± 0.00 c | 2.59 ± 0.09 c | 0.09 ± 0.00 c |
| 2nd FT | 35.14 ± 0.29 b,c | 9.77 ± 0.97 d | 0.69 ± 0.06 b,c | 1.63 ± 0.05 d | 4.79 ± 0.46 a,b | 0.08 ± 0.01 c | 2.70 ± 0.13 b | 0.10 ± 0.00 b |
| 3rd FT | 34.70 ± 0.33 b,c | 7.08 ± 0.23 e | 0.57 ± 0.04 c | 1.55 ± 0.03 d,e | 3.99 ± 0.52 a,b | 0.46 ± 0.04 a | 2.81 ± 0.10 b | 0.08 ± 0.00 e |
| 1MAT | 34.16 ± 0.38 b,c | 5.58 ± 0.24 e,f | 0.53 ± 0.02 c,d | 1.51 ± 0.02 d,e | 3.26 ± 0.42 b | 0.43 ± 0.04 a | 2.65 ± 0.15 b,c | 0.09 ± 0.00 c,d |
| 3MAT | 34.03 ± 0.52 b,c | 5.14 ± 0.11 f | 0.51 ± 0.03 c,d | 1.47 ± 0.02 e | 3.32 ± 0.53 b | 0.43 ± 0.02 a | 2.54 ± 0.14 c | 0.06 ± 0.00 f |
| 6MAT | 34.19 ± 0.43 b,c | 4.52 ± 0.31 f | 0.47 ± 0.01 d | 1.43 ± 0.02 e | 3.33 ± 0.30 b | 0.37 ± 0.01 a | 2.38 ± 0.13 d | 0.08 ± 0.00 e |
| DT | 32.72 ± 0.91 c | 4.16 ± 0.17 f | 0.43 ± 0.01 d | 1.44 ± 0.03 e | 3.25 ± 0.21 b | 0.25 ± 0.02 b | 2.47 ± 0.13 c,d | 0.11 ± 0.01 a |
| Samples | GC | EGC | C | EGCG | EC | GCG | ECG | CG | Total Catechins | Galloylated/Non-Galloylated Catechins |
|---|---|---|---|---|---|---|---|---|---|---|
| FTL | 0.98 ± 0.02 a | 1.36 ± 0.04 a | 0.22 ± 0.01 b | 3.28 ± 0.09 a | 0.62 ± 0.03 a,b | 0.84 ± 0.04 b | 0.94 ± 0.04 a | 0.17 ± 0.01 b,c | 8.41 ± 0.17 a | 1.64 ± 0.02 c,d |
| DTL | 0.91 ± 0.01 b | 1.18 ± 0.05 b | 0.25 ± 0.00 a | 3.03 ± 0.10 a,b | 0.54 ± 0.01 b,c | 1.01 ± 0.07 a | 0.79 ± 0.02 b | 0.20 ± 0.01 a,b | 7.91 ± 0.19 a,b | 1.74 ± 0.02 b |
| RTL | 0.64 ± 0.03 c | 1.37 ± 0.04 a | 0.19 ± 0.01 c | 3.08 ± 0.08 a,b | 0.50 ± 0.01 c | 0.81 ± 0.04 b | 0.83 ± 0.03 b | 0.16 ± 0.01 b,c | 7.58 ± 0.14 b | 1.80 ± 0.03 a |
| CT | 0.65 ± 0.01 c | 1.22 ± 0.03 b | 0.18 ± 0.01 c | 2.85 ± 0.08 b | 0.67 ± 0.03 a | 0.58 ± 0.03 c | 0.82 ± 0.03 b | 0.12 ± 0.01 c | 7.09 ± 0.15 b,c | 1.60 ± 0.03 d |
| 1st FT | 0.56 ± 0.02 d | 1.00 ± 0.06 c | 0.24 ± 0.01 a,b | 2.70 ± 0.12 bc | 0.57 ± 0.03 b | 0.42 ± 0.03 d | 0.75 ± 0.02 b | 0.23 ± 0.02 a | 6.47 ± 0.18 c | 1.73 ± 0.02 b |
| 2nd FT | 0.60 ± 0.01 c,d | 1.00 ± 0.02 c | 0.18 ± 0.01 c | 2.20 ± 0.06 c | 0.43 ± 0.03 d | 0.62 ± 0.03 c | 0.60 ± 0.02 c | 0.11 ± 0.01 c,d | 5.74 ± 0.12 c,d | 1.60 ± 0.04 d,e |
| 3rd FT | 0.40 ± 0.02 e | 0.85 ± 0.04 d | 0.14 ± 0.01 d | 1.62 ± 0.08 d | 0.29 ± 0.03 e,f | 0.48 ± 0.04 d | 0.50 ± 0.01 d | 0.17 ± 0.02 b | 4.45 ± 0.15 d | 1.66 ± 0.01 c |
| 1MAT | 0.38 ± 0.01 e | 0.63 ± 0.02 e | 0.11 ± 0.00 f | 1.06 ± 0.03 e | 0.23 ± 0.02 f | 0.27 ± 0.01 e | 0.36 ± 0.02 e,f | 0.09 ± 0.01 c,d | 3.13 ± 0.08 e | 1.32 ± 0.02 g |
| 3MAT | 0.30 ± 0.01 f | 0.46 ± 0.01 f | 0.11 ± 0.01 e,f | 0.98 ± 0.03 e | 0.32 ± 0.01 e | 0.28 ± 0.01 e | 0.42 ± 0.02 e | 0.08 ± 0.02 d | 2.95 ± 0.07 e | 1.48 ± 0.03 f |
| 6MAT | 0.14 ± 0.01 g | 0.27 ± 0.01 g | 0.13 ± 0.01 d,e | 0.67 ± 0.02 f | 0.29 ± 0.02 e,f | 0.23 ± 0.01 e | 0.32 ± 0.01 f | 0.09 ± 0.02 c,d | 2.14 ± 0.05 f | 1.57 ± 0.04 d,e |
| DT | 0.15 ± 0.03 g | 0.23 ± 0.01 g | 0.09 ± 0.00 f | 0.20 ± 0.02 g | 0.24 ± 0.01 f | 0.14 ± 0.01 f | 0.35 ± 0.02 e,f | 0.08 ± 0.01 c,d | 1.48 ± 0.04 f | 1.09 ± 0.08 h |
| No. | tR(min) | MW | VIP Value | Identification |
|---|---|---|---|---|
| 1 | 6.282 | 458.085 | 5.67 | (−)—Epigallocatechin gallate |
| 2 | 3.141 | 306.074 | 4.19 | (−)—Epigallocatechin |
| 3 | 4.659 | 290.079 | 3.82 | (−)—Catechin |
| 4 | 0.484 | 342.116 | 3.68 | Coniferin |
| 5 | 7.780 | 442.090 | 3.48 | (−)—Epicatechin gallate |
| 6 | 0.934 | 170.022 | 3.24 | Gallic acid |
| 7 | 6.283 | 480.067 | 3.07 | Myricetin-3-O-galatoside |
| 8 | 7.638 | 472.100 | 2.20 | Methoxy-epigallocatechin gallate |
| 9 | 7.780 | 464.072 | 2.05 | Quercetin-3-O-glucopyranoside |
| 10 | 6.282 | 916.168 | 2.04 | Assamicain A/B/C |
| 11 | 6.283 | 456.070 | 1.87 | Methoxy-epicatechin gallate |
| 12 | 0.501 | 174.100 | 1.85 | l-theanine |
| 13 | 0.497 | 534.179 | 1.75 | Unknown |
| 14 | 6.282 | 521.080 | 1.73 | Unknown |
| 15 | 0.483 | 388.122 | 1.66 | Dihydrovomifoliol-O-glucopyranoside |
| 16 | 0.493 | 516.216 | 1.64 | Di-caffeoylquinic acid |
| 17 | 0.484 | 405.112 | 1.64 | Unknown |
| 18 | 3.171 | 307.077 | 1.63 | Unknown |
| 19 | 0.551 | 406.109 | 1.53 | Unknown |
| 20 | 3.027 | 304.058 | 1.48 | Taxifolin |
| 21 | 7.160 | 458.085 | 1.45 | (−)—Gallocatechin gallate |
| 22 | 7.637 | 494.083 | 1.43 | Unknown |
| 23 | 3.143 | 374.061 | 1.43 | Unknown |
| 24 | 1.051 | 344.074 | 1.42 | Theogallin |
| 25 | 0.934 | 126.032 | 1.41 | Unknown |
| 26 | 4.662 | 358.066 | 1.41 | Junipetrioloside A |
| 27 | 6.280 | 548.054 | 1.38 | Unknown |
| 28 | 8.664 | 456.106 | 1.28 | (−)—Epicatechin gallate-3”-O-Me |
| 32 | 0.481 | 182.079 | 1.21 | Unknown |
| 34 | 5.104 | 578.142 | 1.11 | Procyanidin B2 |
| 35 | 6.284 | 938.15 | 1.09 | Unknown |
| 37 | 6.278 | 454.054 | 1.06 | Unknown |
| 38 | 1.044 | 366.056 | 1.05 | Unknown |
| 39 | 6.281 | 478.051 | 1.03 | 7-methoxy-kaempferol-glucopyranoside |
| 41 | 7.790 | 772.206 | 1.01 | Quercetin 3-O-glucopyranosyl-O-glucopyranosyl-O-rhamnoside |
| Marker Compounds | Pearson Correlation Coefficient | ||||
|---|---|---|---|---|---|
| ABTS | DPPH | FRAP | α-Amylase | α-Glucosidase | |
| Myricetin-3-O-galactoside | 0.719 * | 0.713 * | 0.799 ** | 0.552 | 0.724 * |
| Methoxy-epigallocatechin gallate | 0.819 ** | 0.764 ** | 0.829 ** | 0.600 | 0.687 * |
| Quercetin-3-O-glucopyranoside | 0.756 ** | 0.720 * | 0.785 ** | 0.561 | 0.750 ** |
| Assamicain A/B/C | 0.832 ** | 0.765 ** | 0.817 ** | 0.586 | 0.661 * |
| Methoxy-epicatechin gallate | 0.768 ** | 0.734 * | 0.789 ** | 0.595 | 0.738 ** |
| l-theanine | 0.807 ** | 0.743 ** | 0.829 ** | 0.555 | 0.648 * |
| Trihydroxyflavone-di-arabinopyranoside | 0.781 ** | 0.701 * | 0.776 ** | 0.602 * | 0.717 * |
| 521.080 (unknown compound) | 0.828 ** | 0.763 ** | 0.791 ** | 0.615 * | 0.658 * |
| Dihydrovomifoliol-O-glucopyranoside | 0.702 * | 0.645 * | 0.729 * | 0.580 | 0.781 ** |
| Di-O-caffeoylquinic acid | 0.823 ** | 0.746 ** | 0.815 ** | 0.573 | 0.667 * |
| 405.111 (unknown compound) | 0.760 ** | 0.680 * | 0.761 ** | 0.593 | 0.741 ** |
| 307.077 (unknown compound) | 0.886 ** | 0.841 ** | 0.863 ** | 0.701 * | 0.512 |
| 406.108 (unknown compound) | 0.793 ** | 0.721 * | 0.791 ** | 0.705 * | 0.543 |
| Pentahydroxyflavanone | 0.889 ** | 0.840 ** | 0.854 ** | 0.745 ** | 0.423 |
| 494.061 (unknown compound) | 0.793 ** | 0.733 * | 0.822 ** | 0.528 | 0.703 * |
| 374.061 (unknown compound) | 0.880 ** | 0.830 ** | 0.834 ** | 0.713 * | 0.516 |
| Theogallin | 0.108 | 0.075 | 0.242 | 0.045 | 0.764 ** |
| 126.031 (unknown compound) | −0.469 | −0.431 | −0.522 | −0.304 | −0.939 ** |
| Junipetrioloside A | 0.862 ** | 0.795 ** | 0.854 ** | 0.616 * | 0.486 |
| 548.054 (unknown compound) | 0.612 * | 0.658 * | 0.702 * | 0.640 * | 0.679 * |
| (−)—epicatechin gallate-3”-O-Me | 0.791 ** | 0.750 ** | 0.811 ** | 0.598 | 0.720 * |
| 182.079 (unknown compound) | −0.325 | −0.285 | −0.379 | −0.224 | −0.965 ** |
| Procyanidin B2 | −0.704 * | −0.568 | −0.554 | −0.278 | −0.453 |
| 938.150 (unknown compound) | 0.826 ** | 0.765 ** | 0.824 ** | 0.574 | 0.670 * |
| 454.053 (unknown compound) | 0.750 ** | 0.740 ** | 0.783 ** | 0.637 * | 0.708 * |
| 366.056 (unknown compound) | 0.447 | 0.534 | 0.552 | 0.393 | 0.806 ** |
| 7-methoxy-kaempferol-glucopyranoside | 0.585 | 0.589 | 0.716 * | 0.411 | 0.751 ** |
| Quercetin 3-O-glucopyranosyl-O-glucopyranosyl-O-rhamnoside | 0.772 ** | 0.711 * | 0.812 ** | 0.554 | 0.637 * |
| Aqueous extracts | 0.55 | 0.633 * | 0.768 ** | 0.376 | 0.553 |
| Total polyphenols | 0.620 * | 0.599 | 0.727 * | 0.479 | 0.736 ** |
| Total amino acids | 0.592 | 0.566 | 0.717 * | 0.269 | 0.684 * |
| Total flavonoids | 0.683 * | 0.638 * | 0.751 ** | 0.462 | 0.718 * |
| Total soluble sugars | 0.364 | 0.351 | 0.485 | 0.350 | 0.688 * |
| Gallic acid | −0.272 | −0.382 | −0.329 | −0.267 | −0.748 ** |
| Caffeine | 0.207 | 0.294 | 0.406 | 0.084 | 0.199 |
| GC | 0.504 | 0.524 | 0.679 * | 0.204 | 0.753 ** |
| EGC | 0.541 | 0.588 | 0.684 * | 0.447 | 0.689 * |
| C | 0.354 | 0.314 | 0.524 | 0.274 | 0.653 * |
| EGCG | 0.510 | 0.528 | 0.682 * | 0.445 | 0.714 * |
| EC | 0.448 | 0.46 | 0.639 * | 0.477 | 0.684 * |
| GCG | 0.624 * | 0.684 * | 0.754 ** | 0.382 | 0.626 * |
| ECG | 0.531 | 0.53 | 0.661 * | 0.454 | 0.709 * |
| CG | 0.277 | 0.075 | 0.314 | 0.210 | 0.373 |
| Total catechins | 0.535 | 0.555 | 0.698 * | 0.421 | 0.718 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, P.-C.; Qin, C.-Y.; Liu, P.-P.; Feng, L.; Ling, T.-J.; Ning, J.-M.; Zhang, L.; Wan, X.-C. Untargeted Metabolomics Combined with Bioassay Reveals the Change in Critical Bioactive Compounds during the Processing of Qingzhuan Tea. Molecules 2021, 26, 6718. https://doi.org/10.3390/molecules26216718
Zheng P-C, Qin C-Y, Liu P-P, Feng L, Ling T-J, Ning J-M, Zhang L, Wan X-C. Untargeted Metabolomics Combined with Bioassay Reveals the Change in Critical Bioactive Compounds during the Processing of Qingzhuan Tea. Molecules. 2021; 26(21):6718. https://doi.org/10.3390/molecules26216718
Chicago/Turabian StyleZheng, Peng-Cheng, Chun-Yin Qin, Pan-Pan Liu, Lin Feng, Tie-Jun Ling, Jing-Ming Ning, Liang Zhang, and Xiao-Chun Wan. 2021. "Untargeted Metabolomics Combined with Bioassay Reveals the Change in Critical Bioactive Compounds during the Processing of Qingzhuan Tea" Molecules 26, no. 21: 6718. https://doi.org/10.3390/molecules26216718






